Abstract
Stress-related psychiatric disorders, including anxiety, are complex diseases that have genetic, and environmental causes. Stressful experiences increase the release of prefrontal amygdala neurotransmitters, a response that is relevant to cognitive, emotional, and behavioral coping. Moreover, exposure to stress elicits anxiety-like behavior and dendritic remodeling in the amygdala. Members of the miR-34 family have been suggested to regulate synaptic plasticity and neurotransmission processes, which mediate stress-related disorders. Using mice that harbored targeted deletions of all 3 members of the miR-34-family (miR-34-TKO), we evaluated acute stress-induced basolateral amygdala (BLA)-GABAergic and medial prefrontal cortex (mpFC) aminergic outflow by intracerebral in vivo microdialysis. Moreover, we also examined fear conditioning/extinction, stress-induced anxiety, and dendritic remodeling in the BLA of stress-exposed TKO mice.
We found that TKO mice showed resilience to stress-induced anxiety and facilitation in fear extinction. Accordingly, no significant increase was evident in aminergic prefrontal or amygdala GABA release, and no significant acute stress-induced amygdalar dendritic remodeling was observed in TKO mice. Differential GRM7, 5-HT2C, and CRFR1 mRNA expression was noted in the mpFC and BLA between TKO and WT mice. Our data demonstrate that the miR-34 has a critical function in regulating the behavioral and neurochemical response to acute stress and in inducing stress-related amygdala neuroplasticity.
Keywords: miR-34, Stress, Prefrontal cortex, Amygdala, Anxiety
1. Introduction
All stress-related psychiatric disorders, including anxiety, are complex diseases involving genetic, and environmental causes.
MicroRNAs (miRs), a class of small, noncoding RNAs, regulate gene expression at the post-transcriptional level and have been shown to play a crucial role in many neurobiological processes and in regulating stress response (Issler and Chen, 2015; Malan-Müller et al., 2013; O’Connor et al., 2012; Schouten et al., 2013). In particular, human and animal studies indicate that members of the miR-34 family of miRs are involved in several psychopathological phenotypes (Bavamian et al., 2015; Dias et al., 2014a; Dickson et al., 2013; Haramati et al., 2011; Parsons et al., 2008; Zhou et al., 2009). Three miR-34 precursors are produced from two transcriptional units; the human miR-34a is transcribed from chromosome 1, miR-34b and miR-34c precursors are co-transcribed from a region on chromosome 11. MiR-34 has also been proposed to be a target for the actions of mood stabilizers and antidepressants (Bavamian et al., 2015; Bocchio-Chiavetto et al., 2013; Liu et al., 2014; Zhou et al., 2009) and to modulate the dendritic remodeling underlying neuroplasticity (Agostini et al., 2011; Bavamian et al., 2015).
Accumulating evidence also implicates miR-34 in the stress response, particularly in the manifestation of behaviors relevant to fear, anxiety as well as fear memory consolidation (Dias et al., 2014a; Haramati et al., 2011).
A corpus of data implicates dysfunctions of the neural circuit connecting the prefrontal cortex and amygdala in the pathophysiology of fear and anxiety-like disorders induced by stress exposure (Arikav and Moroun, 2007; Holmes, 2008; Shin and Liberzon, 2010). Exposure to stress induces increased neurotransmitters release in this circuit which is considered relevant for cognitive, emotional and behavioral responses (Andolina et al., 2013, 2014; Pascucci et al., 2007) and the increase, stress-induced, of prefrontal catecholamine release is considered a relevant response for cognitive, emotional and behavioral coping (Di Segni et al., 2015; Finlay et al., 1995; Pascucci et al., 2007; Ventura et al., 2013). It has also been demonstrated that aminergic transmission in the medial prefrontal cortex (mpFC), through regulation of subcortical structures such as amygdala, modulates the stress response (Andolina et al., 2013, 2014; Pascucci et al., 2007). A wide projection from mpFC to Basolateral Amygdala (BLA) has been described (Likhtik et al., 2005). We have previously shown that mpFC serotonergic neurotransmission modulates the amygdalar response to stress in mice, and this could be via effects on GABA-ergic inhibitory neurons in the basolateral amygdala (BLA) (Andolina et al., 2013, 2014). These BLA interneurons have a critical influence on behavior, emotionality and stress-induced dendritic plasticity (Andolina et al., 2013, 2014; Rodríguez-Manzanares et al., 2005). Previous work has also shown that stress can induce hypertrophy of BLA neurons (Maroun et al., 2013; Mitra et al., 2005; Rao et al., 2012).
Here, we directly evaluated the role of miR-34 in the mpFC-BLA stress response by using mice carrying a targeted deletion of all three members of the miR-34 family (TKO). First, we tested the hypothesis that miR-34 modulates the behavioral response to stress exposure. Since GABA-ergic neurotransmission within the BLA plays a critical role in regulating stress response and the prefrontal serotonergic input into this region regulates amygdalar GABA-ergic neurotransmission (Andolina et al., 2013, 2014), we evaluate, by in vivo intracerebral microdialysis, the BLA GABA-ergic outflow and mpFC aminergic outflow in Control and TKO mice subjected to acute restraint stress. Furthermore, because numerous genes associated with stress and neuropsychiatric disorders, including metabotropic glutamate receptor 7 (GRM7) (Zhou et al., 2009), synaptotagmin 1(Syt1) (Agostini et al., 2011), Serotonin 2C receptor (5-HT2C), and corticotropin-releasing factor receptor 1 (CRFR1) (Dias et al., 2014a; Haramati et al., 2011) are putative or validated miR-34 targets, we also evaluate the effects of acute stress on mRNA expression of these genes in the mpFC and BLA of WTand TKO mice.
Finally, because stress-induced anxiety behavior has been related, in animal models, to hypertrophy of BLA neurons (Mitra et al., 2005; Maroun et al., 2013; Rao et al., 2012), in the last experiment we also assess dendritic remodeling in the BLA of TKO and WT mice exposed to acute stress.
2. Materials and methods
2.1. Animals
MiR-34 knockout (TKO) mice were generated as described (Concepcion et al., 2012). WT and TKO male mice, aged 8–9 weeks, were used for the experiments. All experiments were conducted in accordance with Italian national laws (D.Lgs no. 116/1992) governing the use of animals for research.
2.2. Stress protocols
The restraint apparatus has been described (Cabib and Puglisi-Allegra, 1991). In the behavioral experiments, mice were restrained for 30 min to induce anxiety-like behavior, based on previous studies (Haramati et al., 2011; MacNeil et al., 1997). In the microdialysis experiments, WT and TKO mice were restrained for 2 h to evaluate the time-dependent changes induced by stress exposure in prefrontal aminergic release and BLA GABAergic outflow.
2.3. Elevated plus maze, dark-light test, and open field test
Based on previous studies (Haramati et al., 2011; Resstel et al., 2009), 24 h after exposure to acute stress (restraint- 30 min), mice were tested individually in a single session of the elevated plus maze (EPM), dark-light test (DLT), or open field (OF) test. The EPM apparatus comprised a central section (5 × 5 cm); 2 opposing open arms (15 × 5 cm), and 2 opposing closed arms (15 × 5 × 15 cm), and the white light that was cast across the arena was 30 lux. The percentage of time spent in the open arms [(time in open/open closed) × 100], the percentage of entries in the open arms [(open entries/open closed) × 100], and the distance traveled in the apparatus were recorded for 5 min. The DLT apparatus was a rectangular box divided by a partition into 2 environments: a dark compartment (35 × 20 × 30 cm; white light: <30 lux) and a brightly illuminated compartment (35 × 20 × 30 cm; white light: 80–110 lux). The compartments were connected by a small passage at the bottom center of the partition. The latency to entry into the dark compartment, the number of visits to the lighted compartment, the percentage of time spent and the distance covered in the aversive lighted compartment were recorded for 15 min.
The open field (OF) apparatus consisted of a circular Plexiglas box (60 cm in diameter and 30 cm in height). The center region (30 × 30 cm) was defined as the central area, and the white light that shone throughout the arena was 30 lux. Latency to the first exit from the center, the number of visits to the center, the percentage of time spent in the center, and the distance traveled in the apparatus were recorded for 5 min.
