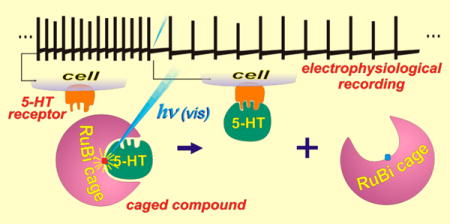
Abstract
Serotonin, or 5-hydroxytryptamine (5HT), is an important neurotransmitter in the nervous system of both vertebrates and invertebrates. Deficits in 5HT signaling are responsible for many disabling psychiatric conditions, and its molecular machinery is the target of many pharmaceuticals. We present a new 5HT phototrigger, the compound [Ru(bpy)2(PMe3)(5HT)]2+, where PMe3 is trimethylphosphine. As with other ruthenium-bipyridyl based caged compounds, [Ru(bpy)2(PMe3)(5HT)]2+ presents activity in the visible region of the spectrum. We characterize and discuss the photochemical properties of the caged compound, and demonstrate its use by modulating the excitability of mouse prefrontal principal neurons.
Keywords: Ruthenium complex, serotonin, phototriggers, neurophysiology
Graphical abstract
INTRODUCTION
A caged compound, or phototrigger, is a molecule reversibly bound to or “caged within” a protecting group that restricts its interactions with cell-membrane receptors, proteins, or other molecules. Entities that have been caged include ions,1 acids,2 bases,3 neurotransmitters,4 fluorophores,5,6 small-molecule drugs,7 gene inducers,8,9 and even nucleic acids10 and proteins.11,12 Phototriggers are light-sensitive, and will release the caged molecule upon irradiation with light of the proper wavelength,13 thus removing the restrictions for the caged entity to interact normally with its targets. As the uncaging spot size can be precisely determined by focusing a low-power laser, this technique can have very high spatial and temporal resolution, making it of great advantage in biological research, for it allows the delivery of molecules without mechanically acting on the preparation, a problem usually associated with more invasive techniques like microinjection.
Most common caged compounds comprise an organic protecting group, usually a nitrobenzyl14 or nitroindonilyl15 which are active in the UV region and therefore can damage the biological tissue. Therefore, new kinds of organic-based protecting groups have been developed, such as coumarine derivatives, which can extend the photoactivity to the violet region, thus reducing cell damage.16
As we have shown in recent years, ruthenium-bipyridyl coordination complexes yield excellent phototriggers suitable for neurophysiology studies. They present unique properties, including nanosecond photoreaction kinetics through heterolytic photocleavage with no secondary reaction products,7,17 and sensitivity to visible light in the blue and green regions.3,6,7,18–20 As an important advantage over other photoprotective groups, Rubipyridyl phototriggers can be used in a two-photon regime.17,21–24 Ruthenium phototriggers can also be used for general delivery of drugs, toward application in therapy. Turro and Bonnet and their co-workers have shown the versatility of Ru(II) polypyridines for caging nitriles and thioethers.25–27 A similar strategy using the photoredox properties of the Ru(II) polypyridyl chemistry was used by Ford and Santana da Silva to release nitric oxide (NO) by visible light, both directly or mediated by energy transfer processes from molecular antennas.28,29
Ruthenium phototriggers are very stable at room temperature, both as a solid or in physiological solutions when kept in the dark, minimizing dark leakage of the caged biomolecule, one of the most common drawbacks of nitrobenzyls and similar caging groups.
Serotonin is one of the most important neuromodulators in a very wide range of animal phyla. It modulates basic behaviors such as feeding and mating,30 and imbalances in its physiology lead to debilitating psychiatric conditions in humans, such as obsessive compulsive disorder, panic disorder, or major depression.31 For this reason, the serotonergic system is the target of many pharmaceuticals such as tricyclic antidepressants (TCAs) selective serotonin reuptake inhibitors (SSRIs) and monoaminooxidase inhibitors (MAOIs), and even many recreational drugs.32 Discerning the effects of 5HT on its various receptor and cellular subtypes and understanding its interactions with other systems of neurotransmitters would be greatly aided by a caged 5HT. As serotonin does not exhibit a carboxylate group, most strategies used to cage neuroactive amino acids cannot be extended directly to cage this amine. Currently, two of the commercially available caged serotonins are NPEC-5HT and BHQ-O-5HT, which use the phenol group of the 5HT for anchoring the photoremovable protecting group and are active under UV light (365 nm). While the first shows negligible absorption in two-photon regime33 and has a very slow response time in the hundreds of milliseconds,34 BHQ-O-5HT can also be used at 740 nm (2P) with good quantum yields.35,36 Other caged 5HT compounds have been described, showing dissimilar performances.36–40
We report the synthesis and chemical properties of the complex [Ru(bpy)2(PMe3)(5HT)]+2, which releases 5HT upon absorption of visible light, and demonstrate its use by modulating the excitability of mouse prefrontal principal cells that have previously been shown to be regulated by 5HT.41
RESULTS AND DISCUSSION
The complex [Ru(bpy)2(PMe3)(5HT)]2+ is yellow-orange in color. Its water solubility is well above the tens of millimolar range. No decomposition was detected in samples stored in the dark for months at room temperature either dry or in solution. Water solutions of this complex present a metal to ligand charge transfer (MLCT) band with a molar extinction coefficient (ε) of 6718 M−1 cm−1 and an absorption maximum in the visible range at 447 nm. Its synthesis is a simple one-pot reaction, and the photolysis is fast and clean, as depicted in Scheme 1.
