Abstract
Cystatin C and beta-2-microglobulin (B2M) are filtration markers associated with adverse outcomes in non-transplant populations, sometimes with stronger associations than for creatinine. We evaluated associations of estimated glomerular filtration rate from cystatin C (eGFRcys), B2M (eGFRB2M), and creatinine (eGFRcr) with cardiovascular outcomes, mortality, and kidney failure in stable kidney transplant recipients using a case-cohort study nested within the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. A random subcohort was selected (N=508; mean age 51.6 years, median transplant vintage 4 years, 38% women, 23.6% non-white race) with enrichment for cardiovascular events (N=306; 54 within the subcohort), mortality (N=208; 68 within the subcohort), and kidney failure (N=208; 52 within the subcohort). Mean eGFRcr, eGFRcys, and eGFRB2M were 46.0, 43.8, and 48.8 mL/min/1.73m2, respectively. After multivariable adjustment, hazard ratios for eGFRcys and eGFRB2M <30 vs. 60+ were 2.02 (95% CI 1.09–3.76; p=0.03) and 2.56 (1.35–4.88; p=0.004) for cardiovascular events; 3.92 (2.11–7.31) and 4.09 (2.21–7.54; both p<0.001) for mortality; and 9.49 (4.28–21.00) and 15.53 (6.99–34.51; both p<0.001) for kidney failure. Associations persisted with additional adjustment for baseline eGFRcr. We conclude that cystatin C and B2M are strongly associated with cardiovascular events, mortality, and kidney failure in stable kidney transplant recipients.
Introduction
Kidney transplant recipients have increased risk of cardiovascular disease, kidney failure and all-cause mortality. Lower levels of kidney function, ascertained with serum creatinine or glomerular filtration rate (GFR) estimated from serum creatinine (eGFRcr) is independently associated with cardiovascular and kidney disease outcomes in a wide range of non-transplant populations,(1–4) and is associated with increased risk of cardiovascular events(5–9), all-cause mortality,(10) and graft loss(11–13) in kidney transplant recipients.
Cystatin C and beta-2 microglobulin (B2M) are low molecular weight serum proteins that are being investigated as novel filtration markers. In non-transplant populations, these filtration markers and their related GFR estimates (eGFRcys, eGFRB2M) are independent predictors of mortality, cardiovascular events, and ESRD, and in some cases show stronger risk associations than those observed for serum creatinine and eGFRcr.(14–21) It is hypothesized that such differences in risk associations among filtration markers reflect differences in factors other than GFR that influence their serum levels. The non-GFR determinants of filtration markers may differ in kidney transplant recipients compared to the non-transplant population, leading to differences in risk associations between these populations. Few studies have evaluated risk associated with low molecular weight serum protein levels in kidney transplant recipients. Prior work suggests that higher B2M is associated with greater eGFR decline 1–2 years post-transplant(22) and that both higher cystatin C and B2M are associated with increased risk of mortality(23) and graft loss(23–25) in transplant recipients. It is not known, however, whether the potential advantage of the low molecular weight serum protein filtration markers over creatinine for estimating cardiovascular and kidney disease risk associations that have been observed in the non-transplant population are present among transplant patients. If the cystatin C and B2M are shown to be strong markers of major clinical outcomes in transplant recipients, they may prove useful in risk prediction and risk stratification in this population.
We have previously demonstrated an independent association of baseline eGFRcr with cardiovascular disease and all-cause mortality in a post-hoc analysis of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial.(8) The aim of the current study is to evaluate risk associations of eGFRcys and eGFRB2M with cardiovascular outcomes, all-cause mortality, and kidney failure in stable kidney transplant recipients in a case-cohort study drawn from the FAVORIT Trial, and whether these associations are independent of eGFRcr.
Materials and Methods
Study population
Our study sample was drawn from the FAVORIT Trial (clinicaltrials.gov: NCT00064753), a multi-center double-blind randomized clinical trial conducted to determine whether lowering homocysteine levels with vitamin therapy reduced the rate of cardiovascular outcomes.(26, 27) The FAVORIT Study was approved by the Institutional Review Boards at participating institutions. All participants provided written informed consent. In brief, 4,110 kidney transplant recipients aged 35–75 years who were at least 6 months post-kidney transplant were enrolled between August 2002 and January 2007 at 30 transplant centers in the United States, Canada, and Brazil. Trial eligibility required an elevated serum homocysteine level (≥11μmol/L [women]; ≥12μmol/L [men]) and stable kidney function (estimated creatinine clearance ≥30mL/min [men] or ≥25mL/min [women]). Participants were followed-up every six months through January 31, 2010 to obtain outcomes through June 24, 2009.