2.4. Fear conditioning and extinction
The fear conditioning and extinction procedure was performed as described by Izquierdo et al., 2006. In brief, to train in fear conditioning (Panlab, HARVARD APPARATUS), mice were placed in the conditioned chamber (25 × 25 × 25 cm, with black and transparent walls and a metal grid floor, cleaned with a 79.5% water/19.5% ethanol/1% lemon extract solution), and after a 120-s acclimation period, they received 3 pairings (with 60–120-s variable interpairing intervals) of a conditioned stimulus (CS, 30 s, 80 dB, 3 kHz tone) and unconditioned stimulus (US, 2 s, 0.6 mA scrambled footshock), in which US was delivered in the last 2 s of presentation of the CS. After a 120-s no-stimulus consolidation period (following the final CS-US pairing), the mice were returned to their home cage.
Twenty-four hours after training, extinction learning was assessed. Mice were placed in a novel context (transparent cylinder in black/white-checkered walls and a solid Plexiglas white floor, cleaned with a 50% v/v ethanol solution). After an initial 120-s acclimation period, the mice were presented with the CS 40 times, each lasting 30 s and separated by a 5-s no-stimulus interval. Twenty-four hours later, the mice were returned to the original training chamber for 5 min to assess contextual fear memory. Freezing (evaluated as the complete absence of voluntary movements, except for respiratory movements) was scored every 5 s by an observer who was blinded to the experimental conditions and converted to percentage [(freezing observations/total observations) × 100]. Freezing during extinction was averaged into 8-trial blocks for statistical analysis.
2.5. In vivo microdialysis
Animals were anesthetized with chloral hydrate (450 mg/kg), mounted in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA) that was equipped with a mouse adapter, and implanted with a microdialysis probe in the mpFC and BLA using a dual-probe in vivo microdialysis procedure (Di Segni et al., 2015). Vertical concentric dialysis probes were prepared with AN69 fibers (Hospal Dasco, Bologna, Italy), according to the method of Di Chiara (Di Chiara et al., 1993). The total length of the probe was 3 mm (dialysis membrane length 2 mm, o.d. 0.24 mm) for the mpFC and 5.5 mm (dialysis membrane length 1 mm, o.d. 0.24 mm) for the BLA. Microdialysis experiments were performed 48 h after surgery. The coordinates from the bregma [measured according to the atlas of Franklin and Paxinos (Franklin and Paxinos, 1997)] were: mpFC: 2.5 AP, 0.6 L and BLA: −1.16 AP, −3,55 L The day before use, the membranes were tested to verify in vitro norepinephrine (NE), dopamine (DA), 5-HT, and GABA recovery.
The microdialysis probe was connected to a CMA/100 pump (Carnegie Medicine, Stockholm, Sweden) through PE-20 tubing and an ultralow-torque dual-channel liquid swivel (Model 375/D/ 22QM, Instech Laboratories, Inc., Plymouth Meeting, PA, USA) to allow free movement.
Artificial cerebrospinal fluid (CSF) was pumped through the dialysis probe at a constant flow rate of 2 μ,L/min. The mean concentration of the 3 samples, collected immediately before treatment (<10% variation), was taken as the basal concentration. Twenty microliters of dialysate sample was analyzed by HPLC with regard to 5-HT, NE, and DA as described (Andolina et al., 2013,2014; Ventura et al., 2013). GABA concentrations in the dialysates were determined as described (Andolina et al., 2013, 2014; Rea et al., 2005). The detection limit of the assay was 4.2 and 0.1 pg per 20 μl (signal-to-noise ratio 2) for GABA and amine (5-HT, DA, NE), respectively.
2.6. Probe placement
At the end of the experiments, the mice were killed by decapitation. Brains were postfixed in 4% paraformaldehyde, and correct probe placement was assessed by visual inspection of the probe tracks on Nissl-stained coronal sections (40 μm). Only mice with correctly placed probes in the BLA and mpFC were considered.
2.7. Plasmatic corticosterone
WT and TKO animals were randomly assigned to “basal” or “stress” conditions; in the former condition, mice were extracted from their home cages and sacrificed immediately, whereas in the latter, mice were sacrificed immediately after the end of an acute stressful experience (restraint) that lasted 30 or 120 min.
Trunk blood samples were collected after decapitation and immediately centrifuged. After blood centrifugation (20 min, 4 °C, 16,000 rpm), serum samples were stored at −80 °C until the assays were conducted. Corticosterone levels were measured using commercial ELISA kits (EIA kit Assay Design) in duplicate.
2.8. RT-qPCR
Sixty minutes after the end of restraint-induced stress (30 min) (Haramati et al., 2011), the brain was removed, and the expression of miR-34 (a,c), CRFR1, GRM7, 5-HT2C, and Syt1 mRNA was measured by RT-qPCR (Concepcion et al., 2012). RNA was extracted from punches of the mpFC and BLA from WT and TKO mice using Trizol (Invitrogen). Punches were obtained from brain slices (coronal sections) that were not thicker than 300 μm. Stainless steel tubes (1.0-mm inside diameter) were used. The coordinates were measured according to the atlas of Franklin and Paxinos (Franklin and Paxinos, 1997). qPCR was performed using primers and probes from Applied Biosystems according to the manufacturer’s instructions. Sno-135 and GAPDH were used to normalize miR-34 (a,c) and CRFR1, GRM7, 5-HT2C, and Syt1 mRNA levels.
2.9. Morphological analysis
Twenty-four hours following exposure to acute stress (restraint, 30 min), the brains of all groups of mice [WT unstressed (US), WT stressed (AS), TKO US, and TKO AS] were impregnated with a standard Golgi-Cox solution as described (Andolina et al., 2011). Coronal sections (120 μm) were sliced on a vibratome, mounted on gelatinized slides, stained according to the Gibb and Kolb method, and covered with Eukitt (Kindler GmbH & Co., Germany) (Gibb and Kolb, 1998). Measurements were made on impregnated neurons under low magnification (20X/0.4 numerical aperture). The analysis of the BLA was restricted to pyramidal-like neurons that lay between −1.34 and −2.06 mm from the bregma [measured according to the atlas of Franklin and Paxinos (Franklin and Paxinos, 1997)]. Golgi-impregnated neurons were selected according to criteria proposed by Vyas et al. (Vyas et al., 2002). An average of 5–6 neurons were analyzed for each mouse and were randomly selected from both hemispheres. A total of 104 neurons were identified and included in the statistical analyses. An experimenter who was blinded to the experimental groups performed the morphological analyses. Dendritic length and number of branch points were analyzed using 3D reconstructions of the selected neurons on a NeuroLucida image analysis system (mbf, Bioscience) that was connected to an Olympus BX53 microscope (100X/1.25 numerical aperture). Using the same NeuroLucida system (100X/ 1.25 numerical aperture, Olympus BX53), all protrusions, irrespective of their morphological characteristics, were counted on each dendritic branch order as spines if they were in direct contact with the dendritic shaft.
3. Statistics
Statistical analyses for the elevated plus maze, the dark-light and open field tests, corticosterone levels (30, 120 min), mRNA expression, and morphological data (spine density, number of branch points, and dendritic length) were performed by two-way ANOVA (genotype, 2 levels: WT, TKO; treatment, 2 levels: stressed, unstressed). For the microdialysis experiments, statistical analyses were performed on raw data (concentration of pg/20 μl). For the microdialysis and fear conditioning/extinction experiments, data were analyzed by repeated-measures ANOVA with 1 between factor (genotype, 2 levels: WT, TKO) and 1 within factor [microdialysis: time, seven levels: 0, 20, 40, 60, 80,100, and 120 min; fear conditioning/extinction: time points (fear conditioning: 3 levels; fear extinction: 8 levels]. For the microdialysis (including basal extracellular levels of 5-HT, NE, and DA in the mpFC and basal extracellular GABA levels in the BLA) and fear conditioning/ extinction experiments, simple effects were assessed by one-way ANOVA for each time point. The effects of restraint (30 min) on the expression of miR-34 (a,c) were analyzed in the mpFC and BLA of unstressed and stressed WT mice by student’s t-test.
For all experiments, individual between-group comparisons were performed, when appropriate, by post hoc test (Duncan’s multiple-range test).
4. Results
4.1. Elevated plus maze, dark-light test, and open field test
We hypothesized that miR-34 modulates the behavioral response to stress. To test this hypothesis, we examined the function of miR-34 in stress-induced anxiety by administering 3 behavioral tests (EPM, DLT, and OF) to miR-34 TKO and WT mice.
Concerning EPM results, two-way ANOVA revealed a no significant genotype (p = 0.49) effect, and a significant treatment (F(1,28) = 4.58; p < 0.05) effect and genotype ×treatment interaction (F(1,28) = 4.99; p < 0.05) with regard to the percentage of time spent in the open arms (Fig. 1A).
Fig. 1.
