Abstract
Introduction
Oncogenic fusion of anaplastic lymphoma kinase (ALK) with echinoderm microtubule-like (EML) protein or other partner genes occurs in 3–6% of lung adenocarcinomas. Although fluorescence in situ hybridization (FISH) is the gold standard for detecting ALK gene rearrangement that gives rise to new fusion genes, not all ALK FISH positive (+) patients respond to ALK inhibitor therapies. We report here an ALK FISH+ patient-derived xenograft (PDX) that is non-responsive to crizotinib therapy.
Methods
The PDX was established in NOD/SCID mice from a resected pT4N1M0 lung adenocarcinoma patient. ALK gene status was investigated using the standard FISH break apart assay, RT-qPCR, RNA sequencing (RNAseq) and immunohistochemistry (IHC) using the 5A4 antibody. PHLC-402 was treated with Crizotinib (50mg/kg) by daily oral gavage.
Results
ALK FISH analysis was positive in both the primary patient tumor and PDX, which were negative for ALK protein expression by IHC. ALK fusion product was not detected by RNAseq and RT-qPCR comparing the 5′ and 3′ ALK transcript levels. Crizotinib treatment of PHLC-402 grown in mice resulted in no tumor response.
Conclusion
ALK protein expression may be necessary for ALK FISH+ lung cancer to be responsive to ALK inhibitor therapy.
Keywords: Lung cancer, patient-derived xenograft, FISH, IHC, Crizotinib
INTRODUCTION
Anaplastic lymphoma kinase (ALK) fusion oncoprotein as a result of genetic rearrangement is expressed in 3–6% of lung adenocarcinomas (ADC) and has been validated as a driver oncogene and therapeutic target. Fluorescence in situ hybridization (FISH) is the “gold” standard for detecting ALK rearrangements, but ALK protein expression detected by immunohistochemistry (IHC) is commonly used for screening and diagnostics based on its very high sensitivity and specificity in detecting ALK FISH positive lung cancers1. However, there have been reports of discrepancy between FISH and IHC results2, and correlation with response to ALK inhibitor therapy in ALK FISH+/IHC- patients remains unclear. Our group conducted a large scale establishment of patient derived xenografts (PDX) from lung cancer patients. We report here the lack of response of an ALK FISH+/IHC- PDX to crizotinib.
MATERIALS AND METHODS
Establishment of lung PDX was described previously3. All animal studies were approved by the University Health Network (UHN) Human Research Ethics and Animal Care Committee. H2228 and H661 cell lines were obtained from ATCC and authenticated by short tandem repeat analysis. Crizotinib was purchased from UHN Shanghai, Inc. (Shanghai, China) with >99% purity and its ALK inhibitor activity validated in vitro in the H2228 cell line (data not shown). Mutation profiling was performed using the OncoCarta v.1 MassArray platform (Sequenom, San Diego, CA).
Fluorescence in situ hybridization
The Vysis ALK Break-Apart FISH Probe Kit (Abbott Molecular, Abbott Park, Illinois, USA) was used for FISH analysis. Slides were examined with an epifluorescence microscope, and images were captured using CCD camera. FISH results were analyzed using Cyto Vision software.
Immunohistochemistry
Formalin-fixed paraffin embedded (FFPE) tissues were stained with antibodies using BenchMark XT autostainer (Ventana Medical System, Tucson, AZ). ALK immunohistochemistry was performed by a clinically optimized and standardized assay using 5A4 antibody (Leica Canada, Concord, ON).4 The pAkt (S473) (clone D9E), and pErk1/2-Y202/T204 (clone D13.14.4E) antibodies were purchased from Cell Signaling (Danvers, MA).
RT-qPCR and RNA sequencing
Total RNA from tumor tissue and cell lines were extracted using TRIzol method according to the manufacturer’s instructions (Life Technologies Inc., Burlington, ON, Canada). RNA was reverse transcribed and qPCR was performed using the following conditions: 94°C for 1 min, 60°C for 30 sec, and 72°C for 30sec for 30 cycles. Primers used included ALK exon14F 5′-TGTGAACAGAAGCGTGCATGA-3′, exon15R 5′-TCTCTCTGGGTGGAACGTGT-3′, exon22F 5′-TGTGCTCTGAACAGGACGAACT-3′, exon 23R 5′-TGAGCTCCAGCAGGATGAACC-3′. RNA sequencing was performed on PDX-isolated mRNA at read depth of 60 million using HiSeq2000 sequencer (Illumina, San Diego).
In vivo therapeutic studies
PHLC-402 that has been cryopreserved at passage 3 was implanted into the flank subcutaneous tissue of non-obese diabetic/severe combined immune deficient (NOD/SCID) mice. Tumors were grown to 150 mm3 prior to treatment initiation. Crizotinib (50mg/kg) was delivered via daily oral gavage for 27 days, and tumor size was monitored twice a week by caliper measurement.