For the present analysis we utilized an established case-cohort study nested within the FAVORIT Trial. The case-cohort study is an efficient design to evaluate novel factors associated with multiple outcomes with a study sample. It includes (1) a random sample of participants drawn from the overall original study (the “sub-cohort”) that is additionally enriched to include (2) all outcomes of interest that occur within the overall original study population. For the FAVORIT case-cohort study, a sub-cohort was selected to include a 15% random sample of participants with non-missing baseline measurements of serum creatinine, eGFRcr, cholesterol, and triglycerides, no missing follow-up, and stored serum and urine samples available (a key component of the case-cohort study for additional novel biomarker measurements); 3530 of the 4110 participants in the overall FAVORIT trial cohort met these criteria, 530 of whom were selected for the sub-cohort.(28–30) For the present analyses, we additionally excluded participants in the sub-cohort with missing serum measurements of cystatin C and B2M or key model covariates at baseline (N=22). Weighted Cox proportional hazards regression analyses for adjudicated cardiovascular events included 306 participants with events (54 events that overlap with the sub-cohort), resulting in an analytic study sample of 760 participants. Analyses for all-cause mortality included 382 participants with events (68 events that overlap with the sub-cohort), resulting in an analytic sample of 822 participants. Analyses for dialysis-dependent kidney failure included 280 participants with events (52 events that overlap with the sub-cohort), resulting in an analytic sample of 736 participants.
Exposure Assessment
Serum creatinine was previously measured in 2011 from frozen samples collected during the baseline study visit following a single thaw. Serum creatinine assays were performed at the United States Department of Agriculture Jean Mayer Human Nutrition Research Center on Aging at Tufts University using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc, Center Valley, PA) instrument (coefficient of variation [CV] intra assay 2.0%; -iInter assay 4.0%), calibrated to an isotope dilution mass spectrometry traceable standard.(8) Serum cystatin C and B2M were measured at the Brigham and Women’s Hospital in 2012 in previously frozen baseline EDTA-treated plasma samples using a Roche Cobas C6000 analyzer (Roche Diagnostics, Indianapolis, IN). Cystatin C assays were calibrated to an IFCC standard. Total imprecision of the B2M assay was 1.7% at 2.42 mg/L and 1.9% at 5.60 mg/L. Total imprecision of the cystatin C assay was 3.7% at 0.78 mg/L and 2.0% at 3.43 mg/L. Filtration markers were transformed to eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine (2009), cystatin C (2012), and B2M (2015) estimation equations(31–33) to allow comparisons across markers on the same scale (mL/min/1.73m2).
Outcome Assessment
Outcomes included adjudicated cardiovascular events, all-cause mortality, and dialysis-dependent kidney failure. Adjudicated cardiovascular events included cases of cardiovascular death, myocardial infarction, resuscitated sudden death, and stroke that were centrally adjudicated and reviewed by the FAVORIT Clinical Endpoints Committee. Dialysis-dependent kidney failure was defined as the need for chronic dialysis as ascertained by local study staff.
Covariate Assessment
Demographic, clinical, and transplant characteristics were assessed at the time of study enrollment. Smoking status was defined based on self-report and categorized as current, former or never smoking status. Transplant characteristics included donor type (living vs. deceased donor) and vintage (years since transplant). Baseline systolic and diastolic blood pressure was defined as the average of two measurements. BMI was calculated as weight/ height2 (kg/m2). Diabetes was defined as the use of insulin or oral hypoglycemic medications or based on self-report. Self-reported prevalent cardiovascular disease at baseline included prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower extremity arterial revascularization or non-traumatic amputation above the ankle. Additional laboratory measurements included serum cholesterol measurements.
