Abstract
Objective
To determine how visceral sensations affect responses to food stimuli in anorexia nervosa (AN).
Methods
Twenty weight-restored, unmedicated adolescent and young adult women with AN and twenty healthy control participants completed an interoceptive attention task during which they focused on sensations from the heart, stomach, and bladder and made ratings of these sensations. They then underwent fMRI scanning while viewing pictures of food and non-food objects. Between-groups t-tests were employed to investigate group differences in the relationship between interoceptive sensation ratings and brain hemodynamic response to food pictures, and, specifically, to highly palatable foods.
Results
In response to food pictures, AN participants exhibited a positive relationship between stomach sensation ratings and posterior insula activation (peak t=4.30). AN participants displayed negative relationships between stomach sensation ratings and amygdala activation (peak t=−4.05) and heart sensation ratings and ventromedial prefrontal cortex activation (peak t=−3.52). In response to highly palatable foods, AN was associated with positive relationships between stomach sensation ratings and activity in the subgenual anterior cingulate (peak t=3.88) and amygdala (peak t=4.83), and negative relationships in the ventral pallidum (peak t=−3.99) and ventral tegmental area (peak t=−4.03). AN participants also exhibited negative relationships between cardiac sensations and activation in response to highly palatable foods in the putamen (peak t=−3.41) and ventromedial prefrontal cortex (peak t=−3.61). Healthy participants exhibited the opposite pattern in all of these regions.
Conclusions
Hedonic and interoceptive inferences made by individuals with AN at the sight of food may be influenced by atypical visceral interoceptive experience, which could contribute to restrictive eating.
Keywords: anorexia nervosa, eating disorder, interoception, food stimuli, reward, fMRI
A person suffering from anorexia nervosa (AN) avoids eating, or if given the option, chooses a food with inadequate caloric content for meeting their energy needs. Continuing this pattern over weeks and months leads to severe malnutrition and to the significant morbidity and mortality associated with AN. Although food avoidance may be driven by a number of factors, including a fear of fatness or desire for control, it may also be that individuals with AN have fundamentally altered interoceptive (i.e., brain-body) signaling that causes them to experience sensations from their bodies (particularly their stomachs) differently, thereby altering the homeostatic significance of food stimuli. Indeed, a recent study provides evidence that individuals with AN exhibit altered activity in the mid-insula, a key interoceptive region in the brain, while attending to stomach sensations (1). Likewise, this same study demonstrated that numerous behavioral markers of illness severity were linearly related to atypical activity in the insula during stomach interoception, but not heart or bladder interoception. These findings are important in light of other evidence demonstrating that gastric sensations are both readily paired with food cues (2) and are related to activity in the mid-insula (1,3), and homeostatic signals related to energy availability modulate the response of interoceptive insula cortex to food (4). Indeed, the mid-insula has long been known to activate to the sight of food (5–9), suggesting that it may play an important role in inferences about the interoceptive consequences of visually perceived foods. In summary, past research suggests that the insula modulates response to food stimuli based on the interoceptive milieu, and that individuals with AN have altered insular processing of interoceptive sensations from the stomach. Taken together, these lines of evidence suggest that altered stomach interoception may form the basis for food avoidance in AN. We thus hypothesized that gastric interoceptive experience may alter the hedonic and interoceptive inferences made by individuals with AN when they are exposed to food cues.
To test this hypothesis, we asked participants with AN and healthy control (HC) participants to undergo functional magnetic resonance imaging (fMRI) while viewing food pictures. Immediately prior to performing the food picture task, participants also performed a visceral interoceptive attention task during which they made subjective intensity ratings for naturally occurring sensations in their heart, stomach, and bladder.
Methods
Participants
20 adolescent and young adult women diagnosed with restricting-type AN (age range: 13 – 24 years; body mass index [BMI] range: 18.6 – 22.7) and 20 HC female participants (age range: 13 – 23 years; BMI range: 19 – 24.1) with no history of psychiatric disorder participated in the study. We recruited participants in this age range because it represents the majority of the demographic of individuals treated for AN. Although this age range represents a mix of adolescents and young adults, it also reduces potential confounding effects that could occur if the sample included individuals with decades of chronic illness.
AN participants were diagnosed using the Structured Interview for Anorexic and Bulimic Disorders (10) and a semi-structured interview with a psychiatrist. The Structured Clinical Interview for DSM-IV Disorders (11,12) was used to screen HC participants and diagnose comorbid disorders in participants with AN. At the time of scanning, all AN participants were weight restored to a BMI of at least 18.5 and were free of psychotropic medications within three weeks prior to scanning (six weeks for fluoxetine). See Table 1 for sample demographics.