Scheme 1.
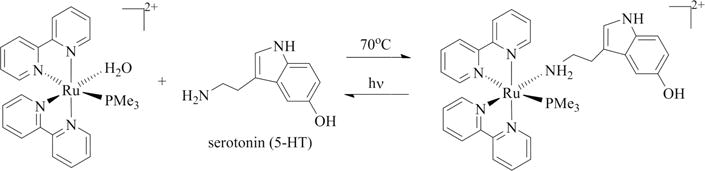
Coordination Reaction between Serotonin and [Ru(bpy)2(PMe3)(H2O)]2+ (Forward) and the Clean, One Step Photorelease Reaction (Reverse) Releasing Serotonin upon Light Absorption
Figure 1 shows the 1H NMR spectra of the complex (top) and of its partial photolysis (bottom). In the first, unphotolyzed spectrum, the aromatic region shows the expected eight doublets and eight triplets that are characteristic of the bipyridines in a cis octahedral complex of the form [Ru-(bpy)2(L1)(L2)]n+, and the four aromatic signals of 5HT. In the aliphatic region (Figure 1, right), besides the PMe3 signal at 0.92 ppm the two aliphatic signals for the amine and methylenes groups of coordinated 5HT can be seen between 3.60 and 1.71 ppm. The fact that proton signals of amine group can be seen in a D2O solution at 3.57 and 2.65 ppm and that the methylene signals shifts and split indicates the coordination of serotonin to the metal atom through its amine nitrogen. Also visible in the first spectrum are the set of signals corresponding to the trans configuration of the complex, although in much smaller proportion. The subtle differences in the photochemical behavior of the cis and trans configurations of the complex are negligible in the scope of this work, and no further distinction between these isomers will be made, although a full characterization of both isomers should be needed for any biomedical uses.
Figure 1.
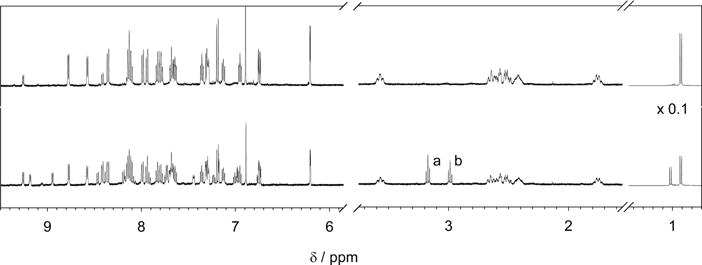
1H NMR spectra of a 5 mg/mL solution of [Ru(bpy)2(PMe3)(5HT)]Cl in D2O before irradiation (upper trace); and after 60 s irradiation with 525 nm green LED inside the NMR tube (lower trace). Note that after irradiation the triplets a and b appear, characteristics of free serotonin.
Once irradiated inside the NMR tube for 60 s, the photolysis progressed around 40%. The signals of the bipyridines in the original complex appear diminished, while a new set of signals corresponding to the aquo-complex are evident. In the aliphatic region, the two characteristic triplets of the methylenes of free 5HT appear at 3.17 and 2.98 ppm (Figure 1, signals a and b). Furthermore, amine protons are rapidly exchanged for deuterium and these signals are not present in the free 5HT. The signal from PMe3 protons is now shifted to higher fields and corresponds to that of the newly formed aquo complex.
To measure the quantum efficiency of photolysis, a water solution of the complex was irradiated with a 8.56 mW, 405 nm (violet) diode laser at pH = 7 and T = 24 °C. Figure 2 shows the UV-vis spectra during the progression of the photolysis reaction. As is deduced from the presence of an isosbestic point at 434 nm, the reaction yields just one absorbing product and proceeds to completion in around 12 min. The spectrum of the photoproduct corresponds to that of the aquo complex [Ru(bpy)2(PMe3)(H2O)]2+ accordingly with the known photochemistry of the complexes of the form [Ru(bpy)2(PMe3)-(L)]n+ in water at neutral pH.17,22,23 At pH > 12, a broader and red-shifted absorption spectrum is obtained, which corresponds in this case to the hydroxo complex [Ru(bpy)2(PMe3) (OH)]+.
Figure 2.