Statistical Analyses
Baseline demographic and clinical characteristics are presented for the sub-cohort of 508 participants, overall and by eGFRcys and eGFRB2M categories. We calculated Pearson correlation coefficients between eGFRcys, eGFRB2M, and eGFRcr within the sub-cohort. Associations of eGFRcys, eGFRB2M, and eGFRcr with adjudicated cardiovascular events, all-cause mortality, and dialysis-dependent kidney failure were first evaluated using Kaplan-Meier survival curves with log-rank tests in the sub-cohort (N=508). We then used weighted Cox proportional hazards regression in the full analytic samples for each outcome to evaluate the association of eGFRcys, eGFRB2M, and eGFRcr with events to account for the weighted study design, similar to prior work in this case-cohort.(28–30, 34) Model adjustment included (1) adjustment for demographic and transplant characteristics (age, sex, race, country, randomized treatment assignment, donor type, graft vintage); (2) additional multivariable adjustment for baseline cardiovascular disease, diabetes, smoking status, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index, and natural log-transformed urinary albumin to creatinine ratio; and (3) additional adjustment for eGFRcr in models with eGFRcys or eGFRB2M as the primary predictor of interest. Exposures were modeled categorically and continuously in analyses. Statistical analyses were performed in Stata, Version 12.1 (StataCorp LP) and SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline Characteristics of the Sub-Cohort
In the sub-cohort (n=508), mean age was 51.6±9.2 years, 38.0% were female, 23.6% were of non-white race, and 68.5%, 13.5%, and 17.9% were recruited from centers in the United States, Canada, and Brazil, respectively (Table 1); characteristics of the sub-cohort are similar to those observed in the larger FAVORIT study cohort.(8) Participant characteristics across eGFRcys, eGFRB2M, and eGFRcr groups are presented in Tables 1, S1, and S2, respectively. A strong correlation was observed between eGFRcys and eGFRB2M (Pearson correlation coefficient=0.87, p<0.001); eGFRcys and eGFRB2M were more moderately correlated with eGFRcr (Pearson correlation coefficients of 0.61 and 0.55, respectively, both p<0.001). Table S3 compares demographic characteristics and outcomes in the random sub-cohort to those who were not included in the subcohort. Serum creatinine was significantly higher and there was a trend toward a higher proportion of participants who developed kidney failure in participants selected in the sub-cohort.
Table 1.
Baseline characteristics of the FAVORIT sub-cohort, overall and by cystatin C-based estimated glomerular filtration rate (eGFRcys) group.
| Characteristics | Overall | eGFRcys Group (mL/min/1.73m2) | |||
|---|---|---|---|---|---|
| <30 | 30 to <45 | 45 to <60 | 60+ | ||
| N | 508 | 96 | 207 | 125 | 80 |
| Age | 51.6 (9.2) | 52.0 (8.7) | 52.6 (9.7) | 51.0 (8.9) | 49.4 (8.6) |
| Women | 193 (38.0%) | 40 (41.7%) | 83 (40.1%) | 42 (33.6%) | 28 (35.0%) |
| Race | |||||
| White | 388 (76.4%) | 70 (72.9%) | 157 (75.9%) | 97 (77.6%) | 64 (80%) |
| Black | 87 (17.1%) | 16 (16.7%) | 39 (18.8%) | 21 (16.8%) | 11 (13.75%) |
| Other | 33 (6.5%) | 10 (10.4%) | 11 (5.3%) | 7 (5.6%) | 5 (6.25%) |
| Treatment Group | |||||
| High Dose Vitamin | 253 (49.8%) | 47 (48.96%) | 110 (53.1%) | 58 (46.4%) | 38 (47.5%) |
| Low Dose Vitamin | 255 (50.2%) | 49 (51.0%) | 97 (46.9%) | 67 (53.6%) | 42 (52.5%) |
| Country | |||||
| United States | 348 (68.5%) | 63 (65.6%) | 142 (68.6%) | 88 (70.4%) | 55 (68.75%) |