Table 1.
Demographic and clinical characteristic of the study samples
| AN (n = 20) | HC (n = 20) | t | p | |
|---|---|---|---|---|
| Age, M (SD), years | 17 (3) | 18 (3) | −1.43 | 0.16 |
| Current BMI, M (SD) | 19.84 (0.87) | 21.30 (1.55) | −3.69 | <.001 |
| Lowest BMI, M (SD) | 14.62 (1.77) | – | ||
| HAM-A, M (SD) | 6.05 (3.55) | 0.70 (0.86) | 6.55 | <.001 |
| EDI-EDRCa, M (SD) | 45.69 (6.63) | 31.55 (5.96) | 6.23 | <.001 |
| EDI-GPMCa, M (SD) | 41.92 (5.54) | 31.00 (4.36) | 6.00 | <.001 |
| Current comorbid diagnoses, nb | ||||
| Depressive disorder | 7 | – | ||
| GAD | 3 | – | ||
| Social phobia | 3 | – | ||
| OCD | 1 | – | ||
| PTSD | 1 | |||
| Specific phobia | 1 | – |
Data unavailable for 7 AN participants (n=13).
Values represent unique diagnoses rather than unique individuals. Nine AN participants did not have any comorbid diagnoses, and 5 AN participants had two comorbid diagnoses (typically an anxiety and depressive disorder).
AN=anorexia nervosa; HC=healthy control; BMI=body mass index; HAM-A=Hamilton Anxiety Scale; EDI-EDRC=Eating Disorders Inventory-3, Eating Disorders Risk Composite (T-score); EDI-GPMC=Eating Disorders Inventory-3, General Psychological Maladjustment Composite (T-score); GAD=generalized anxiety disorder; OCD=obsessive-compulsive disorder; PTSD=posttraumatic stress disorder.
Study procedures were reviewed and approved by The University of Oklahoma Health Sciences Center Institutional Review Board and the Western Institutional Review Board. Adult participants and parents/guardians of minor participants provided written informed consent, and minor participants provided written informed assent. Data were collected from December 2010 to May 2016.
Task
All experimental sessions took place in the afternoon to control for time-of-day effects on response to food cues. Participants first performed an interoceptive attention task during which they focused attention on naturally occurring sensations in the stomach, heart, and bladder. In fMRI studies, this task has been repeatedly demonstrated to activate interoceptive cortex in the insula (1,3,13,14). Following half of the interoceptive attention trials, participants made ratings on a scale numbered from 1 (little sensation) to 7 (extremely intense sensation) indicating the intensity of sensations they felt from the specified organ during that trial. In total, nine intensity ratings were made for each body part. The ratings for each body part were then averaged for each participant, providing an index of the current level of interoceptive sensation being experienced during the experimental session. Interoceptive sensation rating group averages are provided in Supplemental Digital Content 1.
Participants then completed a task in the scanner during which they viewed pictures of food and non-food objects (Figure 1). Participants were given instructions to press a button when they saw two pictures of the same food or object presented consecutively (for example, two different pictures of bananas presented in a row). For the purpose of data analyses, food pictures were classified into two categories based on the type of food presented: high palatability foods (45 pictures per run; examples: ice cream, bacon, fruit) and low palatability foods (15 pictures per run; examples: beans, soup). Object pictures (15 pictures per run; examples: broom, wrench) consisted of common household and office products. The food picture task is the same task used in previous studies by our lab, with food and non-food pictures matched for naming accuracy and picture typicality (4). Please see Supplemental Digital Content 1 for additional details regarding stimulus norming.
Figure 1. Food image task design.
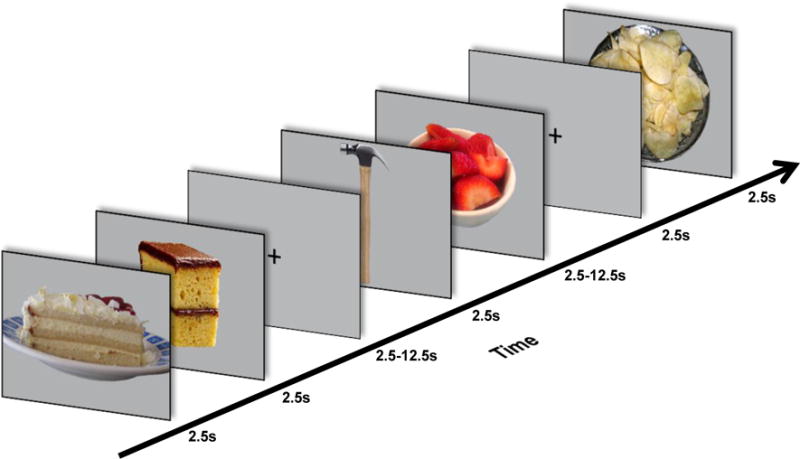
While in the scanner, images of food and non-food objects were displayed to participants for 2.5s each with variable interstimulus intervals of 2.5–12.5s.