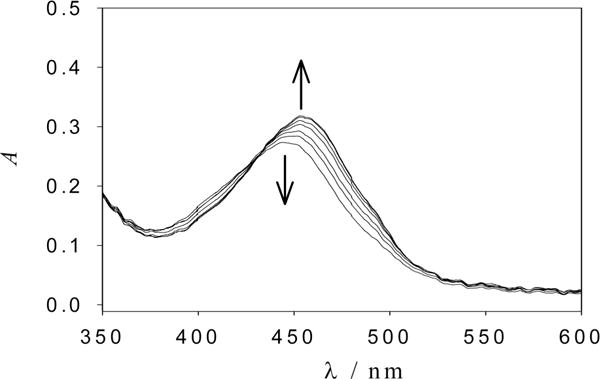
Subset of UV-vis spectra of [Ru(bpy)2(PMe3)(5HT)]2+ acquired in aqueous solution at pH = 7 while irradiating with a 405 nm laser diode. The arrows indicate photolysis progress.
The progression of the photoreaction is shown in Figure 3 as the amount of photoreleased 5HT versus the total number of photons absorbed. Although the quantum efficiency of the phototrigger can be directly obtained from the initial slope of this graph, a more precise value was obtained by fitting the complete curve, as described below.
Figure 3.
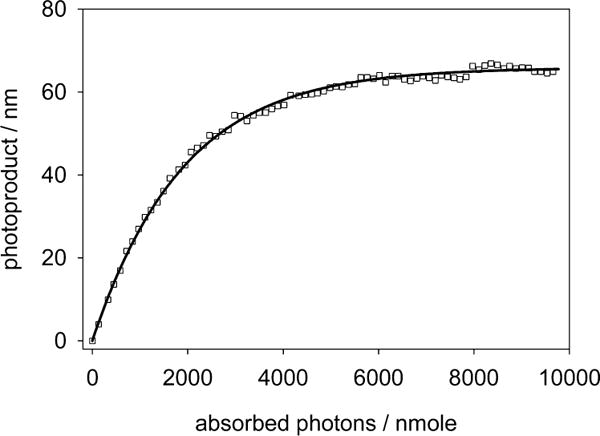
Progression of the UV-vis spectra during a photolysis reaction against absorbed photons. The continuous line is the theoretical fit according to eq 1. The initial slope of the curve represents the quantum yield ϕPD = 0.034.
Given the power of the irradiation beam, its optical path, and the amount of complex in the solution, it is possible to calculate the differential amount of product as
| (1) |
where np is the number of the moles of uncaged product, Ibeam is the intensity of the incident light in Einsteins/s, AbsT and AbsR are the total solution’s absorbance and the reactant’s absorbance, respectively, and ϕPD is the quantum yield of photouncaging. The expression has nonlinear factors, and adjusting the value of ϕPD by iterating a finite differences algorithm over the entire obtained spectra converges to the unique solution for the integrated expression of eq 1.
Cyclic voltammetry was used to further characterize the synthesized complex. Figure 4 shows a cyclic voltammogram of [Ru(bpy)2PMe3(5HT)]+2 recorded in acetonitrile. Ferrocene was used as internal reference. The Ru(III)/Ru(II) couple is observed at 1.07 V vs NHE (0.67 V vs Fc/Fc+). Other two irreversible processes are evident at higher potentials and correspond to the oxidation of the coordinated amine, a behavior also found in all other similar compounds.17
Figure 4.
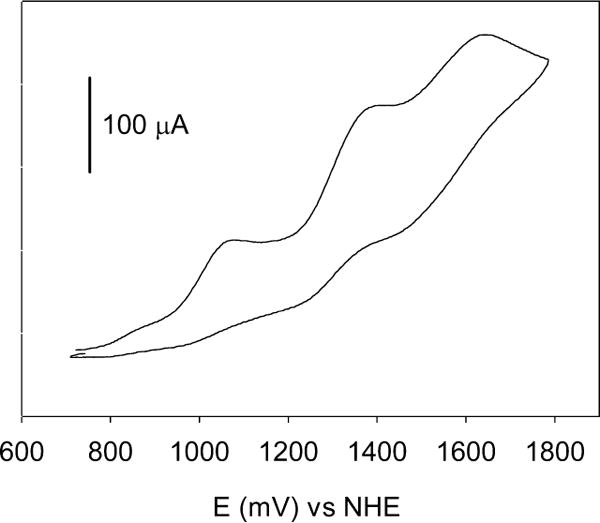
Cyclic voltammogram of [Ru(bpy)2PMe3(5HT)]+2 recorded in acetonitrile at 100 mV/s on Pt wire electrode in CH3CN containing 100 mM TBAPF6 as supporting electrolyte.
To confirm the biocompatibility of this complex and evaluate its performance as a serotonin phototrigger, a series of electrophysiology experiments in acute brain slices were performed.
In the mouse prefrontal cortex, separate populations of pyramidal neurons that project either subcortically to the pons (corticopontine, CPn) or to the contralateral cortex (commissural, COM) differentially express hyperpolarization-activated cyclic nucleotide–gated (HCN) channels. HCN channel expression can be assessed electrophysiologically by hyperpolarizing neurons in current clamp recordings. HCN channels activate slowly over 10s of ms in response to neuronal hyperpolarization. Thus, cells with high HCN expression levels (e.g., CPn neurons) respond to hyperpolarization with a characteristic “sag” during and “rebound” following current pulses as the voltage overshoots steady-state membrane potential in each direction. This overshoot is indicative of the delayed activation and closure of HCN channels.42 COM neurons express lower levels of HCN and display little to no sag, while CPn neurons express high levels of HCN and display significant sag. 5HT has differential effects on firing properties in these pyramidal neuron classes.41 In CPn neurons, 5HT reduces neuronal excitability through 5HT1A-dependent signaling. In COM neurons, this 5HT1A-dependent reduction in excitability is often followed by a slower, 5HT2A-dependent increase in activity.41 As such, 5HT uncaging should result in both reductions and delayed increases in these two cell classes.