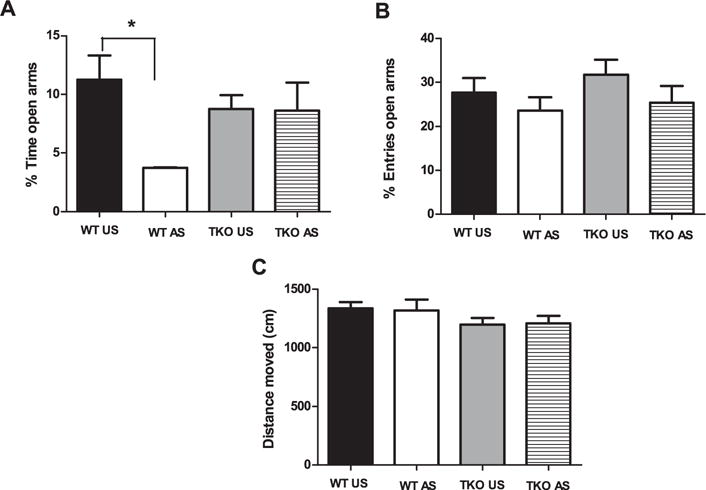
Effects of stress exposure (30 min of restraint) on (A) the percentage of time spent in the open arms (B) the percentage of entries in the open arms, (C) the traveled distance in the apparatus in elevated plus maze in WT (WT Unstressed (US) n = 8; WT stressed (WT AS) n = 8) and TKO (TKO US n = 8; TKO AS n = 8) mice. Data are expressed as mean ± SE. *P < 0.05.
Two-way ANOVA revealed no significant genotype (p = 0.41) or treatment (p = 0.75) effect and genotype × treatment interaction (p = 0.15) concerning the percentage of entries in the open arms (Fig. 1B). Finally, the effects of genotype (p = 0.08) and treatment (p = 0.96) and the genotype × treatment interaction (p = 0.82) were not significant for the total distance covered (cm) over the EPM apparatus (Fig. 1C).
By Duncan’s test, we noted a significant decrease in the percentage of time spent in the open arms in stressed versus unstressed WT mice (df = 7.53; p < 0.05), whereas restraint exposure had no significant effect in TKO mice (p = 0.9) (Fig. 1A). Although the percentage of time spent in the open arms in the EPM levels was low, several groups have reported similar levels in mice (i.e. Abbas et al., 2015; Matsuo et al., 2009; Mozhui et al., 2010; Tsujimura et al., 2008).
For DLT experiment, two-way ANOVA, revealed a no significant effect of genotype (p = 0.08) or treatment (p = 0.78) and a significant genotype × treatment interaction (F(1,25) = 8.54; p < 0.01) regarding the latency to entry into the dark compartment (Fig. 2A).
Fig. 2.
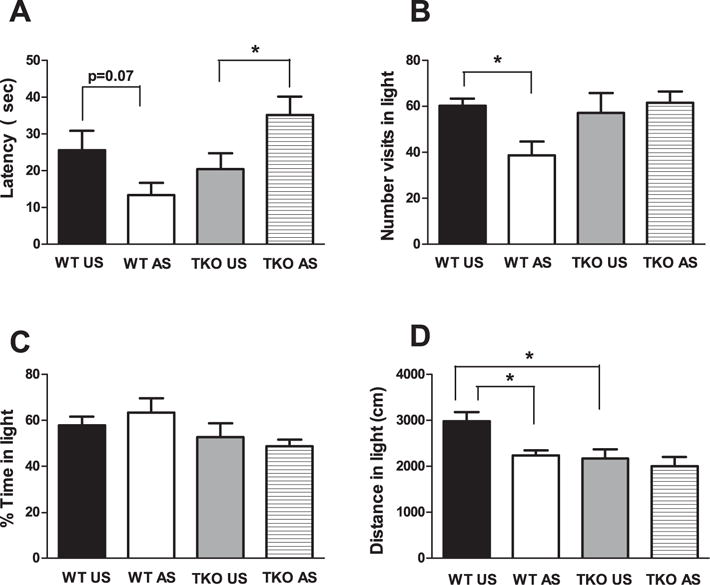
Effects of stress exposure (30 min of restraint) on (A) the latency to entry in the dark compartment, (B) the number of visits in the lighted compartment, (C) the percentage of time spent in the lighted compartment and (D) the distance covered in the lighted compartment in the dark/light test in WT (WT Unstressed (US) n = 7; WTstressed (WT AS) n = 7) and TKO (TKO US n = 7; TKO AS n = 8) mice. Data are expressed as mean = SE. *P < 0.05.
Regarding the number of visits to the lighted compartment, two-way ANOVA revealed no significant genotype (p = 0.1) or treatment (p = 0.15) effect and a significant genotype × treatment interaction (F(1,25) = 4.75; p < 0.05) (Fig. 2B).
No significant genotype (p = 0.05) or treatment (p = 0.88) effect or genotype × treatment interaction (p = 0.33) was observed concerning the percentage of time spent in the lighted compartment (Fig. 2C). The genotype (F(1,25) = 8.08; p < 0.01) and treatment (F(1,25) = 6.02; p < 0.05) effects were significant but the genotype × treatment interaction was not (p = 0.13) for the distance (cm) that was covered in the light compartment (Fig. 2D).
Duncan’s test showed that the acute restraint elicit a no significant decrease of the latency (p = 0.07) (Fig. 2A) but significantly decreased the number of visits (df = 7.73; p < 0.05) (Fig. 2B) and the distance covered (df = 4.95; p < 0.05) (Fig. 2D) in the lighted compartment in stressed WT compared with unstressed WT mice. No significant effect of restraint exposure was evident in TKO mice, except for latency, for which stressed TKO mice showed an increased levels versus unstressed TKO mice (Fig. 2A).
In the DLT, the effect of genotype (WT, TKO) was not significant except for the distance in lighted compartment, wherein unstressed WT mice had significantly higher levels compared with unstressed TKO mice (df = 813.3; p < 0.05) (Fig. 2D). Concerning the OF, two-way ANOVA, revealed a significant genotype effect (F(1,24) = 9; p < 0.01), no significant treatment effect (p = 0.67), and a significant genotype × treatment interaction (F(1,24) = 16.24; p < 0.01) for the latency to exit from the center (Fig. 3A).
Fig. 3.
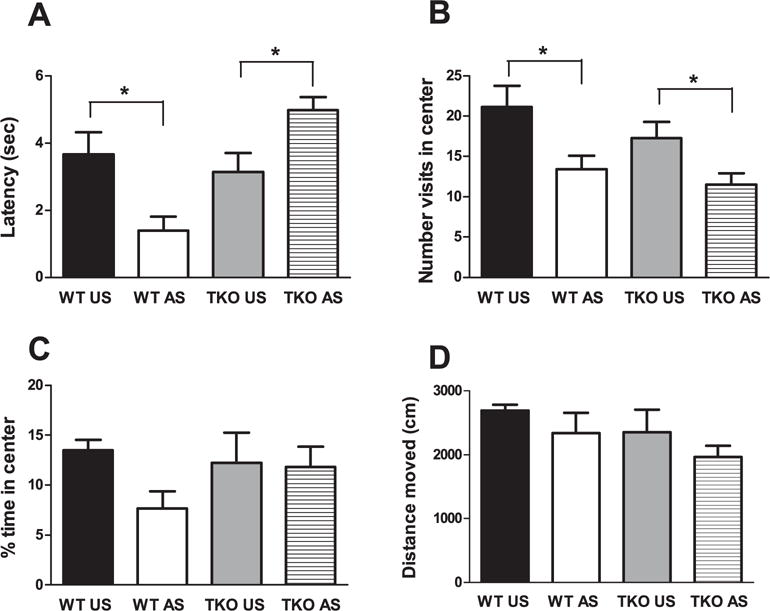
Effects of stress exposure (30 min of restraint) on (A) the latency to exit from the center, (B) the number of visit in the center, (C) the percentage of time spent in the center and (D) the traveled distance in the apparatus in WT (WT Unstressed (US) n = 6; WT stressed (WT AS) n = 7) and TKO (TKO US n = 7; TKO AS n = 8) mice. Data are expressed as mean ± SE. *P < 0.05.
Two-way ANOVA revealed a no significant genotype effect (p = 0.14), a significant treatment effect (F(1,24) = 12.54; p < 0.01), and a no significant genotype × treatment interaction (p = 0.61) for the number of visits to the center (Fig. 3B). The genotype (p = 0.51) and treatment effects (p = 0.16) and the genotype × treatment interaction (p = 0.22) were not significant with regard to the percentage of time spent in the center was evident. However, a reduction in time spent in the center in stressed versus unstressed WT, and no effect in TKO mice was evident (Fig. 3C). Regarding the traveled distance (cm) in the apparatus, two-way ANOVA revealed a no significant genotype (p = 0.19) and treatment effects (p = 0.15) and the genotype × treatment interaction (p = 0.89) was not significant (Fig. 3D).