Optseq2 (http://surfer.nmr.mgh.harvard.edu/optseq/) was used to optimize picture presentation for fMRI in pseudorandom order. Each picture was presented for 2.5 s, with variable interstimulus intervals of 2.5–12.5 s each (M = 3.95 s), during which a fixation mark was presented on the screen. Each participant completed three runs of the food image task.
MRI data acquisition
A General Electric Discovery MR750 whole-body 3 Tesla MRI scanner was utilized for acquisition of structural and functional brain images. MRI signal reception utilized a receive-only 32-element head coil optimized for parallel imaging (Nova Medical Inc.). Blood oxygenation level-dependent fMRI scans used a single-shot gradient-recalled EPI sequence with sensitivity encoding (SENSE), and a T1-weighted MRI scan with magnetization-prepared rapid gradient echo (MPRAGE) sequence with SENSE was obtained as an anatomical reference for fMRI analyses.
The current study utilized the following EPI parameters: FOV/slice/gap=220/2.9/0mm, 44 axial slices per volume, acquisition matrix=96×96, repetition/echo time TR/TE=2500/22ms, SENSE acceleration factor R=2 in the phase encoding (anterior-posterior) direction, flip angle=70°, sampling bandwidth=250kHz, number of volumes 139, scan time 347.5 sec. EPI images were reconstructed into a 128×128 matrix; fMRI voxel volume equaled 1.72×1.72×2.9 mm3.
Scan parameters for the anatomical scan were FOV=240 mm, axial slices per volume=130, slice thickness=1.1 mm, image matrix=256×256, voxel volume 0.94×0.94×1.1 mm3, TR/TE=5/1.948 ms, SENSE acceleration factor R=2, flip angle=8°, inversion/delay time TI/TD=725/1400 ms, sampling bandwidth=31.25 kHz, scan time=305 sec.
Data preprocessing and analysis
AFNI (http://afni.nimh.nih.gov/afni) was utilized for preprocessing of fMRI data and subsequent statistical analyses. AFNI’s anatomical-to-epi alignment procedure registered the anatomical scan to the first volume of the EPI data, followed by spatial transformation to the stereotaxic array of Talairach and Tournoux (15) using AFNI’s automated algorithm. In order to allow the fMRI signal to reach steady state, voxel-wise the first 4 volumes were excluded from analysis. Spatial transformation and motion correction were both implemented in a single image transformation. The EPI data were resampled to a 1.75mm3 grid and smoothed with a 6mm full-width at half-maximum Gaussian kernel. The signal value for each EPI volume was normalized to percent signal change using each voxel’s average signal across the time course.
Statistical analyses
A multiple linear regression model was used to estimate effects at the participant level prior to the group analysis. Regressors of interest were each of four food categories (high-fat/high-sweet, low-fat/high-sweet, high-fat/low-sweet, and low-fat/low-sweet) and the objects. All five task regressors were modeled by a gamma-variate hemodynamic response function beginning at the onset of stimulus presentation. Regressors of non-interest were six motion parameters (3 translations, 3 rotations) computed during the image registration preprocessing and regressors to account for each run’s mean, linear, quadratic, and cubic signal trends.
Corrections for multiple comparisons utilized cluster-size correction within anatomically-defined ROIs chosen a priori due to their known involvement in reward processing and interoception: orbitofrontal cortex, ventromedial prefrontal cortex (vmPFC), amygdala, insula, ventral striatum, caudate, putamen, ventral pallidum, and ventral tegmental area (VTA). Please see the Supplemental Digital Content 2 for the anatomical markers used to define each of these regions in the present analyses. For all analyses, a voxelwise threshold of p < .005 was set within the predefined ROIs, with a voxelwise threshold of p < .001 set for the rest of the brain. Cluster-size thresholds corrected for multiple comparisons at p < .050 were calculated for each a priori defined ROI and the remainder of the brain using AFNI’s 3dClustSim procedure. In order to protect against inflated family-wise error rates (16), we followed procedures currently recommended by the developers of AFNI. For each t-test, we obtained an output of the residuals. These residuals were then entered into 3dFWHMx with the ‘-ACF’ option in order to obtain model parameters for the spatial autocorrelation of the data for each ROI and the rest of the brain. These parameters were then entered in 3dClustSim in order to obtain the cluster-size thresholds for each ROI and the rest of the brain.