RESULTS
Case Report
A 62 year old male was presented with shortness of breath, cough, intermittent hemoptysis and weight loss. He was a smoker of approximately 50 pack years, but no other significant prior medical history. Radiographic imaging of the chest showed a large solitary hilar mass involving superior vena cava (SVC). An endobronchial ultrasound-guided biopsy was performed and revealed an adenocarcinoma. There was no evidence of systemic metastasis. The patient underwent a pneumonectomy with SVC resection. Histological examination revealed a solid predominant adenocarcinoma with minor acinar component, measuring 7.0 cm in greatest diameter (Fig. 1A). The tumor has invaded the vena cava and hilum of the right upper, middle and lower lobes. The tumor was pathologically staged as pT4N1M0. Unfortunately, the patient developed post-operative complication and death.
FIGURE 1.
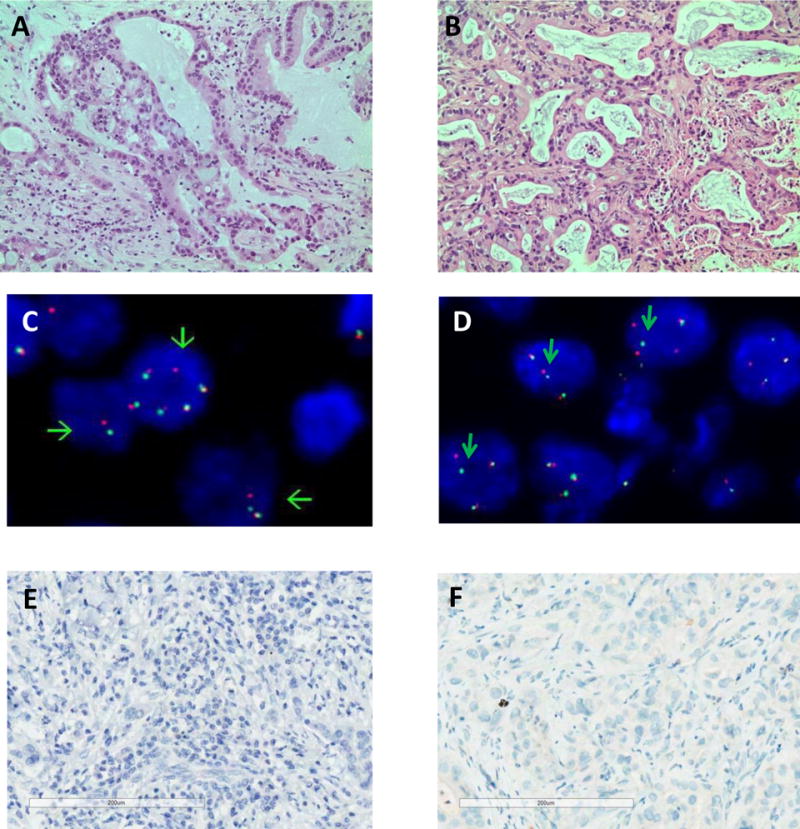
Histopathology, fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) of PHLC-402 lung adenocarcinoma. (A) Hematoxylin and eosin stain of the patient tumor and (B) its matching PDX passage 3 shows the histological preservation of the PDX tumor. (C) FISH showed ALK break-apart signals in 68% of tumor cells in the patient tumor, and (D) 82% of tumor cells in the PDX tumor. ALK IHC showed lack of staining in both the (E) patient and (F) PDX tumors.
Pathology, molecular characteristics and Crizotinib response
The histology of PHLC-402 PDX largely recapitulated the histology of the primary tumor showing a mix of solid and acinar components (Fig.1A & 1B). Molecular characterization of the surgically resected tumor by Oncocarta (Sequenom, San Diego) and direct sequencing confirmed the absence of EGFR, KRAS, or c-MET mutations. FISH analyses of both the primary resected tumor and PDX identified ALK rearrangement with split 3′ALK-5′ALK signals (Fig. 1C & 1D), but absence of ALK protein expression was noted by IHC (Fig. 1E and 1F). To determine 5′ and 3′ ALK transcriptional differences, RT-qPCR was performed in PDX 402, along with ALK negative control cell line H661 and ALK fusion positive control cell line H2228. Results show that PDX 402 mRNA expression is low with no differences in 5′ and 3′ ALK mRNA expression, while H2228 3′ ALK mRNA expression is significantly higher than that of 5′ ALK (Fig.2). To identify other potential ALK fusion mRNA transcripts, RNA sequencing was performed on PDX-isolated mRNA. However, no ALK fusion was detected.