Duncan’s test showed that, acute restraint exposure produced a significant decrease of the latency (df = 1.69; p < 0.05) (Fig. 3A) and the number of visits to the center (df = 7.73; p < 0.05) (Fig. 3B) in stressed WT compared with unstressed WT mice.
No significant effect of restraint exposure was evident in TKO mice, except for latency (df = 1.83; p < 0.05) (Fig. 3A) and the number of visits to the center (df = 5.78; p < 0.05) (Fig. 3B), for which stressed TKO mice showed increased and decreased values, respectively, versus unstressed TKO mice. The effect of genotype (WT, TKO) was not significant for latency (p = 0.56) (Fig. 3A).
4.2. Fear conditioning and extinction
Because dysfunction of the prefrontal cortex-amygdala neural circuit is associated with alterations in the fear responsed—a process that is dysregulated in certain anxiety disordersd—we also evaluated the effects of miR-34 lack on fear conditioning/extinction.
Two-way ANOVA revealed no significant genotype effect (p = 0.15), a significant time effect (F(3,56) = 29.78; p < 0.01) and no significant genotype × time interaction (p = 0.24) for freezing during the fear conditioning phase. No significant genotype effect (p = 0.16) and a significant time effect (F(7,98) = 11.82; p < 0.01) as well as significant genotype × time interaction (F(7,98) = 2.95; P < 0.01) were evident between TKO and WT mice for freezing during the extinction phase (Fig. 4). One-way ANOVA for each time point showed that although freezing progressively decreased in both WT and TKO mice over the extinction trial blocks, WT mice showed significantly higher freezing than TKO mice on the fifth (F(1,14) = 6.04; p < 0.05) and seventh (F(1,14) = 7.74; p < 0.05) extinction trial blocks and a significant trend on the second (p = 0.07), third (p = 0.1) and eighth (p = 0.1) extinction trial blocks (Fig. 4). One-way ANOVA for freezing in the contextual fear memory test carried out 24 h after extinction procedure shows no significant difference between WT and TKO mice (p = 0.43).
Fig. 4.
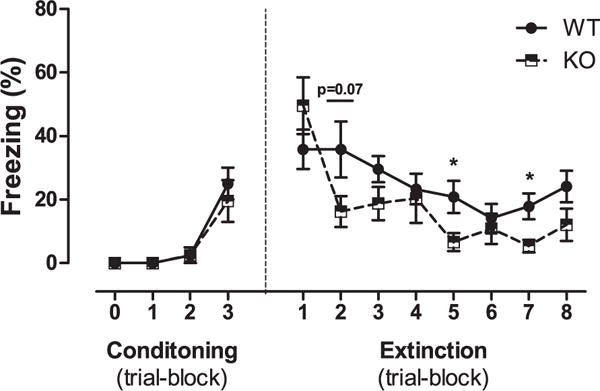
Effects of the genotype (WT, n = 8; TKO, n = 8) on fear memory extinction following contextual and cued fear conditioning training. Data are expressed as mean ± SE of percent freezing. *P < 0.05.
4.3. In vivo microdialysis
Because GABAergic transmission in the BLA has a critical function in regulating stress responses and because we have demonstrated that prefrontal serotoninergic transmission governs amygdalar GABAergic transmission, we evaluate BLA GABAergic and mpFC aminergic outflow in TKO and WT mice subjected to acute restraint stress by in vivo intracerebral microdialysis. Two-way ANOVA, revealed a no significant genotype (p = 0.8) or time (p = 0.26) effect but a significant genotype × time interaction (F(6,102) = 2.32; P < 0.05) for prefrontal 5-HT (Fig. 5A). Regarding NE, two-way ANOVA revealed a no significant genotype effect (p = 0.69) but time effect (F(6,96) = 11.51; p < 0.01) and the genotype × time interaction (F(6,96) = 2.60; P < 0.05) were significant (Fig. 5B). The amygdalar GABA analysis revealed a no significant genotype effect (p = 0.39), a significant time effect (F(6,96) = 1.87; p < 0.05), and a significant genotype × time interaction (F(6,96) = 2.91; p < 0.05) (Fig. 5D) for release induced by restraint exposure. Consistent with previous reports, restraint stress induced a significant time-dependent increase in prefrontal 5-HT, NE, and DA and GABA release in WT mice compared with basal levels (Andolina et al., 2013, 2014; Ventura et al., 2013). In contrast, prefrontal 5-HT and amygdala GABA release in TKO animals were unchanged versus basal values (Fig. 5A, D). Concerning NE outflow, TKO animals showed significantly greater release compared with basal values only at the first time point (20 min) (Fig. 5B).
Fig. 5.
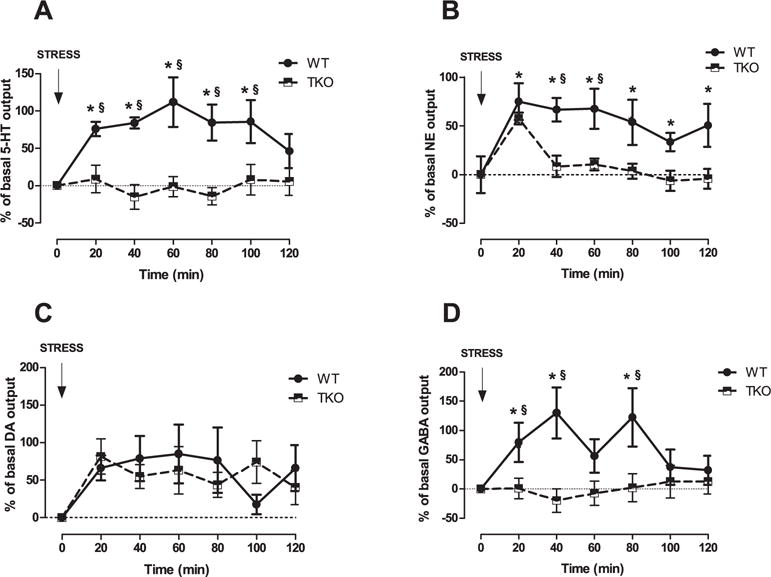
Effects of stress exposure (120 min of restraint) on (A) Serotonin (5-HT), (B) Norepinephrine (NE), (C) Dopamine (DA) outflow in the medial prefrontal cortex (mpFC) and (D) GABA outflow in the Basolateral Amygdala (BLA) of WT (NE, n = 9; DA, n = 8; 5-HT, n = 7; GABA, n = 9) and TKO (NE, n = 9; DA, n = 7; 5-HT, n = 12; GABA, n = 9) mice. Results are expressed as percent changes (mean ± SE) from basal values. Statistical analyses were performed on raw data. § P < 0.05 in comparison with the corresponding time point of miR-34s TKO group. *P < 0.05 from basal values.
Finally, no significant genotype effect (p = 0.2), a significant time effect (F(6,78) = 3.25; p < 0.01), and a no significant the genotype × time interaction (p = 0.45) were evident in prefrontal DA release (Fig. 5C). Prefrontal 5-HT, NE, and DA levels and BLA GABA basal levels were similar between WT and TKO mice (5-HT: WT = 0.73 ± 0.05, TKO = 0.84 ± 0.08; NE: WT = 0.77 ± 0.06, TKO = 1.02 ± 0.09; DA: WT = 0.29 ± 0.07, TKO = 0.39 ± 0.07; GABA: WT = 39.3 ± 5.31, TKO = 47.07 ± 9.83).
4.4. Plasmatic corticosterone
Peripheral corticosterone (CORT) levels were evaluated in baseline and after 30 and 120 min of exposure to stress in the WT and TKO groups to assess common peripheral stress responses.
Two-way ANOVA revealed significant genotype (F(1,36) = 16.46; p < 0.01) and treatment F(2,36) = 31.40; P < 0.01) effect but their interaction was not significant (p = 0.31) (Fig. 6). Duncan test, revealed that TKO animals had significant higher levels of corticosterone under basal (unstressed) conditions versus WT animals (df = 267.21; p < 0.05) (Fig. 6).
Fig. 6.