Two conditions were of interest in the group analysis: response to food pictures and the differential response to high palatability foods (foods high in sugar and/or fat) versus low palatability foods (foods low in both sugar and fat). Object pictures served as a baseline. For the first condition, betas for the response to object pictures were subtracted from betas for the response to all food pictures (regardless of fat and sugar content) at the individual participant level prior to group comparisons. We therefore had a beta value for each participant that represented the participant’s differential response to food pictures as compared to pictures of objects. Similarly, for our other condition of interest (high versus low palatability foods), the betas for low palatability foods were first subtracted from those for high palatability foods in order to examine the differential response to the foods with high fat and/or sugar content. Please see Supplemental Digital Content 1 for an analysis of between-group main effects.
We then used these beta values to determine if AN and HC participants exhibited differences in the association between visceral sensation intensity ratings and brain activity to food pictures. Specifically, we examined whether there was a difference between groups in the slope of the regression line describing the relationship between response to food pictures and stomach, heart, and bladder intensity ratings. Because the effect of interest was a group difference in slopes, each participant’s data (both intensity ratings and activation betas) were first ranked within each group to provide a more robust estimation of between-group differences (17). Ranking of fMRI data was achieved by first creating a single dataset for each group containing each participant’s data in a separate sub-brick, then using the “rank” option in 3dTsort to convert the beta value for each voxel to its within-group rank. For each organ (stomach, heart, bladder), two between-groups t-tests, one examining the response to all food pictures and the other examining the differential response to high palatability versus low palatability foods, were conducted using 3dttest++ with each participant’s average intensity rating for that organ entered as a covariate. Entering covariates in 3dttest++ results in output including a t-value for the group difference between slopes for the linear relationship between the ratings (stomach, heart, or bladder) and beta values for the response to food stimuli. These t-values were then submitted to significance testing, including corrections for multiple comparisons (described above), in order to determine brain regions exhibiting a group difference in the relationship between interoceptive sensation ratings and response to food images.
Results
Stomach sensation ratings
Two regions exhibited group differences in the slope of the relationship between stomach sensation ratings and activation in response to food pictures: the amygdala and posterior insula (Figure 2, Table 2). In the amygdala, AN participants exhibited a negative relationship between stomach sensation intensity and activation to food pictures, while HC participants exhibited a positive relationship. In contrast, in the posterior insula AN participants exhibited a positive relationship between stomach sensation intensity and activity to food pictures, while HC participants exhibited a negative relationship.
Figure 2. Relationship between interoceptive sensation ratings and activation in response to images of food compared to non-food objects.
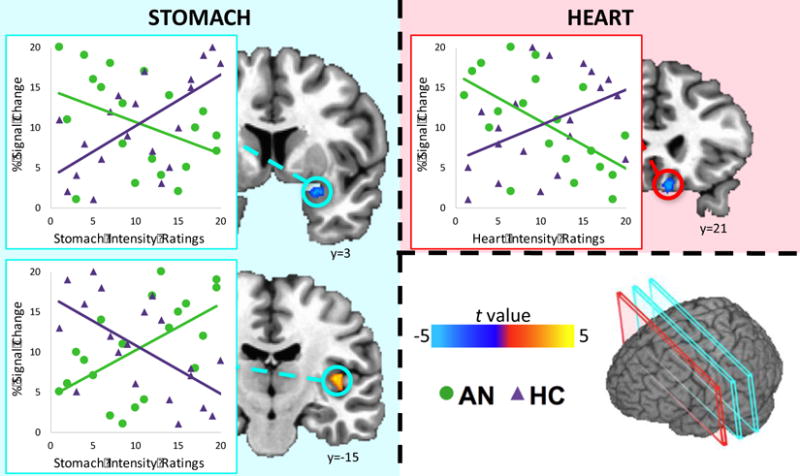
AN participants (green) exhibited a positive relationship between stomach sensation intensity ratings and food-image evoked activation in the posterior insula and a negative relationship between stomach sensation intensity ratings and activation in the amygdala, while healthy participants (purple) exhibited the opposite pattern. AN participants’ ratings of heart sensation intensity were negatively correlated with activation in the ventromedial prefrontal cortex, while healthy participants exhibited positive relationships with heart sensation ratings in this area. Scatterplot data are within-group ranked values and provided for visualization purposes only. Coordinates are in Talairach space.
Table 2.