| Canada | 69 (13.5%) | 13 (13.5%) | 24 (11.6%) | 14 (11.2%) | 18 (22.5%) |
| Brazil | 91 (17.9%) | 20 (20.8%) | 41 (19.8%) | 23 (18.4%) | 7 (8.75%) |
| Graft vintage (yr), median (25th, 75th) |
4 [2, 7] | 5 [2, 9] | 4 [2, 7] | 3 [1, 5] | 4 [2, 7] |
| Living donor kidney (%) | 214 (42.1%) | 28 (29.2%) | 83 (40.1%) | 67 (53.6%) | 36 (45.0%) |
| History of cardiovascular disease (%) | 96 (18.9%) | 25 (26.0%) | 37 (17.9%) | 25 (20.0%) | 9 (11.25%) |
| History of diabetes mellitus (%) | 184 (36.2%) | 38 (39.6%) | 69 (33.3%) | 53 (42.4%) | 24 (30.0%) |
| Smoking (%) | |||||
| Never | 254 (50.0%) | 48 (50%) | 100 (48.3%) | 62 (49.6%) | 44 (55.0%) |
| Current | 56 (11.0%) | 15 (15.6%) | 26 (12.6%) | 10 (8.0%) | 5 (6.25%) |
| Former | 192 (37.8) | 32 (33.3%) | 79 (38.2%) | 51 (40.8%) | 30 (37.5%) |
| Body mass index (kg/m2) | 28.9 (5.9) | 29.2 (5.7) | 29.6 (6.4) | 28.6 (5.5) | 27.2 (4.9) |
| SBP (mm Hg) | 136 (20) | 138 (24) | 136 (20) | 137 (19) | 129 (17) |
| DBP (mm Hg) | 79 (13) | 80 (14) | 80 (14) | 79 (10) | 78 (11) |
| Total Cholesterol (mg/dL) | 188 (43) | 190 (51) | 188 (44) | 186 (38) | 187 (35) |
| LDL cholesterol (mg/dL) | 103 (33) | 102 (37) | 103 (33) | 104 (32) | 104 (31) |
| HDL cholesterol (mg/dL) | 47 (14) | 42 (16) | 47 (13) | 47 (14) | 50 (15) |
| Triglycerides (mg/dL) | 167 [114, 243] | 189 [142, 274] | 167 [117, 240] | 155 [107, 229] | 133 [96, 219] |
| Creatinine (mg/dL) | 1.8(0.6) | 2.4 (0.7) | 1.9 (0.6) | 1.5 (0.3) | 1.3 (0.3) |
| Cystatin C (mg/L) | 1.5 (0.4) | 2.2 (0.3) | 1.6 (0.1) | 1.2 (0.1) | 1.0 (0.1) |
| B2M (mg/L) | 3.8 (1.8) | 6.5 (2.1) | 3.8 (1.0) | 2.8 (0.6) | 2.2 (0.4) |
| eGFRcr (mL/min/1.73m2) | 46.0 (18.2) | 30.7 (12.3) | 41.4 (13.2) | 54.8 (15.2) | 62.8 (19.4) |
| eGFRcys (mL/min/1.73m2) | 43.8 (15.0) | 26.1 (5.9) | 38.4 (7.1) | 52.5 (7.7) | 65.5 (11.5) |
| eGFRB2M (mL/min/1.73m2) | 48.8 (16.4) | 28.8 (6.9) | 44.4 (8.9) | 57.2 (9.9) | 71.1 (12.8) |
| Urine Albumin to Creatinine Ratio (mg/g) | 25 [10, 110] | 64 [18, 435] | 29 [10, 112] | 15 [7, 47] | 16 [8, 58] |
Note: Continuous variables are presented as mean (standard deviation) or as median [25th percentile, 75th percentile].
Adjudicated Cardiovascular Events
Over median follow-up of 3.6 years in the sub-cohort, 54 (10.6%) adjudicated cardiovascular events occurred. Kaplan-Meier survival analyses plots of the relationship between eGFRcys, eGFRB2M, or eGFRcr and cardiovascular outcomes in the sub-cohort are presented in Figure 1. In the case-cohort analysis in the full cardiovascular events analytic sample (N=760) with weighted Cox proportional hazards regression, lower eGFRcys and lower eGFRB2M were each significantly associated with an increased risk of cardiovascular events after adjustment for demographic and transplant characteristics (Table 2; both p-trend<0.001 when modeled categorically and p<0.001 when modeled continuously). Results were similar with additional multivariable adjustment (HR for eGFR<30 vs. eGFR 60+ for eGFRcys and eGFRB2M of 2.02 [95% CI 1.09–3.76; p=0.03] and 2.56 [95% CI 1.35–4.88; p=0.004]), and associations of eGFRcys and eGFRB2M with cardiovascular events persisted in models that further adjusted for eGFRcr. Lower eGFRcr categories were associated with a trend for increased risk of cardiovascular events with demographic and transplant characteristic adjustment (p-trend=0.02), although this was not significant after additional multivariable adjustment (p-trend=0.32); no significant associations were observed with continuous eGFRcr (Table 2).