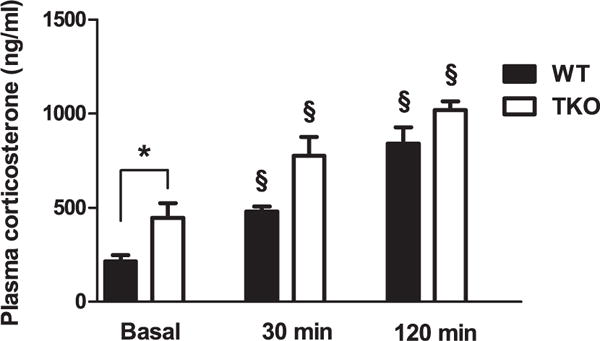
CORT levels in basal condition and after 30 or 120 min of acute restraint stress exposure in WT (WT basal, n = 8; WT stressed (AS) 30 min, n = 8; WT AS 120 min, n = 6) and TKO mice (TKO basal, n = 8; TKO stressed (AS) 30 min, n = 7; TKO AS 120 min, n = 6 mice). Data are expressed as mean ± SE. *P < 0.05. § P < 0.05 from basal values.
The WT and TKO groups showed a significant rise in CORT levels after 30 min [WT unstressed (US) vs. WT stressed (AS) (30 min): df = 290.64; p < 0.05; TKO US vs. TKO AS (30 min): df = 373.27, p < 0.05] and 120 min [WT US vs. WT AS (120): df = 681.69, p < 0.05; TKO US vs. TKO AS (120 min): df = 792.14; p < 0.05] of restraint compared with the respective unstressed groups (Fig. 6).
4.5. RT-qPCR
To verify the increase in stress-induced miR-34 (a,c) expression, we performed RT-qPCR in stressed and unstressed WT mice. Also, the mRNA levels of GRM7, Syt1, 5-HT2C, and CRFR1 were measured in the mpFC and BLA of unstressed and stressed WTand TKO mice.
Student’s t-test for the mpFC and BLA, revealed a significant increase expression of miR-34a in the mpFC (t = 2.22, df = 0.451 p < 0.05) (Fig. 7A), as did miR-34c in the BLA (t = 3.4, df = 2.14 p < 0.01) (Fig. 7B) of stressed WT versus unstressed WT. miR-34a levels in the BLA (p = 0.19) (Fig. 7A) and miR-34c in the mpFC (p = 0.98) were unchanged between stressed and unstressed WT mice (Fig. 7B). The absolute and relative levels of miR-34a and miR-34c in the mpFC and BLA of unstressed and stressed WT and TKO mice are reported in Supplementary Table 1.
Fig. 7.
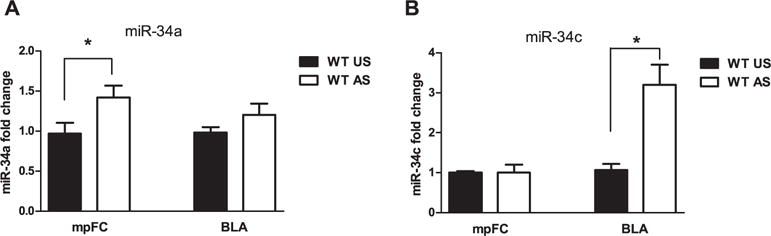
Effects of stress exposure (30 min of restraint) on (A) miR-34a and (B) miR-34c expression in the medial prefrontal cortex (mpFC) and Basolateral Amygdala (BLA) of WT (WT Unstressed (US), mpFC miR-34a, n = 9; mpFC miR-34c, n = 8; BLA miR-34a, n = 8; BLA miR-34c, n = 8); WTstressed (AS), mpFC miR-34a, n = 8; mpFC miR-34c, n = 6; BLA miR-34a, n = 9; BLA miR-34c, n = 9)) mice. Data are expressed as mean ± SE. *P < 0.05.
Concerning mRNA levels, two-way ANOVA revealed a significant effect of genotype on mpFC 5-HT2C (F(1,12) = 30.31; p < 0.01) and BLA CRFR1 levels (F(1,13) = 4.92; p < 0.05) and a nearly significant effect on mpFC GRM7 (p = 0.08). There was no significant effect of treatment (mpFC = CRFR1: p = 0.92; GRM7: p = 0.72; SYT1: p = 0.66; 5-HT2C: p = 0.56. BLA = CRFR1: p = 0.33; GRM7: p = 0.75; SYT: p = 0.28; 5-HT2C: p = 0.46) or the genotype × treatment interaction for the mRNA levels (mpFC = CRFR1: p = 0.34; GRM7: p = 0.23; SYT1: p = 0.63; 5-HT2C: p = 0.51. BLA =CRFR1: p = 0.14; GRM7: p = 0.52; SYT: p = 0.89; 5-HT2C: p = 0.62) (Fig. 8). Duncan’s test, showed significantly higher mRNA levels of 5-HT2C (df = 0.263; p < 0.05) in the mpFC of unstressed WTcompared with unstressed TKO mice. Moreover, stressed WT mice had significantly higher levels of 5-HT2C mRNA in mpFC than stressed TKO animals (df = 0.238; p < 0.05) (Fig. 8A). Regarding the BLA, Duncan’s test showed significantly higher levels of mRNA CRFR1 in stressed WT versus stressed TKO mice (df = 0.278; p < 0.05) (Fig. 8B).
Fig. 8.
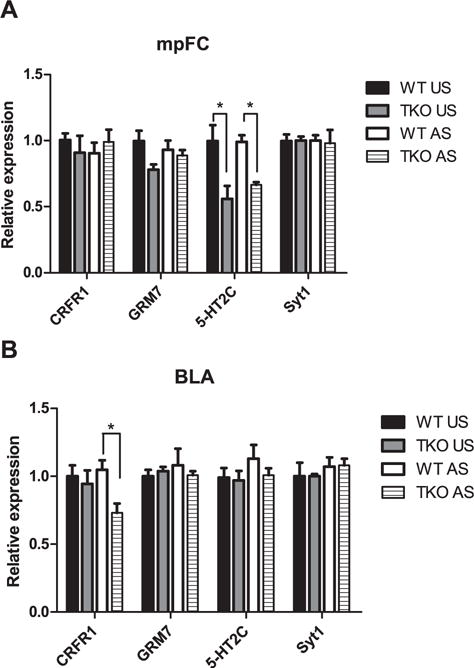
Effect of the stress exposure (30 min of restraint) on genes mRNA expression in the (A) medial prefrontal cortex (mpFC) and (B) Basolateral amygdala (BLA) of WT (WT Unstressed (US), mpFC n = 4, BLA n = 4; WT stressed (AS), mpFC n = 4, BLA n = 5) and TKO (TKO US, mpFC n = 4, BLA n = 4; TKO AS, mpFC n = 4, BLA n = 4) mice. Data are expressed as mean ± SE. *P < 0.05.
4.6. Morphological analysis
Because stress-induced anxiety has been linked to hypertrophy of BLA neurons in animal models, we decided to examine dendritic remodeling induced by stress in the BLA neurons of TKO and WT mice. Two-way ANOVA, revealed a significant genotype (F(1,100) = 5.26; p < 0.05) and treatment (F(1,100) = 21.79; p < 0.01) effect and genotype × treatment interaction (F(1,100) = 20.27; p < 0.01) with regard to the density of dendritic spines (Fig. 9A, D). Concerning the number of branch points, two-way ANOVA revealed no significant genotype effect (p = 0.31) and a significant treatment effect (F(1,100) = 7.91; p < 0.01) and genotype × treatment interaction (F(1,100) = 18.31; p < 0.01) (Fig. 9B, E). Two-way ANOVA revealed no significant genotype (p = 0.32) and treatment (p = 0.13) effects and a significant genotype × treatment interaction (F(1,100) = 11.77; p < 0.01) for dendritic length (Fig. 9C, E). Single comparisons between groups showed that whereas restraint exposure induced a significant increase in all morphological parameters [numbers of dendritic spines (df = 1.68; p < 0.05) (Fig. 9A), number of branch points (df = 5.03; p < 0.05) (Fig. 9B), and dendritic length (df = 355.49; p < 0.05) (Fig. 9C)] in the BLA neurons of stressed WT mice versus unstressed WT, the effect of stress was no significant between stressed and unstressed TKO mice [density of dendritic spines (p = 0.9) (Fig. 9A), number the of branch points (p = 0.27) (Fig. 9B), and dendritic length (p = 0.13) (Fig. 9C)]. Moreover, between unstressed groups, there were significantly more branch points (df = 2.32; p < 0.05) in TKO versus WT mice (Fig. 9B) and no significant difference in density of dendritic spines (p = 0.13) (Fig. 9A) or dendritic length (p = 0.12) (Fig. 9C).
Fig. 9.