Brain regions exhibiting group differences in the relationship between interoceptive sensation ratings and activation in response to food pictures
| Region | Coordinates
|
Peak t | f2 | Volume (mm3) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Foods – Objects | ||||||
| Stomach | ||||||
| R posterior insula | +39 | −17 | +9 | 4.30 | 0.58 | 161 |
| R amygdala | +27 | +4 | −14 | −4.05 | 0.40 | 59 |
| Heart | ||||||
| R vmPFC | +8 | +20 | −15 | −3.52 | 0.38 | 96 |
| High palatability – Low palatability foods | ||||||
| Stomach | ||||||
| L amygdala | −22 | −3 | −19 | 4.83 | 0.53 | 230 |
| L sgACC | −1 | +18 | −10 | 3.88 | 0.39 | 107 |
| L ventral pallidum | −15 | −3 | −1 | −3.99 | 0.40 | 86 |
| VTA | +3 | −15 | −7 | −4.03 | 0.42 | 43 |
| Heart | ||||||
| R vmPFC | +4 | +15 | −17 | −3.61 | 0.40 | 107 |
| L putamen | −25 | +3 | +2 | −3.41 | 0.32 | 75 |
R=Right; L=left; vmPFC=ventromedial prefrontal cortex; VTA=ventral tegmental area. Coordinates are in Talairach space.
No statistically significant clusters were found outside of the anatomically defined a priori regions of interest. Modified f2 values were calculated using program at http://hermanaguinis.com/mmr/fsquared/based on procedures described in Aguinis H, Pierce CA. Computation of effect size for moderating effects of categorical variables in multiple regression. Appl Psychol Meas 2006;30:440–442.
Four regions displayed group differences in slopes describing the relationship between stomach sensation ratings and activity in response to seeing high palatability foods (as opposed to low palatability foods; Figure 3). AN was associated with a negative relationship between stomach sensation ratings and activation in the ventral pallidum and VTA, while HC participants exhibited positive relationships in these regions. AN participants exhibited a positive relationship between stomach sensation intensity ratings and high palatability food picture-evoked activity in the subgenual anterior cingulate cortex (sgACC) and left amygdala. HC participants, in contrast, exhibited a negative relationship in these regions.
Figure 3. Relationship between interoceptive sensation ratings and activation in response to high palatability food images compared to low palatability food images.
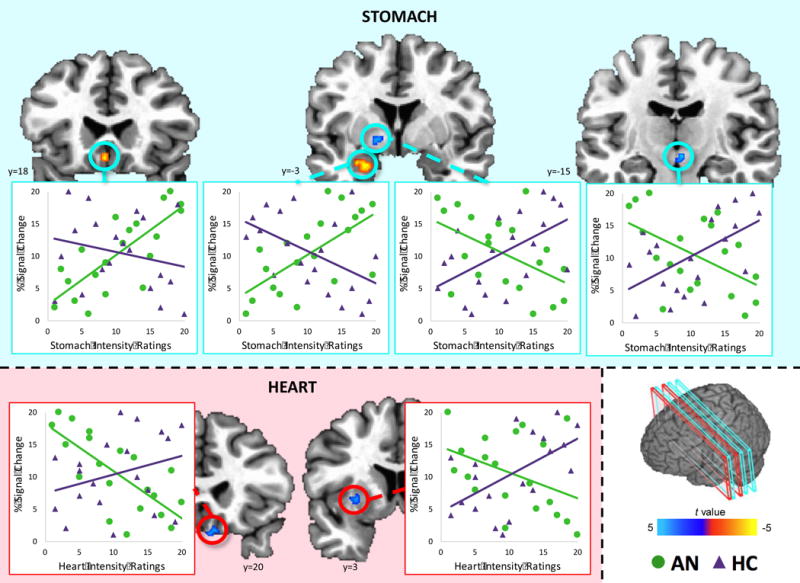
AN participants (green) exhibited a positive relationship between stomach sensation intensity ratings and activation in response to high palatability foods in the subgenual anterior cingulate cortex and amygdala, while HC participants (purple) exhibited a negative relationship in these regions. In contrast, AN was associated with a negative relationship between stomach sensation intensity ratings and activation in the ventral pallidum and ventral tegmental area, while HC participants displayed the opposite pattern. Scatterplot data are within-group ranked values and provided for visualization purposes only. Coordinates are in Talairach space.
Heart and bladder sensation ratings
AN participants exhibited a negative relationship between heart intensity ratings and brain activation in the vmPFC in response to all food pictures and specifically to high palatability food pictures, while HC participants displayed a positive relationship (Figure 2). AN participants also exhibited a negative relationship between heart intensity ratings and activation in the putamen evoked by high palatability food images. There were no regions with group differences in slopes for the relationship between bladder intensity ratings and response to all foods or high palatability foods.