To determine whether uncaging of [Ru(bpy)2(PMe3)-(5HT)] could alter pyramidal cell firing rates, we made recordings from COM and CPn neurons in mouse prefrontal cortex (Figure 5). When the caged-5HT was added to the slice in the dark, we observed no change in electrophysiological measures including resting membrane potential and input resistance, suggesting that the RuBi-5HT is not toxic in its caged form.
Figure 5.
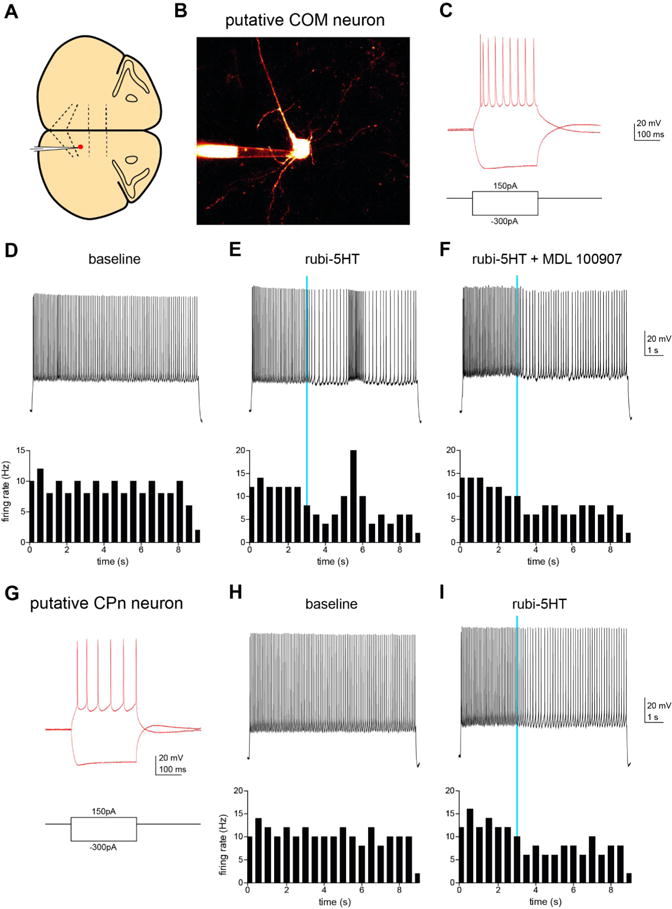
(A) Schematic indicating location of recorded neurons in coronal slice from mPFC of mouse. (B) Two-photon z-stack image of recorded neuron filled with Alexa-594. (C) Voltage responses of putative CPn neuron to hyperpolarizing and depolarizing current steps. (D–F) A depolarizing current step was delivered to the CPn neuron to elicit spiking. Top: Firing of CPn neuron at baseline (D), using a pulse of blue light (470 nm, 5 ms, 3.6 mW) to release 5HT (E), and with 5HT uncaging in the presence of a specific 5HT2A receptor antagonist (1 μM MDL100907, F). Bottom: Peristimulus time histograms of firing rate of the neuron in 200 ms bins. (G) Voltage responses of putative COM neuron to hyperpolarizing and depolarizing current steps. (H, I) Top: Firing of COM neuron at baseline (H) and with uncaging of 5HT (I). Bottom: Peristimulus time histograms of firing rate of the neuron in 200 ms bins.
Consistent with previous work, firing rate was lowered by 5HT uncaging in a CPn neuron (Figure 5I).41 A neighboring COM neuron exhibited a characteristic biphasic modulation of firing rate after [Ru(bpy)2(PMe3)(5HT)] uncaging with an initial depression of firing rate followed by a brief increase in firing (Figure 5E). In a subsequent experiment, we selectively blocked the delayed excitation with the 5HT2A receptor antagonist (1 μM MDL 100907) (Figure 5F). This experimental design thus provided an internal control that 5HT photolysis occurred (by the generation of the initial decrease in firing rate) while also demonstrating that changes in firing rate were indeed due to activation of 5HT receptors (by blocking the increase in firing rate with MDL 100907).