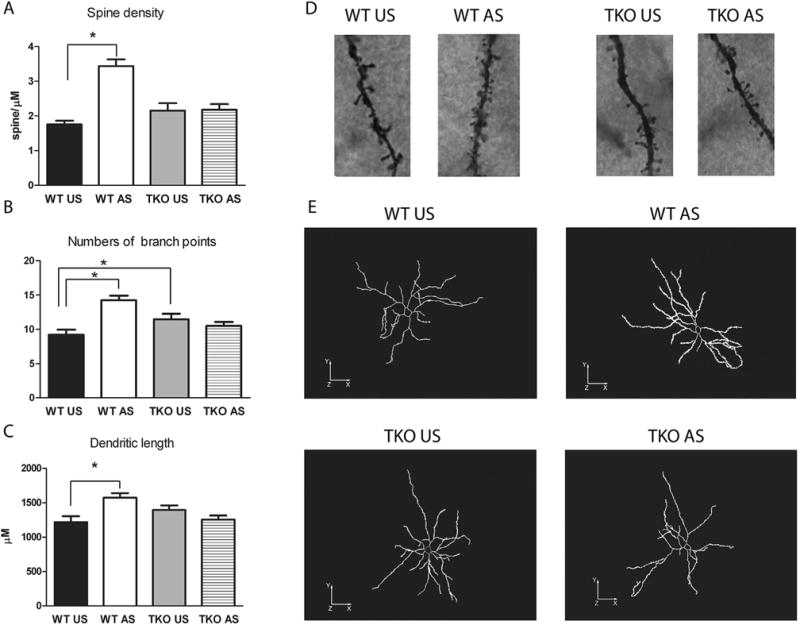
Effect of the stress exposure (30 min of restraint) on (A) spines density, (B) numbers of branch points, and (C) dendritic length in the Basolateral Amygdala (BLA) of WT (WT Unstressed (US), n = 5/neurons = 26; WT stressed (AS), n = 5/neurons = 26) and TKO (TKO Unstressed (US), n = 5/neurons = 25; TKO stressed (AS), n = 5/neurons = 27) mice. (D) High-power photomicrographs of the representative dendritic segment in the BLA of WT and TKO mice. (E) Representative camera lucida drawing the neurons of WT and miR-34 TKO mice. Data are expressed as mean ± SE. *P < 0.05.
5. Discussion
We have found that mice that lack miR-34 (TKO mice) show a strong reduction in behavioral, morphological, and neurochemical responses to acute stress compared with the WT group, suggesting a sort of resilience to the aversive effects of stress.
Overall, stress induced anxiety-like behaviors and changes in morphology of BLA neurons in WT mice but the impact of stressful experiences was mitigated in TKO. The TKO mice also showed facilitation of extinction fear memory, supporting our interpretation that absence of miR-34 confers a phenotype that is resilient to stress.
Under unstressed conditions, WT and TKO mice had similar profiles in anxiety-like behavior and locomotor activity, as measured by the EPM, DLT, and OF. The sole difference between unstressed WTand TKO mice concerned the distance covered in the lighted compartment in the dark-light test. Although unstressed TKO mice showed a reduction in distance moved versus WT mice, this parameter was unaffected by exposure to stress. Because the distance traveled in the dark compartment can not be evaluated in our DLTapparatus, the distance measured in the light compartment is merely a partial index of general locomotor activity. However, the overall comparison between WT and TKO mice in all behavioral tests (EPM, DLT, OF) suggests that small differences in basal locomotion and anxiety are unlikely to explain the robust differences between WT and TKO after stress.
Stress significantly altered anxiety-like behavior in WT mice, as evaluated by the DLT, EPM, and OF. Although the percentage of time spent in the open arms in the EPM levels was low, several groups have reported similar levels in mice (i.e. Abbas et al., 2015; Matsuo et al., 2009; Mozhui et al., 2010; Tsujimura et al., 2008). In contrast, there was no significant effect on anxiety-like behavioral parameters in stressed versus unstressed TKO animals, except for the latency, wherein stressed TKO mice increased the time to exit from aversive zone versus unstressed TKO mice (indicating reduced anxiety-like behavior) and for number of visits to the center in the OF, wherein stressed TKO mice made fewer visits versus unstressed TKO mice. Although the last result could suggest a weak effect of stress on anxiety-like behavior in TKO mice, all of the anxiety-related parameters observed in the EPM, DLT, and OF tests make difficult to supports this interpretation. Note that although elevated plus maze, dark-light box and open-field tests all assess anxietylike behaviors, they do that in differing way, exploring different aspects of the same phenotype. This could explain the not totally overlapping results obtained (in this and other studies) using different anxiety-based behavioral tests.
Concerning DLT, whereas stress decreased the latency to enter in the dark compartment, the number of visits, and the distance traveled in the light compartment in WT mice, only latency to enter the dark compartment rose significantly in stressed TKO mice compared with unstressed animals, indicating a strongly reduced effect of stress in animals that lack miR-34. Also, no significant effect of stress on the percentage of time spent in the light compartment was observed in WTor TKO mice. This result could be explained by the genetic background or the type of stress. Conflicting data have been reported depending on stress exposure protocol (acute vs repeated) and genetic background (Bluett et al., 2014; Chotiwat and Harris, 2006; Delgado-Morales et al., 2012; Ihne et al., 2012; Mozhui et al., 2010). Consistent with the DLT results, the EPM data showed a significant effect of stress on the percentage of time spent in the open arms only in WT mice. Finally concerning OF results, while an decrease of the latency to exit from the center, a reduced number of visits as well as the time spent in the center during the open field test were evident in stressed WT compared with unstressed animals, stress only reduced the number of visits to the center in TKO mice and significantly increased the latency to exit from the center, the latter suggesting a reduced impact of stress in TKO compared to WT mice.
Stress exposure induces anxiety disorders through elaborate mechanisms that are related to neural plasticity in brain regions that are involved in emotionality. Several studies have reported that neurons into the BLA that are highly sensitive to stress and anxiety-inducing stimuli undergo significant remodeling on exposure to stress. Further, acute stress increases anxiety and induces dendritic hypertrophy in the BLA (Grillon et al., 2007; Haramati et al., 2011; Maroun et al., 2013; Mitra et al., 2005; Rao et al., 2012). Our data clearly indicate selective effects of stress exposure on morphology only in WT mice, paralleling the behavioral results.
No significant difference was observed in morphological parameters in the BLA of unstressed WT and TKO groups, except for a slight increase in branch node number in the TKO group versus WT mice. However, this effect was unrelated to changes in basal anxiety-like behavior. Consistent with these data, the inhibition of miR-34a expression increases the number of branch nodes in cortical neurons (Agostini et al., 2011) and is unrelated to alterations in basal anxiety-like behavior in unstressed mice (Dias et al., 2014a). In the stressed groups, the number of dendritic spines, dendritic length, and number of dendritic nodes increased significantly in the BLA neurons of WTanimals compared with unstressed WT mice, but there was no significant effect on these morphological parameters in stressed versus unstressed TKO animals.
Our data on increased anxiety-like behavior and morphological alterations in the BLA of stressed WT mice are consistent with other evidence (Grillon et al., 2007; Haramati et al., 2011; Maroun et al., 2013; Mitra et al., 2005; Rao et al., 2012). However, they contrast other studies that have reported delayedd—or a lack of—dbehavioral and morphological modifications (Mitra et al., 2005) and dendritic retraction in the right hemisphere (Maroun et al., 2013) on exposure to stress. Several factors can explain this discrepancy. In fact, stress-induced modifications of BLA dendritic neurons are “sensitive” to the type and duration of stress (Maroun et al., 2013; Mitra et al., 2005; Vyas et al., 2006), the animal’s age (Padival et al., 2015), and the strain (Mozhui et al., 2010). Notably, the results inTKO mice conflict with a previous work that showed that lentivirally mediated overexpression of miR34c in the central amygdala (CeA) induces anxiolytic behavior after challenge (Haramati et al., 2011). This discrepancy might be attributed to the site of manipulation and disparate compensatory mechanisms. Whereas Haramati and colleagues overexpressed miR-34c in the central amygdala, we studied the effects of stress on the BLA, and it has been suggested that stress differentially affects different areas of the amygdala (central vs basolateral amygdala) (Andolina et al., 2013; Quirk et al., 2003). Moreover, because we used miR-34 TKO mice (instead of lentivirally mediated overexpression of miR34), compensatory mechanisms that are related to development and that affect our results can not be ruled out.