Discussion
The findings reported here demonstrate for the first time that the typical relationships between interoceptive signals from the viscera and the brain’s response to food stimuli do not hold in AN. Importantly, by testing the relationships between response to foods and sensations from three different visceral organ systems (heart, stomach, and bladder), we have demonstrated that AN patients’ response to foods may be related to broadly altered brain-body signaling. Most of the group differences, however, were for perceived interoceptive sensations from the stomach, particularly in response to high palatability foods, indicating altered gastric interoception specifically may play a key role in restrictive eating behaviors in AN.
AN participants exhibited a number of atypical relationships between their brain response to food pictures and their reported stomach sensations. HC participants who reported high intensity stomach sensations exhibited greater amygdala activity to food pictures. In contrast, AN participants who reported high intensity stomach sensations exhibited weaker amygdala activity to food pictures. The amygdala has long been implicated in fear processing (18–22). It is interesting, therefore, that while stomach sensations were negatively correlated with right amygdala response to all foods, they were positively correlated with the differential activation of the left amygdala to high palatability (versus low palatability) foods. It is possible that in the presence of high intensity gastric sensations, individuals with AN perceive many low-calorie foods as “safe” options, leading to less amygdala activation when these foods are included in the analyses. In contrast, foods high in fat and sugar content may be more likely to activate fear circuitry, reflected in the positive correlation between stomach sensations and left amygdala activation to these foods. Additionally, there is some evidence for functional lateralization of the amygdala (23,24), which may contribute to the differences in activation between left and right amygdala found in our study.
AN participants also exhibited an altered relationship between stomach interoceptive sensations and posterior insula activation, suggesting that increased salience of gastric sensations was associated with heightened interoceptive processing in response to food pictures. Moreover, the posterior insula has been found to be particularly responsive to painful stimuli (23,25,26), suggesting that food pictures evoked a neural response suggestive of an aversive interoceptive experience. Additional evidence for altered processing in the posterior insula is evidenced by two prior studies (27,28) that reported AN was associated with attenuated posterior insula activity during receipt of a thermal nociceptive stimulus. Another possibility is that posterior insula activation in our sample could be related to hunger and satiety levels. Further research is needed to clarify the exact nature of altered functioning in the posterior insula in AN. A tentative inference, however, is that for some individuals suffering from AN, heightened gastrointestinal sensations result in food stimuli being perceived as an interoceptive threat, an idea which is certainly supported by clinical experience.
Because a central characteristic of AN is the avoidance of energy-dense foods, we also compared the relationships between interoceptive experience and response to high palatability and low palatability foods. Our results revealed two brain regions where AN participants displayed a positive relationship between stomach sensation intensity and response to highly palatable foods – the amygdala and sgACC. Both regions have been strongly implicated in emotional processing, with the amygdala being involved in the salience of an emotional stimulus (18,19), and the sgACC involved in the regulation of emotional responses (29), possibly through its role as a key visceromotor control region as part of a larger interoceptive network (30). Hyperactivity of the sgACC has been found in depressed individuals during anticipation of emotional stimuli (31) and in individuals with AN in response to the sight of chocolate (32). Together with our findings, this suggests that intense gastric sensations contribute to a dysfunctional emotional response to high palatability food stimuli in individuals with AN, which may result in anxiety and subsequent avoidance.
In contrast to the above regions, AN participants displayed negative relationships between stomach sensation intensity ratings and activation in the ventral pallidum and VTA. Both of these regions are centrally involved in the representation and hedonic experience of reward (33–38). The VTA receives endocrine and metabolic signals (e.g., ghrelin) from the gastrointestinal tract and may be especially involved in the intake of palatable foods (39), and decreased VTA activation has been associated with the long-term success of weight loss interventions (40). The VTA may therefore play an important role in the reward processing of highly palatable foods, and our results suggest that gastric sensations may interfere with this reward processing in individuals with AN, possibly via influence of projections from interoceptive cortex to the reward system (41).
AN participants also displayed atypical relationships between heart sensation ratings and response to food images in the vmPFC. Past research using a food choice task revealed that the vmPFC activates late in the choice process, suggesting its role in integrating interoceptive and memory information with value during decision making (42). In healthy individuals, interoceptive signals of autonomic arousal, such as cardiac sensations, might therefore be integrated with valuation in the vmPFC, resulting in an appraisal of the high palatability food stimulus as rewarding. In AN participants, however, increased perception of heartbeat sensations may contribute to the appraisal of food stimuli as threatening. A similar process may also occur in another reward-related region, the putamen, which exhibited both a main effect for group and a moderation effect of group for the relationship between heartbeat sensations and response to highly palatable foods.