CONCLUSIONS
We have developed [Ru(bpy)2(PMe3)(5HT)]2+, a ruthenium bipyridil-based 5HT phototrigger which has its absorption maximum well into the visible range. Due to its absorption spectrum, 5HT can be released by irradiating with readily available low-power visible wavelength laser diodes at 405, 445, and even 532 nm. The photoprocess is very clean, showing no sign of other photoproducts besides free serotonin and the corresponding aquo complexes, as is expected from the known photochemistry of this kind of compounds. Its redox potential falls into the expected values for a complex of this family, and is highly related with the position of the MLCT band.43 However, its quantum efficiency of photorelease is somewhat lower that expected. The analogue complexes [Ru(bpy)2PMe3(Dopa)]+ (Dopa = dopamine)23 and [Ru(bpy)2PMe3(GABA)]+ present the same absorption44 but three times more activity than [Ru(bpy)2PMe3(5HT)]+, suggesting that some degree of recaptation after uncaging may be occurring. On the other hand, the sensitivity of this new caged serotonin is high enough to allow clean and fast uncaging in biological models, as is demonstrated by confirming neuronal characterization experiments while uncaging with a 5 ms 470 nm LED light source.
This phototrigger can be used with harmless visible light instead of UV light as is needed with the more traditional organic protecting groups. The water solubility of its chloride salt [Ru(bpy)2(PMe3)(5HT)]Cl2 is around 200 mM, much higher than needed for biological experiments, and the excellent stability of its solutions at neutral pH has been confirmed.
METHODS
Syntheses
All chemicals were purchased from Sigma-Aldrich and used as received without further purification. Ru(bpy)2Cl2 was synthesized according to literature45 and from this [Ru(bpy)2(PMe3) (Cl)]PF6 was obtained as previously described.17
For synthesis of [Ru(bpy)2(PMe3)(5HT)]Cl2, 207 mg (320 μmol) of [Ru(bpy)2(PMe3) (Cl)]PF6 was dissolved in 2 mL of acetone and 10 mL of water. The mixture was stirred with Cl−-loaded Dowex ionexchange resin for 15–30 min. After evaporation of acetone, the resultant solution of [Ru(bpy)2(PMe3) (Cl)]Cl was heated at 70 °C to obtain the aquo complex [Ru(bpy)2(PMe3)(H2O)]Cl. Then 320 mg (1500 μmol) of serotonin hydrochloride salt was introduced under N2 atmosphere in a Schlenk flask and the pH was raised to 9–10 by adding 1400 μL of NaOH 1 M. When the formation of serotonin complex was confirmed by UV-visible spectroscopy, the mixture was cooled in an ice bath and HCl 1 M was added to lower the pH and prevent possible oxidation of serotonin by air during the subsequent procedures. The solution was filtered, and after the addition of 1500 μL of KPF6 0.5 M the complex [Ru(bpy)2(PMe3)(5HT)](PF6)2 precipitates as an orange powder. Anal. Calcd for C, 41.47; H, 3.90; N, 8.79. Found: C, 41.2; H, 4.1; N, 8.5. For the purification of the serotonin complex, hexafluorophosphate anion was interchanged for chloride using Dowex-22 ion-exchange resin. The complex was reprecipitated with KPF6 0.5 M at pH 3, the anion interchanged again, and pH adjusted between 7–8. The lyophilized complex [Ru(bpy)2(PMe3)(5HT)]Cl was characterized by NMR spectroscopy. 1H NMR (D2O, K2CO3) δ, ppm = 8.78 (1H, d, bpy), 8.57 (1H, d, bpy), 8.36 (1H, d, bpy), 8.13 (1H, d, bpy), 8.10 (1H, t, bpy), 7.99 (1H, d, bpy), 7.94 (1H, d, bpy), 7.83 (1H, t, bpy), 7.79 (1H, t, bpy), 7.69 (1H, t, bpy), 7.64 (1H, t, bpy), 7.36 (1H, t, bpy), 7.31 (1H, d, bpy), 7.29 (1H, d, bpy), 7,19 (1H, d, J = 8.5 Hz, 5HT), 7.13 (1H, t, bpy), 6.95 (1H, t, bpy), 6.89 (1H, s, 5HT), 6.63 (1H, dd, J = 8.5 Hz and J = 2 Hz, 5HT), 6.21 (1H, d, J = 2 Hz, 5HT), 3.57 (1H, t, 5HT), 2.65 (1H, t, 5HT), 2.61–2.47 (2H, m, 5HT), 2.42 (1H, m, 5HT), 1.76 (1H, m, 5HT), 0.92 (9H, d, PMe3).
Spectroscopic Measurements and Photolysis
The optical bench used for UV–vis measurements consists, briefly, of a set of collinear lasers directed toward a four-faceted cuvette, stirred and thermostated at 25 °C. The solution’s absorbance is monitored perpendicularly to the laser path via an OceanOptics PC2000 diode-array spectrophotometer run by OOIChem sofware.
Quantum yield measurements of photouncaging were performed recording full absorption spectra while the photoreaction occurs. Then, the quantum yield of photolysis (ΦPD) was adjusted as a parameter to fit eq 1 to the measured spectra.
NMR spectra were obtained with a 500 MHz Bruker AM-500. The compound was photolyzed inside the unopened NMR test tube with an own-designed illuminator (see Supporting Information, Figure S2) using an array of high power LEDs centered at 450 ± 20 nm fwhm.