FIGURE 2.
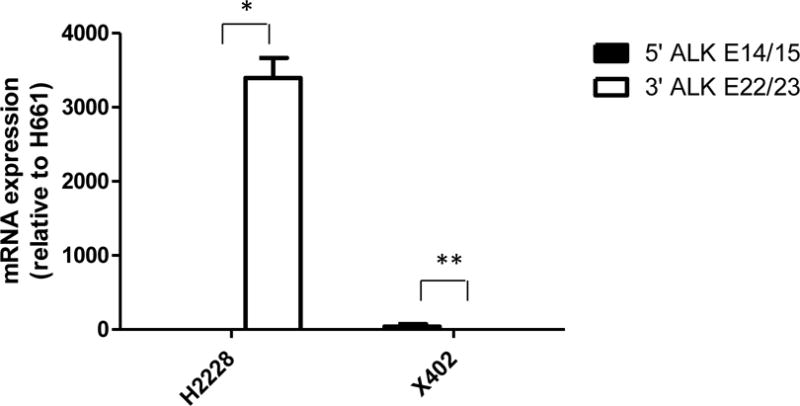
Lack of ALK fusion mRNA expression by RT-qPCR of 5′ (exon 14–15) and 3′ (exon 22–23) ALK in cell line H2228 and PDX 402 relative to H661 ALK mRNA expression. H2228 harboring EML4-ALK fusion expresses higher 3′ mRNA expression than that of 5′ region, therefore this indicates the presence of ALK fusion. PDX 402 not only expresses low levels of ALK but also shows no differences between 5′ and 3′ ALK mRNA, indicating the absence of ALK fusion. *p<0.05, **p>0.05.
Compared to untreated control mice, crizotinib treatment of mice with growing PHLC-402 PDX did not result in tumor growth inhibition over 4 weeks of treatment period (Fig.3A). There was also no inhibition of pAkt and pErk1/2 in both untreated control and crizotinib treated tumors (Fig.3B & 3C).
FIGURE 3.
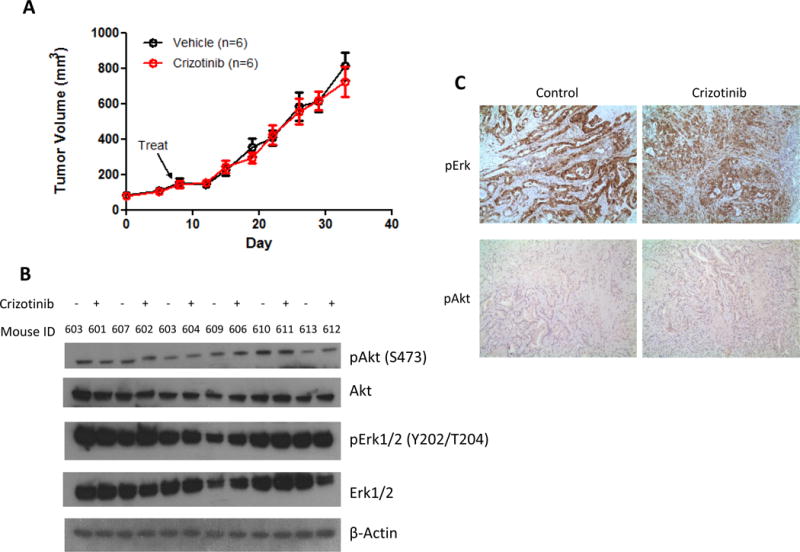
Lack of response of PDX-402 to crizotinib therapy. (A) Crizotinib was administered via oral gavage five times a week at 50mg/kg for 27 days. No tumor shrinkage can be observed in the crizotinib treated group. Experiments were performed in duplicate.*p>0.05. (B) Western blots show the absence of changes to phosphorylation status of ALK downstream signaling proteins after crizotinib treatment. β-Actin was used as a loading control. (C) Representative IHC images of pAkt and pErk1/2 in control and crizotinib treated groups, showing no differences in antibody staining intensity can be observed between control and treated groups.
DISCUSSION
Although there is a high percentage of concordance between FISH and IHC for ALK testing, not all FISH+ patients respond to Crizotinib. Moreover, 20% of FISH+ patients do not respond to ALK targeted therapies due to unknown reasons8. This lack of response could be due to false positive FISH results. A study has shown that tumors that predominantly harbor isolated 3′ ALK pattern may be more frequently associated with false positivity as determined by next generation sequencing9. However in the case of PHLC 402, we were unable to detect any ALK mRNA or protein even though a split 3′ and 5′ ALK signal by FISH was detected. Speculations for the false positivity could potentially be the genetic rearrangement fusing the ALK gene to a region that cannot be transcribed, the breakpoint occurring outside of the ALK gene or post-transcriptional mechanisms deleting the ALK mRNA product.