The results of our study reveal that AN is associated with an atypical relationship between visceral interoceptive sensations and response to food pictures in brain regions related to emotional, interoceptive, and reward processing. Remarkably, the relationships between brain activation and interoceptive sensations were nearly all opposite of the relationships exhibited by healthy individuals. This study provides empirical evidence in line with theories that individuals with AN may experience heightened interoceptive processing when exposed to food stimuli, which could contribute to restrictive eating patterns (43).
A possible limitation of this study is that patients were only asked about the overall intensity of their stomach sensations, rather than what specific type (e.g., hunger, nausea, fullness) of sensation(s) they were experiencing. AN is associated with difficulty in discriminating between different types of sensations, such as hunger and fullness (44), and asking these participants to make such discriminations would therefore likely introduce a confounding effect between our two groups. We thus sought to determine how the overall intensity of their interoceptive sensations affected their neural responses to food stimuli, without introducing a possible confound related to interoceptive accuracy or ability to specifically label their interoceptive experiences.
An additional limitation is that while our study provides evidence that gastric sensations differentially affect the brain’s response to food stimuli in AN, future research is needed in order to determine whether these sensations differentially influence actual eating behaviors in this population. It is also unknown whether our findings reflect stable inter-individual differences in average overall visceral sensitivity, or if they reflect intra-individual variations over time.
Although we examined possible effects for three types of sensations (heart, stomach, and bladder), the results were largely specific to stomach sensations. This suggests that even though AN patients may have general interoceptive deficits, or deficits for specific types of sensations such as heartbeat (45), altered stomach interoception in particular may be a driving force in behavioral patterns of restricted food intake. A direct clinical implication of our findings might be the need to provide patients with psychoeducation regarding how their brains may not process interoceptive signals from the gastrointestinal tract in the same way as healthy individuals. Interventions such as acceptance and commitment therapy, which encourages behavior change in spite of the presence of aversive emotions such as anxiety, could be tailored for AN patients to have a stronger emphasis on aversive interoceptive sensations. Therapy may also focus on interpretation of interoceptive experience, for example an increased heart rate as indicative of excitement rather than anxiety. Additionally, simply assessing patients’ own perception of the intensity of their current stomach sensations might also be helpful to predict when they are most at risk for restrictive eating behaviors, although future studies will be necessary to determine if these are state or trait deficits in AN. Similar to mechanisms in exposure therapy, sustained, regular ingestion of high calorie foods may serve to dampen the aversive interoceptive predictions they trigger and ultimately contribute to stable recovery. Our results also underscore the importance of attending to gastrointestinal symptoms during treatment for AN and utilizing pharmacological measures to reduce gastric discomfort when appropriate.
A central finding of the present study is that those participants with AN who reported the greatest stomach sensations in a visceral interoceptive attention task subsequently exhibited the greatest activity in a region of the posterior insula implicated in pain perception. But this activity in the AN patients was not measured in response to thermal pain to the arm (46,47), or the face (48), or to gastric distention (49), that have all activated this region in earlier studies. Rather, it was simply to the sight of food. This fact may tell us something important about the physical/embodied experience of these young women when they are confronted with food stimuli, and why they develop such incapacitating food anxiety: this activity may be a marker of a conditioned aversive interoceptive response to food cues.
Supplementary Material
Supplemental Digital Content 1. Text that describes food stimulus norming procedures and supplemental analyses; Table S1 showing brain activation correlates of anxiety and BMI; Figures S1 and S2 showing data for AN participants with and without comorbid diagnoses; Table S2 displaying group comparisons of visceral sensation ratings. pdf
Supplemental Digital Content 2. Text and figure that illustrate anatomical region-of-interest definitions. pdf
Acknowledgments
The authors thank Joel Barcalow and Jennifer Dobson for conducting participant recruitment and assessment, the staff of the Laureate Eating Disorders Program for their help in coordinating and supporting patient participation, and the research participants and their families for their time and effort.
Source of Funding: This research was supported by a NARSAD Young Investigator Award to WKS; the National Institute of Mental Health (grant number K01MH096175-01 to WKS); the Oklahoma Center for the Advancement of Science and Technology (grant number HR10-141 to WKS); the Laureate Institute for Brain Research; and The William K. Warren Foundation.