Voltammetry
Cyclic voltammetry was recorded at 100 mV/s scan rate in acetonitrile solution and tetrabutylammonium salt as supporting electrolyte, using a three-electrode potentiostat based on an operational amplifier TL071 in current–voltage configuration. A Pt wire with a diameter of 500 μm was used as a working electrode and acquisition software was written in QuickBasic 4.5. Ferrocene was used as internal reference.
Electrophysiology
Slice Preparation
Coronal brain slices (250 μm) containing medial prefrontal cortex were made from a P45 C57BL/6 male mouse. Slicing solution was chilled to 4 °C and contained (in mM): 234 sucrose, 26 NaHCO3, 11 glucose, 10 MgSO4, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, bubbled with 5% CO2/95% O2. Slices were incubated in artificial cerebrospinal fluid (aCSF) at 32 °C for 30 min and then at room temperature until recording in aCSF containing (in mM): 123 NaCl, 26 NaHCO3, 11 glucose, 3 KCl, 2 CaCl2, 1.25 NaH2PO4, 1 MgCl2, also bubbled with 5% CO2/95% O2.
Serotonin Uncaging and Patch Clamp Electrophysiology
[Ru(bpy)2(PMe3)(5HT)]Cl was applied as a powder to the aCSF bath solution (100 μM) and was photolyzed by 1 photon excitation with a single 5 ms light flash of a 470 nm LED (~3.6 mW).
Neurons were visualized using DODT contrast microscopy on an upright microscope (Bruker). Recordings were made using a Multiclamp 700B (Molecular Devices) amplifier and acquired with IgorPro. Patch pipettes (2–5 MΩ tip resistance, Schott 8250) were filled with the following (in mM): 130 KGluconate, 10 KCl, 10 HEPES, 10 mM Tris-phosphocreatine, 1.1 EGTA, 2 MgCl2, 2 MgATP, 0.3 Na3GTP, and 0.1 Alexa-594. All recordings were made at 32–34 °C. Series resistance ranged from 10 to 15 MΩ, and pipette capacitance was compensated.
Current pulses were applied through the somatic patch pipette for electrophysiological characterization (−300 and +150 pA in 250 ms duration). The neuron that displayed voltage sag at step onset and rebound at step offset when hyperpolarized (indicative of high expression of HCN channels) was identified as a putative corticopontine (CPn) projecting neuron.41,42,46 The neuron lacking significant sag and rebound was identified as a putative commissural (COM) projecting neuron.41,42,47 A 9 s positive current step was then applied to elicit spiking during uncaging trials (100 and 75 pA in CPn and COM cells, respectively). One μM MDL100907 (stock in DMSO), a potent 5HT2A receptor antagonist, was applied before uncaging for some experiments.
Supplementary Material
Acknowledgments
Funding
This research was supported by the National Agency for Science and Technology Promotion, CONICET, and the University of Buenos Aires (R.E); NIH R01NS099254, NSF 1604544, and the Brain Research Foundation (K.E.P.); NIH R01 DA035913 (K.J.B.), and NRSA F31 MH111219-01 (J.A.).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneur-o.7b00083.
1H NMR spectra of [Ru(bpy)2(PMe3)(5HT)]Cl in D2O; scheme of the setup used to perform the uncaging procedure into the NMR tubes (PDF)
ORCID
Roberto Etchenique: 0000-0002-5485-8710
Author Contributions
R.C.: Chemical synthesis, data analysis, discussion, and writing. O.F.: Data analysis, discussion, and writing. B.G.-A.: Chemical synthesis, data analysis, discussion, and writing. J.A.: Electro-physiological experiments, discussion, and writing. K.J.B.: Electrophysiological experiments, discussion, and writing. K.E.P.: Electrophysiological experimental design, discussion, and writing. R.E.: Chemical setups, data analysis, discussion, and writing.
Notes
R.E. is a member of CONICET.
The authors declare no competing financial interest.