Figure 1.
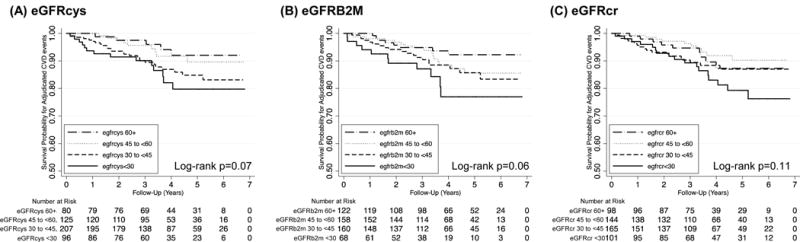
Kaplan Meier survival curves for adjudicated cardiovascular events in the sub-cohort, by (A) cystatin C-based eGFR, (B) beta-2 microglobulin-based eGFR, and (C) creatinine-based eGFR. The sub-cohort includes 508 participants with 54 adjudicated cardiovascular events. eGFR, estimated glomerular filtration rate.
Table 2.
Association of filtration markers with risk of adjudicated cardiovascular events (N=306 events) in stable kidney transplant recipients in FAVORIT
| Demographic and Transplant Characteristic Adjusted* | Multivariable Adjusted^ | Multivariable Adjusted^ + eGFRcr | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| eGFRcys | ||||||
| <30 | 3.58 (2.08, 6.15) | <0.001 | 2.02 (1.09, 3.76) | 0.03 | 3.05 (1.41, 6.57) | 0.005 |
| 30 to <45 | 1.78 (1.07, 2.97) | 0.03 | 1.56 (0.89, 2.77) | 0.13 | 2.09 (1.07, 4.06) | 0.03 |
| 45 to <60 | 1.32 (0.75, 2.29) | 0.33 | 0.93 (0.49, 1.79) | 0.83 | 1.04 (0.54, 2.02) | 0.90 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | 0.03 | p-trend | 0.008 | |
| per 10 mL/min/1.73m2 | 0.72 (0.63, 0.82) | <0.001 | 0.78 (0.68, 0.90) | <0.001 | 0.68 (0.56, 0.83) | <0.001 |
| eGFRB2M | ||||||
| <30 | 3.66 (2.19, 6.13) | <0.001 | 2.56 (1.35, 4.88) | 0.004 | 3.16 (1.54, 6.50) | 0.002 |
| 30 to <45 | 2.10 (1.34, 3.29) | 0.001 | 1.71 (0.96, 3.03) | 0.07 | 1.99 (1.06, 3.74) | 0.03 |
| 45 to <60 | 1.39 (0.87, 2.23) | 0.17 | 1.16 (0.64, 2.08) | 0.63 | 1.24 (0.68, 2.24) | 0.48 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | 0.01 | p-trend | 0.006 | |
| per 10 mL/min/1.73m2 | 0.75 (0.67, 0.83) | <0.001 | 0.80 (0.70, 0.91) | <0.001 | 0.74 (0.63, 0.87) | 0.003 |
| eGFRcr | ||||||
| <30 | 1.26 (0.78, 2.05) | 0.34 | 1.16 (0.66, 2.03) | 0.61 | – | – |
| 30 to <45 | 1.51 (0.97, 2.34) | 0.07 | 1.29 (0.76, 2.18) | 0.34 | – | – |
| 45 to <60 | 0.84 (0.52, 1.34) | 0.45 | 0.83 (0.48, 1.44) | 0.51 | – | – |
| 60+ | Reference | – | Reference | – | – | – |
| p-trend | 0.02 | p-trend | 0.32 | – | – | |
| per 10 mL/min/1.73m2 | 0.94 (0.86, 1.03) | 0.20 | 0.96 (0.88, 1.06) | 0.46 | – | – |
Note: Weighted Cox regression models were performed in the cardiovascular events analytic sample of 760 participants with 306 events. Results in the shaded area are not presented because models with eGFRcr as the primary exposure cannot be additionally adjusted for eGFRcr.