Several miRs have been shown to modulate synaptic plasticity under various stress conditions in several brain areas (Malan-Müller et al., 2013; Schouten et al., 2013), and miRs levels have been reported to be altered in patients who are affected by depression and anxiety and in preclinical models using psychological stress (Issler and Chen, 2015; Malan-Müller et al., 2013; O’Connor et al., 2012; Schouten et al., 2013). Clinical studies have reported strong downregulation in peripheral levels of miRs-34c due to antidepressant treatment in depressed patients (Bocchio-Chiavetto et al., 2013) and have suggested dysregulation of miR-34a in the pathogenesis of bipolar disorder (Bavamian et al., 2015). Accordingly, preclinical studies have reported that the antidepressant effects of 7-CTKA (an NMDA receptor antagonist) are mediated by alterations in miR-34a levels (Liu et al., 2014) and that mood stabilizers downregulate hippocampal expression of miR-34a (Zhou et al., 2009). Moreover, stressful challenges also alter miR expression in various brain structures (Issler and Chen, 2015; Malan-Müller et al., 2013; O’Connor et al., 2012; Schouten et al., 2013). We found that acute stress increases miR-34c expression in the BLA of WT mice, whereas, consistent with other reports (Dias et al., 2014a), no significant increase in miR-34a was evident. Notably, one of the major targets of miR-34 is corticotrophin-releasing factor receptor 1 (CRFR1) mRNA (Dias et al., 2014a; Haramati et al., 2011). The BLA expresses high levels of CRFR1, and the CRF system, including CRFR1, is involved in amygdalar synaptic plasticity and anxiety-like behavior. The decrease in anxiety-like behavior is associated with low levels of CRFR1 mRNA in the BLA of adult mice (Sztainberg et al., 2010), and CRFR1 agonist (BLA infusion) and antagonist treatment increase and reduce BLA neuronal plasticity and anxiety-like behavior, respectively (Rainnie et al., 2004; Sandi et al., 2008). Accordingly, we found that TKO mice showed a decrease in stress-induced BLA CRFR1 mRNA, anxiety-like behavior, and dendritic remodeling in BLA neurons compared with WT mice. Thus, it is possible to hypothesize that miR-34c in the BLA, acting through CRFR1 mRNA, promote neuro-plastic stress-induced alterations, mediating the anxiety-like behavior in WT mice. Further experiments are in progress in our laboratory to test this hypothesis.
An alternative hypothesis concerning corticosterone levels has to be considered. Elevated levels of corticosterone that precede acute stress prevent the stress effects on BLA synaptic connectivity and anxiety-like behavior (Rao et al., 2012). Consistent with this evidence, we found that TKO mice, which do not increase anxietylike behavior or BLA spine density following restraint-induced stress, had higher corticosterone levels under basal (unstressed) conditions versus WT mice. Although unstressed TKO mice have higher blood corticosterone levels than unstressed WT animals, blood corticosterone levels were higher in both groups of stressed mice (WT, TKO) at 30 and 120 min compared with the respective unstressed groups.
Concerning the neurochemical response induced by restraint stress, a time-dependent increase in 5-HT, DA, and NE output in the mpFC and in GABA in the BLA of WT mice was observed, consistent with previous reports (Andolina et al., 2013, 2014; Di Segni et al., 2015; Ventura et al., 2013). However, TKO mice showed only a significant increase in prefrontal DA in response to restraint exposure and no significant increase in 5-HT or NE outflow in the mpFC as well as in GABA in the BLA. Prefrontal NE release was similar between WT and TKO mice at the first time point (20 min). The increased prefrontal NE at this time point could be needed to redirect attention toward particularly arousing events (Chandler et al., 2014; Sara and Bouret, 2012; Ventura et al., 2013) to process salient or potentially dangerous stimuli. The prefrontal cortex and amygdala are key structures in the stress response and in anxiety behavior (Holmes, 2008; Sara and Bouret, 2012; Shin and Liberzon, 2010). Stress-induced alterations in GABAergic transmission in the amygdala are an important pathophysiological mechanism that underlies anxiety and stress disorders. Moreover, the mpFC is a critical regulator of BLA activity (Andolina et al., 2013, 2014; Likhtik et al., 2005). The pFC is involved in the integration and subsequent regulation of stimulus-driven responses in the amygdala, via glutamatergic projections to a neuronal population in the amygdala (Likhtik et al., 2005; Quirk et al., 2003). Changes in prefrontal aminergic neurotransmission modify the function of specific prefrontal cellular networks (Del Arco and Mora, 2009) and, consequently, the function of subcortical structures, including the BLA (Andolina et al., 2013, 2014). Dysfunctions in such regulation are involved in stress-related psychopathologies (Akirav and Maroun, 2007; Holmes, 2008).
Our results point to a modulatory function for miR-34 on mpFC 5-HT and NE and BLA GABAergic transmission under stress, consistent with recent studies that have suggested specific functions for various miRs in regulating brain neurotransmitters activity (Baudry et al., 2010; Issler et al., 2014; Launay et al., 2011). MiR-34 regulates the expression of proteins that are involved in neurotransmitter processes, having a key function in stress-related disorders. Differential expression of mpFC 5-HT2C and GRM7 mRNA was evident in unstressed TKO mice versus unstressed WT mice, although the difference in GRM7 was not significant. GRM7 and 5-HT2C regulate neurotransmission in the mammalian CNS. For instance, it has been reported that selective GRM7 agonists produce modifications in 5-HT and NE function (Pelkey et al., 2007; Sukoff Rizzo et al., 2011), and 5-HT2C receptors mediate the release of 5-HT in response to acute stress and anxiety (Liu et al., 2007; Mongeau et al., 2010). Thus, miR-34 could modulate prefrontal 5-HT and NE release by differentially regulating prefrontal 5-HT2C and GRM7 in stressed WT and TKO and thus governing amygdalar GABAergic release (Andolina et al., 2013, 2014). Our results implicate miR-34 in the development and expression of stress-induced psychopathologic phenotypes, such as anxiety-like behavior.
Although stress is one of the most important risk factors of several psychiatric disorders, interindividual differences exist to stress response underlie the vulnerability or resilience to negative stress effects. The mechanisms of the resilience to stress effects involve the complex interplay between genetic, and environmental factors (Feder et al., 2009; Russo et al., 2012). Our data, consistent with recent studies indicating an important function of miR in response to stress effects (Dias et al., 2014b; Issler et al., 2014), strongly suggest that the lack of miR-34 renders mice more resistant to behavioral aversive stress effects.
Supplementary Material
Acknowledgments
We thank Dr Sergio Papalia for his skillful assistance. This research was supported by ‘Ricerca Corrente’, Italian Ministry of Health (grant no: RC14C) and Ateneo 2011, Sapienza University of Rome and SIR “RBSI14G1HH”, Italian Ministry of Education, Universities and Research.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2016.03.044.