References
- 1.Adams SR, Kao JP, Grynkiewicz Y, Minta GA, Tsien RY. Biologically useful chelators that release Ca2+ upon illumination. J Am Chem Soc. 1988;110:3212–3220. [Google Scholar]
- 2.Barth A, Corrie JE. Characterization of a new caged proton capable of inducing large pH jumps. Biophys J. 2002;83:2864–2871. doi: 10.1016/S0006-3495(02)75295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filevich O, Carrone G, Andino Pavlovsky V, Etchenique R. Fast Optical pH Manipulation and Imaging. Anal Chem. 2012;84:5618–5624. doi: 10.1021/ac300608q. [DOI] [PubMed] [Google Scholar]
- 4.Ellis-Davies GCR, Matsuzaki M, Paukert M, Kasai H, Bergles DE. 4-Carboxymethoxy-5,7-Dinitroindolinyl-Glu: An Improved Caged Glutamate for Expeditious Ultraviolet and Two-Photon Photolysis in Brain Slices. J Neurosci. 2007;27(25):6601–6604. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belov VN, Wurm CA, Boyarskiy VP, Jakobs S, Hell SW. Angew Chem, Int Ed. 2010;49:3520–3523. doi: 10.1002/anie.201000150. [DOI] [PubMed] [Google Scholar]
- 6.del Marmol J, Filevich O, Etchenique R. A Ruthenium-Rhodamine Complex as an Activatable Fluorescent Probe. Anal Chem. 2010;82:6259–6264. doi: 10.1021/ac1012128. [DOI] [PubMed] [Google Scholar]
- 7.Filevich O, Salierno M, Etchenique R. A caged nicotine with nanosecond range kinetics and visible light sensitivity. J Inorg Biochem. 2010;104:1248–1251. doi: 10.1016/j.jinorgbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K, Hashimoto K, Kusaka N, Yamazoe A, Fukaki H, Tasaka M, Nozaki H. Caged gene-inducer spatially and temporally controls gene expression and plant development in transgenic Arabidopsis plant. Bioorg Med Chem Lett. 2006;16:2470–2474. doi: 10.1016/j.bmcl.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 9.Young DD, Deiters A. Photochemical Activation of Protein Expression in Bacterial Cells. Angew Chem, Int Ed. 2007;46:4290–4292. doi: 10.1002/anie.200700057. [DOI] [PubMed] [Google Scholar]
- 10.Shestopalov IA, Sinha S, Chen JK. Light-controlled gene silencing in zebrafish embryos. Nat Chem Biol. 2007;3:650–651. doi: 10.1038/nchembio.2007.30. [DOI] [PubMed] [Google Scholar]
- 11.Chou C, Deiters A. Light-Activated Gene Editing with a Photocaged Zinc-Finger Nuclease. Angew Chem, Int Ed. 2011;50:6839–6842. doi: 10.1002/anie.201101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer G, Heckel A. Biologically active molecules with a “light switch. Angew Chem, Int Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 13.Patchornik A, Amit B, Woodward RB. Photosensitive protecting groups. J Am Chem Soc. 1970;92:6333–6335. [Google Scholar]
- 14.Wieboldt R, Gee KR, Niu L, Ramesh D, Carpenter BK, Hess GP. Photolabile precursors of glutamate: synthesis, photochemical properties, and activation of glutamate receptors on a microsecond time scale. Proc Natl Acad Sci U S A. 1994;91(19):8752–8756. doi: 10.1073/pnas.91.19.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canepari M, Nelson L, Papageorgiou G, Corrie JET, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods. 2001;112:29–42. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- 16.Senda N, Momotake A, Arai T. Synthesis and Photocleavage of 7-[{Bis(carboxymethyl)amino}coumarin-4-yl]-methyl-Caged Neurotransmitters. Bull Chem Soc Jpn. 2007;80(12):2384–2388. [Google Scholar]
- 17.Salierno M, Marceca E, Peterka DS, Yuste R, Etchenique R. A fast ruthenium polypyridine cage complex photoreleases glutamate with visible or IR light in one and two photon regimes. J Inorg Biochem. 2010;104:418–422. doi: 10.1016/j.jinorgbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with visible light using an inorganic caging group. Front Neural Circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Rode DL, Peterka DS, Yuste R, Rothman SM. Optical control of focal epilepsy in vivo with caged γ-aminobutyric acid. Ann Neurol. 2012;71(1):68–75. doi: 10.1002/ana.22596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalifoux JR, Carter AG. GABAB Receptor Modulation of Voltage-Sensitive Calcium Channels in Spines and Dendrites. J Neurosci. 2011;31(11):4221–4232. doi: 10.1523/JNEUROSCI.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolenko V, Yuste R, Zayat L, Baraldo LM, Etchenique R. Two-photon uncaging of neurochemicals using inorganic metal complexes. Chem Chem Commun. 2005:1752–1754. doi: 10.1039/b418572b. [DOI] [PubMed] [Google Scholar]