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage, prevalent cardiovascular disease, diabetes, smoking status, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index, and natural log transformed urinary albumin to creatinine ratio.
All-Cause Mortality
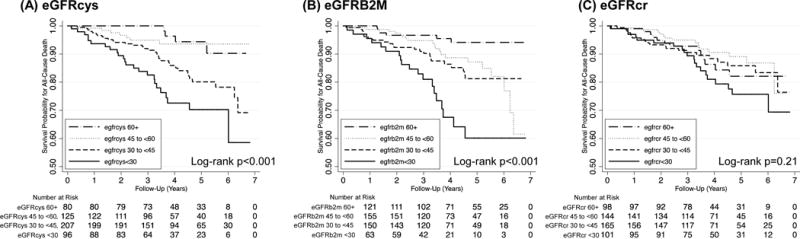
Over median follow-up of 3.8 years, 68 (13.3%) participants within the sub-cohort died. In Kaplan-Meier survival analyses, significant differences were observed across eGFRcys and eGFRB2M but not eGFRcr categories within the sub-cohort (Figure 2). In the case-cohort analysis in the full all-cause mortality analytic sample (N=822) with weighted Cox proportional hazards regression, lower eGFRcys and lower eGFRB2M were each significantly associated with an increased all-cause mortality risk after accounting for demographic and transplant characteristics (Table 3; both p-trend<0.001 when modeled categorically and p<0.001 when modeled continuously). Results were similar with additional multivariable adjustment and persisted in models that further adjusted for eGFRcr (Table 3; HR for eGFR<30 vs. eGFR 60+ 3.92 [95% CI 2.11–7.31] and 4.09 [95% CI 2.21–7.54]; both p<0.001). In contrast, no significant trend or associations were observed with all-cause mortality for eGFRcr when modeled categorically or continuously.
Figure 2.
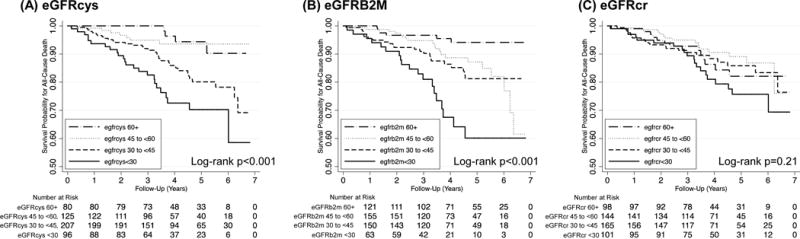
Kaplan Meier survival curves for all-cause mortality in the sub-cohort, by (A) cystatin C-based eGFR, (B) beta-2 microglobulin-based eGFR, and (C) creatinine-based eGFR. The sub-cohort includes 508 participants with 68 all-cause mortality events. eGFR, estimated glomerular filtration rate.
Table 3.