Glossary
- AN
anorexia nervosa
- BMI
body mass index
- fMRI
functional magnetic resonance imaging
- HC
healthy control
- MPRAGE
magnetization-prepared rapid gradient echo
- SENSE
sensitivity encoding
- sgACC
subgenual anterior cingulate cortex
- vmPFC
ventromedial prefrontal cortex
- VTA
ventral tegmental area
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology. 2016;41:521–8. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia J, Kimeldorf DJ, Koellino RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–8. [PubMed] [Google Scholar]
- 3.Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol Psychiatry. 2014;76:258–26. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simmons WK, Rapuano KM, Kallman SJ, Ingeholm JE, Miller B, Gotts SJ, Avery JA, Hall KD, Martin A. Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci. 2013;16:1551–2. doi: 10.1038/nn.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. Neural mechanisms underlying food motivation in children and adolescents. Neuroimage. 2005;27:669–76. doi: 10.1016/j.neuroimage.2005.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons WK. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15:1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 7.van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuroimage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 8.St-Onge M-P, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135:1014–8. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 9.Wang G-J, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–7. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Fichter MM, Herpertz S, Quadflieg N, Herpertz-Dahlmann B. Structured Interview for Anorexic and Bulimic disorders for DSM-IV and ICD-10: updated (third) revision. Int J Eat Disord. 1998;24:227–49. doi: 10.1002/(sici)1098-108x(199811)24:3<227::aid-eat1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) New York, NY: 2002. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York NY: 2002. [Google Scholar]
- 13.Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–58. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avery JA, Kerr KL, Ingeholm JE, Burrows K, Bodurka J, Simmons WK. A common gustatory and interoceptive representation in the human mid-insula. Hum Brain Mapp. 2015;36:2996–3006. doi: 10.1002/hbm.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York, NY: 1988. [Google Scholar]
- 16.Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113:7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conover WJ, Iman RL. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–9. [Google Scholar]
- 18.Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?”. Neuropsychologia. 2010;48:3416–29. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 22.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–80. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duerden EG, Albanese MC. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp. 2013;34:109–49. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reber J, Tranel D. Sex differences in the functional lateralization of emotion and decision making in the human brain. J Neurosci Res. 2017;95:270–8. doi: 10.1002/jnr.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frot M, Faillenot I, Mauguière F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp. 2014;35:5486–99. doi: 10.1002/hbm.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, Kaye WH. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: evidence of interoceptive dysregulation. Int J Eat Disord. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bär K-J, Berger S, Schwier C, Wutzler U, Beissner F. Insular dysfunction and descending pain inhibition in anorexia nervosa. Acta Psychiatr Scand. 2013;127:269–78. doi: 10.1111/j.1600-0447.2012.01896.x. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16:419–29. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:2529–53. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Cowdrey FA, Park RJ, Harmer CJ, McCabe C. Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol Psychiatry. 2011;70:736–43. doi: 10.1016/j.biopsych.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–37. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beaver JD. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 37.Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev. 2014;45:350–68. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–35. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meye FJ, Adan RA. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci. 2014;35:31–40. doi: 10.1016/j.tips.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Murdaugh DL, Cox JE, Cook, Edwin WI, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–21. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris A, Adolphs R, Camerer C, Rangel A. Dynamic construction of stimulus values in the ventromedial prefrontal cortex. PLoS One. 2011;6:e21074. doi: 10.1371/journal.pone.0021074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 44.Halmi KA, Sunday S, Puglisi A, Marchi P. Hunger and satiety in anorexia and bulimia nervosa. Ann N Y Acad Sci. 1989;575:431–45. doi: 10.1111/j.1749-6632.1989.tb53264.x. [DOI] [PubMed] [Google Scholar]
- 45.Pollatos O, Kurz A-L, Albrecht J, Schreder T, Kleemann AM, Schöpf V, Kopietz R, Wiesmann M, Schandry R. Reduced perception of bodily signals in anorexia nervosa. Eat Behav. 2008;9:381–8. doi: 10.1016/j.eatbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–9. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 47.Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–12. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- 48.Brooks JCW, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–9. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 49.Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC. Neuronal correlates of gastric pain induced by fundus distension: A 3T-fMRI study. Neurogastroenterol Motil. 2004;16:575–87. doi: 10.1111/j.1365-2982.2004.00562.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Text that describes food stimulus norming procedures and supplemental analyses; Table S1 showing brain activation correlates of anxiety and BMI; Figures S1 and S2 showing data for AN participants with and without comorbid diagnoses; Table S2 displaying group comparisons of visceral sensation ratings. pdf
Supplemental Digital Content 2. Text and figure that illustrate anatomical region-of-interest definitions. pdf