- 22.Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: two-photon and visible-light photoactivation of neurons and dendritic spines. Front Neural Circuits. 2009;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araya R, Andino-Pavlovsky V, Yuste R, Etchenique R. Two-Photon Optical Interrogation of Individual Dendritic Spines with Caged Dopamine, ACS Chem. ACS Chem Neurosci. 2013;4(8):1163–1167. doi: 10.1021/cn4000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci U S A. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garner RN, Gallucci JC, Dunbar KR, Turro C. [Ru(bpy)2(5-cyanouracil)2] 2+ as a Potential Light-Activated Dual-Action Therapeutic Agent. Inorg Chem. 2011;50:9213–9215. doi: 10.1021/ic201615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoll JD, Turro C. Control and Utilization of Ruthenium and Rhodium Metal Complex Excited States for Photoactivated Cancer Therapy. Coord Chem Rev. 2015;282–283:110–126. doi: 10.1016/j.ccr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahreman A, Cuello-Garibo JA, Bonnet S. Yellow-light sensitization of a ligand photosubstitution reaction in a ruthenium polypyridyl complex covalently bound to a rhodamine dye. Dalton Trans. 2014;43:4494–4505. doi: 10.1039/c3dt52643g. [DOI] [PubMed] [Google Scholar]
- 28.da Rocha ZN, Marchesi MS, Molin JC, Lunardi CN, Miranda KM, Bendhack LM, Ford PC, da Silva RS. The inducing NO-vasodilation by chemical reduction of coordinated nitrite ion in cis-[Ru(NO)2L(bpy)2]+ complex. Dalton Trans. 2008:4282–7. doi: 10.1039/b803441a. [DOI] [PubMed] [Google Scholar]
- 29.Franco LP, Cicillini SA, Biazzotto JC, Schiavon MA, Mikhailovsky A, Burks P, Garcia J, Ford PC, da Silva RS. Photoreactivity of a quantum dot-ruthenium nitrosyl conjugate. J Phys Chem A. 2014;118:12184–91. doi: 10.1021/jp5111218. [DOI] [PubMed] [Google Scholar]
- 30.Blundell JE. Serotonin and appetite. Neuropharmacology. 1984;23:1537–1551. doi: 10.1016/0028-3908(84)90098-4. [DOI] [PubMed] [Google Scholar]
- 31.Pigott TA, Seay SM. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry. 1999;60:101–106. doi: 10.4088/jcp.v60n0206. [DOI] [PubMed] [Google Scholar]
- 32.Gillette R. Evolution and Function in Serotonergic Systems. Integr Comp Biol. 2006;46:838–846. doi: 10.1093/icb/icl024. [DOI] [PubMed] [Google Scholar]
- 33.Palma-Cerda F, Auger C, Crawford DJ, Hodgson ACC, Reynolds SJ, Cowell JK, Swift KAD, Cais O, Vyklicky L, Corrie JET, Ogden D. New caged neurotransmitter analogs selective for glutamate receptor sub-types based on methoxynitroindoline and nitrophenylethoxycarbonyl caging groups. Neuropharmacology. 2012;63:624–634. doi: 10.1016/j.neuropharm.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Corrie JET, Desantis A, Katayama Y, Khodakhah K, Messenger JB, Ogden DC, Trentham DR. Postsynaptic Activation At The Squid Giant Synapse By Photolytic Release Of L-Glutamate From A ‘Caged’ L-Glutamate. J Physiol. 1993;465:1–8. doi: 10.1113/jphysiol.1993.sp019662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenberg LN, Blackiston DJ, Rea AC, Dore TM, Levin M. Left-right patterning in Xenopus conjoined twin embryos requires serotonin signaling and gap junctions. Int J Dev Biol. 2014;58:799–809. doi: 10.1387/ijdb.140215ml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rea AC, Vandenberg LN, Ball RE, Snouffer AA, Hudson AG, Zhu Y, McLain DE, Johnston LL, Lauderdale JD, Levin M, Dore TM. Light Activated Serotonin for Exploring Its Action in Biological Systems. Chem Biol. 2013;20:1536–1546. doi: 10.1016/j.chembiol.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Breitinger HGA, Wieboldt R, Ramesh D, Carpenter BK, Hess GP. Synthesis and Characterization of Photolabile Derivatives of Serotonin for Chemical Kinetic Investigations of the Serotonin 5-HT3 Receptor. Biochemistry. 2000;39:5500–5508. doi: 10.1021/bi992781q. [DOI] [PubMed] [Google Scholar]
- 38.Boahen YO, MacDonald GM. A concise approach to caged serotonin for Fourier transform infrared (FT-IR) difference photolysis studies. J Ghana Sci Assoc. 2005;7:54–59. [Google Scholar]
- 39.Zayat L, Salierno M, Etchenique R. Ruthenium-(II) Bipyridyl Complexes as Photolabile Caging Groups for Amines. Inorg Chem. 2006;45:1728–1731. doi: 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- 40.McLain DE, Rea AC, Widegren MB, Dore TM. Photoactivatable, biologically-relevant phenols with sensitivity toward 2-photon excitation. Photochem Photobiol Sci. 2015;14:2151–2158. doi: 10.1039/c5pp00334b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits. 2012;6:12. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–37. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinnick DV, Durham B. Photosubstitution Reactions of Ru(bpy)2XYn+ Complexes. Inorg Chem. 1984;23:1440–1445. [Google Scholar]
- 44.Filevich O, Etchenique R. RuBiGABA-2: a hydrophilic caged GABA with long wavelength sensitivity. Photochem Photobiol Sci. 2013;12:1565–1570. doi: 10.1039/c3pp25248e. [DOI] [PubMed] [Google Scholar]
- 45.Viala C, Coudret C. An Expeditious Route to cis-Ru(bpy)2Cl2 (bpy: 2,2′-bipyridine) Using Carbohydrate as Reducers. Inorg Chim Acta. 2006;359:984–989. [Google Scholar]
- 46.Gee S, Ellwood I, Patel T, Luongo F, Deisseroth K, Sohal VS. Synaptic activity unmasks dopamine D2 receptor modulation of a specific class of layer V pyramidal neurons in prefrontal cortex. J Neurosci. 2012;32:4959–71. doi: 10.1523/JNEUROSCI.5835-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J Neurosci. 2012;32:10516–21. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