FISH has been regarded as the gold standard for diagnosis of ALK rearranged lung cancer and is used for diagnostic screening in the United States, while many other countries have used IHC for the ALK diagnostic screen. FISH is limited by low throughput, relatively high cost, and potential mis-interpretation of break apart signals, leading to false positive or negative results. IHC is an additional method for ALK detection and is easily scalable, cost-effective, and efficient. While multiple antibodies (ALK1, D5F3, and 5A4) are available for ALK IHC and only one FDA-approved diagnostic ALK IHC assay is available, stringent optimization and standardization of the staining protocol can provide near 100% sensitivity and specificity for ALK IHC to detect ALK FISH positive lung cancers.1,4
Studies have shown that the concordance of FISH and IHC approaches 100%4,5,6. However, there are cases in which FISH results do not correlate with IHC results. Reports have shown that the rate of discordance between ALK FISH and IHC ranges from 0.3 to 2%10, and that patients with ALK FISH−/IHC+ lung adenocarcinoma responded well to Crizotinib7, but how patients with ALK FISH+/IHC− lung cancers respond to Crizotinib is unclear.
Previous reports have shown that the EML4-ALK fusion positive cell lines, H2228 and H3122, are sensitive to Crizotinib in vitro and in vivo11. We showed that PDX 402 is resistant to Crizotinib despite being positive for ALK rearrangement by FISH. The lack of response is justified by the absence of ALK fusion transcript by RNA-Seq, ALK kinase domain mRNA expression by RT-PCR and ALK protein expression by IHC. Our results demonstrate the advantage of including IHC for ALK testing in lung adenocarcinoma.
Acknowledgments
We would like to thank Jing Xu and Wendy So for all IHC staining. This work was supported by a Canadian Cancer Society Research Institute grant #701595. RS is partially supported by The Ontario Student Opportunity Trust Fund (OSOTF). FISH analyses were supported by the University of Colorado Cancer Center Molecular Pathology Shared Resource (CCSG P30CA046934).
Footnotes
Disclosure: The authors declare no conflict of interest related to this work.
References
- 1.Lantuéjoul S, Varella-Garcia M, Thunnissen E, et al. Comparison of different assay platforms for ALK Testing. In: Tsao MS, Hirsch FR, Yatabe Y, editors. IASLC ATLAS of ALK testing in lung cancer. IASLC Press (Aurora, CO); 2013. pp. 44–52. Chapter 6. [Google Scholar]
- 2.Ilie MI, Bence C, Hofman V, et al. Discrepancies between FISH and immunohistochemistry for assessment of the ALK status are associated with ALK ‘borderline’-positive rearrangements or a high copy number: a potential major issue for anti-ALK therapeutic strategies. Annals of Oncology. 2015;26:238–244. doi: 10.1093/annonc/mdu484. [DOI] [PubMed] [Google Scholar]
- 3.John T, Kohler D, Pintilie M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early stage Non-small cell lung cancer. Clinical cancer research. 2011;17(1):134–141. doi: 10.1158/1078-0432.CCR-10-2224. [DOI] [PubMed] [Google Scholar]
- 4.Cutz JC, Craddock KJ, Torlakovic E, et al. Canadian anaplastic lymphoma kinase study: A model for multicenter standardization and optimization of ALK testing in lung cancer. Journal of Thoracic Oncology. 2014;9:1255–1263. doi: 10.1097/JTO.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 5.McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH Testing for ALK Gene Rearrangement in Lung Adenocarcinomas in a Routine Practice, a French study. Journal of Thoracic Oncology. 2012;7:348–354. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 6.Cabillic F, Gros A, Dugay F, et al. Parallel FISH and Immunohistochemical Studies of ALK Status in 3244 Non–Small-Cell Lung Cancers Reveal Major Discordances. Journal of Thoracic Oncology. 2014;9:295–306. doi: 10.1097/JTO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 7.Sun JM, Choi YL, Won JK, et al. A dramatic response to Crizotinib in a non-small cell lung cancer patient with IHC-positive and FISH-negative ALK. Journal of Thoracic Oncology. 2012;7(12):e36–8. doi: 10.1097/JTO.0b013e318274694e. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New England Journal of Medicine. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Sholl LM, Nishino M, et al. Clinical implications of variant ALK FISH rearrangement patterns. Journal of Thoracic Oncology. 2015;10:1648–52. doi: 10.1097/JTO.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatabe Y. ALK FISH and IHC you cannot have one without the other. Journal of Thoracic Oncology. 2015;10(4):548–550. doi: 10.1097/JTO.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 11.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discovery. 2014;4(6):662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]