References
- Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T. Comprehensive behavioral analysis of male Ox1r (−/−) mice showed implication of orexin receptor-1 in mood, anxiety, and social behavior. Front Behav Neurosci. 2015;9:324. doi: 10.3389/fnbeh.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW, Ventura A, Concepcion CP, Han YC, Candi E, Knight RA, Mak TW, Melino G. microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A. 2011;108:21099–21104. doi: 10.1073/pnas.1112063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina D, Conversi D, Cabib S, Trabalza A, Ventura R, Puglisi-Allegra S, Pascucci T. 5-Hydroxytryptophan during critical postnatal period improves cognitive performances and promotes dendritic spine maturation in genetic mouse model of phenylketonuria. Int J Neuropsychopharmacol. 2011;14:479–489. doi: 10.1017/S1461145710001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina D, Maran D, Valzania A, Conversi D, Puglisi-Allegra S. Prefrontal/amy gdalar system determines stress coping behavior through 5-HT/ GABA connection. Neuropsychopharmacology. 2013;38:2057–2067. doi: 10.1038/npp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina D, Maran D, Viscomi MT, Puglisi-Allegra S. Strain-dependent variations in stress coping behavior are mediated by a 5-HT/GABA interaction within the prefrontal corticolimbic system. Int J Neuropsychopharmacol. 2014;18(3) doi: 10.1093/ijnp/pyu074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Madison JM, Zhou F, Rueckert EH, Barker D, Perlis RH, Sur M, Haggarty SJ. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry. 2015;20:573–584. doi: 10.1038/mp.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry. 2014;4:e408. doi: 10.1038/tp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M. Blood microRNA changes in depressed patients during antidepressant treatment. Eur J Neuropsychopharmacol. 2013;23:602–611. doi: 10.1016/j.euroneuro.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Genotype-dependent effects of chronic stress on apomorphine-induced alterations of striatal and mesolimbic dopamine metabolism. Brain Res. 1991;542:91–96. doi: 10.1016/0006-8993(91)91002-i. [DOI] [PubMed] [Google Scholar]
- Chandler DJ, Waterhouse BD, Gao WJ. New perspectives on catechol-aminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Front Neural Circuits. 2014;8:53. doi: 10.3389/fncir.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiwat C, Harris RB. Increased anxiety-like behavior during the post-stress period in mice exposed to repeated restraint stress. Horm Behav. 2006;50:489–495. doi: 10.1016/j.yhbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Concepcion CP, Han YC, Mu P, Bonetti C, Yao E, D’Andrea A, Vidigal JA, Maughan WP, Ogrodowski P, Ventura A. Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet. 2012;8:e1002797. doi: 10.1371/journal.pgen.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex-limbic system interactions: implications for plasticity and psychiatric disorders. J Neural Transm. 2009;116:941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Delgado-Morales R, del Río E, Gomez-Roman A, Bisagno V, Nadal R, de Felipe C, Armario A. Adrenocortical and behavioural response to chronic restraint stress in neurokinin-1 receptor knockout mice. Physiol Behav. 2012;105:669–675. doi: 10.1016/j.physbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Frau R, Carboni E. On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology (Berlin) 1993;112:398–402. doi: 10.1007/BF02244939. [DOI] [PubMed] [Google Scholar]
- Segni DiM, Andolina D, Luchetti A, Babicola L, D’Apolito LI, Pascucci T, Conversi D, Accoto A, D’Amato FR, Ventura R. Unstable maternal environment affects stress response in adult mice in a genotype-dependent manner. Cereb Cortex. 2015:1–11. doi: 10.1093/cercor/bhv204. http://dx.doi.org/10.1093/cercor/bhv204. [DOI] [PubMed]
- Dias C, Feng J, Sun H, Shao NY, Mazei-Robison MS, Damez-Werno D, Scobie K, Bagot R, LaBonte B, Ribeiro E, Liu X, Kennedy P, Vialou V, Ferguson D, Pena C, Calipari ES, Koo JW, Mouzon E, Ghose S, Tamminga C, Neve R, Shen L, Nestler EJ. P-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature. 2014b;516:51–55. doi: 10.1038/nature13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Goodman JV, Ahluwalia R, Easton AE, Andero R, Ressler KJ. Amygdala-dependent fear memory consolidation via miR-34a and Notch signaling. Neuron. 2014a;83:906–918. doi: 10.1016/j.neuron.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson JR, Kruse C, Montagna DR, Finsen B, Wolfe MS. Alternative polyadenylation and miR-34 family members regulate tau expression. J Neurochem. 2013;127:739–749. doi: 10.1111/jnc.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. Stereotaxic Coordinates. Academic; San Diego: 1997. The mouse brain. [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute stress potentiates anxiety in humans. Biol Psychiatry. 2007;62:1183–1186. doi: 10.1016/j.biopsych.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramati S, Navon I, Issler O, Ezra-Nevo G, Gil S, Zwang R, Hornstein E, Chen A. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J Neurosci. 2011;31:14191–14203. doi: 10.1523/JNEUROSCI.1673-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihne JL, Fitzgerald PJ, Hefner KR, Holmes A. Pharmacological modulation of stress-induced behavioral changes in the light/dark exploration test in male C57BL/6J mice. Neuropharmacology. 2012;62:464–473. doi: 10.1016/j.neuropharm.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, Gil S, Mayberg HS, Dunlop BW, Menke A, Awatramani R, Binder EB, Deneris ES, Lowry CA, Chen A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron. 2014;83:344–360. doi: 10.1016/j.neuron.2014.05.042. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay JM, Mouillet-Richard S, Baudry A, Pietri M, Kellermann O. Raphe mediated signals control the hippocampal response to SRI antidepressants via miR-16. Transl Psychiatry. 2011;1:e56. doi: 10.1038/tp.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Seroto-nin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BB, Luo L, Liu XL, Geng D, Liu Q, Yi LT. 7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress. Psychopharmacology (Berlin) 2014;232:541–550. doi: 10.1007/s00213-014-3690-3. [DOI] [PubMed] [Google Scholar]
- MacNeil G, Sela Y, McIntosh J, Zacharko RM. Anxiogenic behavior in the light-dark paradigm following intraventricular administration of cholecystokinin-8S, restraint stress, or uncontrollable footshock in the CD-1 mouse. Pharmacol Biochem Behav. 1997;58:737–746. doi: 10.1016/s0091-3057(97)00037-3. [DOI] [PubMed] [Google Scholar]
- Malan-Müller S, Hemmings SM, Seedat S. Big effects of small RNAs: a review of microRNAs in anxiety. Mol Neurobiol. 2013;47:726–739. doi: 10.1007/s12035-012-8374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL. Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci. 2013;38:2611–2620. doi: 10.1111/ejn.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Tanda K, Nakanishi K, Yamasaki N, Toyama K, Takao K, Takeshima H, Miyakawa T. Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: decreased social contact duration in two social interaction tests. Front Behav Neurosci. 2009;3:3. doi: 10.3389/neuro.08.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Martin CB, Chevarin C, Maldonado R, Hamon M, Robledo P, Lanfumey L. 5-HT(2c) receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J Neurochem. 2010;115:438–449. doi: 10.1111/j.1471-4159.2010.06932.x. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RM, Dinan TG, Cryan JF. Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry. 2012;17:359–376. doi: 10.1038/mp.2011.162. [DOI] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Vantrease JE, Rosenkranz JA. Qualitatively different effect of repeated stress during adolescence on principal neuron morphology across lateral and basal nuclei of the rat amygdala. Neuroscience. 2015;291:128–145. doi: 10.1016/j.neuroscience.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Grimm CH, Paya-Cano JL, Sugden K, Nietfeld W, Lehrach H, Schalkwyk LC. Using hippocampal microRNA expression differences between mouse inbred strains to characterise miRNA function. Mamm Genome. 2008;19:552–560. doi: 10.1007/s00335-008-9116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, Latagliata EC, Cabib S, Puglisi-Allegra S. The medial prefrontal cortex determines the accumbens dopamine response to stress through the opposing influences of norepinephrine and dopamine. Cereb Cortex. 2007;17:2796–2804. doi: 10.1093/cercor/bhm008. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Yuan X, Lavezzari G, Roche KW, McBain CJ. mGluR7 undergoes rapid internalization in response to activation by the allosteric agonist AMN082. Neuropharmacology. 2007;52:108–117. doi: 10.1016/j.neuropharm.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72:466–475. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Cremers TI, Westerink BH. HPLC conditions are critical for the detection of GABA by microdialysis. J Neurochem. 2005;94:672–679. doi: 10.1111/j.1471-4159.2005.03218.x. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS. 5 HT1A receptors are involved in the cannabidiol induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;56:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior-modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76:130–141. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Schouten M, Aschrafi A, Bielefeld P, Doxakis E, Fitzsimons CP. microRNAs and the regulation of neuronal plasticity under stress conditions. Neuroscience. 2013;241:188–205. doi: 10.1016/j.neuroscience.2013.02.065. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Leonard SK, Gilbert A, Dollings P, Smith DL, Zhang MY, Di L, Platt BJ, Neal S, Dwyer JM, Bender CN, Zhang J, Lock T, Kowal D, Kramer A, Randall A, Huselton C, Vishwanathan K, Tse SY, Butera J, Ring RH, Rosenzweig-Lipson S, Hughes ZA, Dunlop J. The metabotropic glutamate receptor 7 allosteric modulator AMN082: a monoaminergic agent in disguise? J Pharmacol Exp Ther. 2011;338:345–352. doi: 10.1124/jpet.110.177378. [DOI] [PubMed] [Google Scholar]
- Sztainberg Y, Kuperman Y, Tsoory M, Lebow M, Chen A. The anxiolytic effect of environmental enrichment is mediated via amygdalar CRF receptor type 1. Mol Psychiatry. 2010;15:905–917. doi: 10.1038/mp.2009.151. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Matsuki M, Takao K, Yamanishi K, Miyakawa T, Hashimoto-Gotoh T. Mice lacking the kf-1 gene exhibit increased anxiety-but not despair-like behavior. Front Behav Neurosci. 2008;8:2–4. doi: 10.3389/neuro.08.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Coccurello R, Andolina D, Latagliata EC, Zanettini C, Lampis V, Battaglia M, D’Amato FR, Moles A. Postnatal aversive experience impairs sensitivity to natural rewards and increases susceptibility to negative events in adult life. Cereb Cortex. 2013;23:1606–1617. doi: 10.1093/cercor/bhs145. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances syn-aptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