Association of filtration markers with risk of all-cause mortality (N=382 events) in stable kidney transplant recipients in FAVORIT
| Demographic and Transplant Characteristic Adjusted* | Multivariable Adjusted^ | Multivariable Adjusted^ + eGFRcr | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| eGFRcys | ||||||
| <30 | 5.88 (3.35, 10.34) | <0.001 | 3.92 (2.11, 7.31) | <0.001 | 8.55 (3.84, 19.03) | <0.001 |
| 30 to <45 | 2.03 (1.18, 3.49) | 0.01 | 1.76 (0.97, 3.21) | 0.06 | 3.10 (1.52, 6.33) | 0.002 |
| 45 to <60 | 1.59 (0.87, 2.88) | 0.13 | 1.33 (0.67, 2.63) | 0.42 | 1.75 (0.86, 3.57) | 0.13 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | <0.001 | p-trend | <0.001 | |
| per 10 mL/min/1.73m2 | 0.62 (0.54, 0.71) | <0.001 | 0.67 (0.58, 0.78) | <0.001 | 0.51 (0.41, 0.63) | <0.001 |
| eGFRB2M | ||||||
| <30 | 5.53 (3.33, 9.20) | <0.001 | 4.09 (2.21, 7.54) | <0.001 | 6.10 (3.06, 12.19) | <0.001 |
| 30 to <45 | 2.56 (1.62, 4.04) | <0.001 | 2.38 (1.37, 4.13) | 0.002 | 3.28 (1.79, 6.02) | 0.001 |
| 45 to <60 | 1.53 (0.95, 2.47) | 0.08 | 1.37 (0.77, 2.45) | 0.28 | 1.62 (0.91, 2.90) | 0.10 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | <0.001 | p-trend | <0.001 | |
| per 10 mL/min/1.73m2 | 0.66 (0.59, 0.74) | <0.001 | 0.69 (0.61, 0.79) | <0.001 | 0.61 (0.52, 0.71) | <0.001 |
| eGFRcr | ||||||
| <30 | 1.23 (0.76, 1.99) | 0.40 | 1.09 (0.63, 1.87) | 0.76 | – | – |
| 30 to <45 | 1.37 (0.89, 2.12) | 0.15 | 1.21 (0.74, 1.98) | 0.49 | – | – |
| 45 to <60 | 0.99 (0.63, 1.56) | 0.98 | 1.03 (0.62, 1.72) | 0.90 | – | – |
| 60+ | Reference | – | Reference | – | – | – |
| p-trend | 0.30 | p-trend | 0.85 | – | – | |
| per 10 mL/min/1.73m2 | 0.95 (0.88, 1.04) | 0.28 | 0.97 (0.88, 1.06) | 0.49 | – | – |
Note: Weighted Cox regression models were performed in the all-cause mortality analytic sample of 822 participants with 382 events. Results in the shaded area are not presented because models with eGFRcr as the primary exposure cannot be additionally adjusted for eGFRcr.
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage, prevalent cardiovascular disease, diabetes, smoking status, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index, and natural log transformed urinary albumin to creatinine ratio.
Dialysis-Dependent Kidney Failure
Over a median follow-up of 3.6 years, 52 (10.2%) of participants in the sub-cohort experienced dialysis-dependent kidney failure. In Kaplan-Meier survival analyses significant differences were observed across eGFRcys, eGFRB2M, and eGFRcr groups curves within the sub-cohort (all log-rank p<0.001; Figure 3). In case-cohort analyses in the full kidney failure analytic sample (N=736) with weighted Cox proportional hazards regression, lower eGFR based on all three filtration markers was associated with increased risk of kidney failure when modeled categorically (all p-trend<0.001) or continuously (all p<0.001, Table 4) after accounting for demographic and transplant characteristics. Results were similar with additional multivariable adjustment (HR for eGFR<30 vs. eGFR 60+ of 9.49 [95% CI 4.28–21.00] for eGFRcys and 15.53 [95% CI 6.99–34.51] for eGFRB2M; both p<0.001), and persisted for both eGFRcys and eGFRB2M in models that further adjusted for eGFRcr (Table 4).
Figure 3.
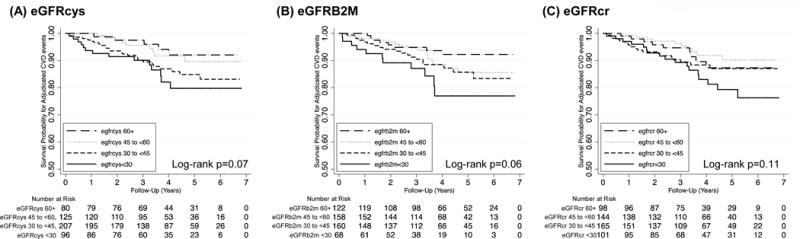
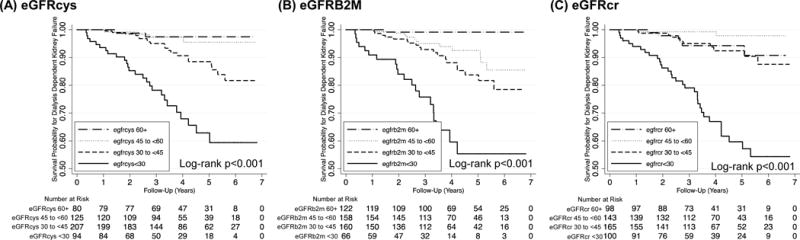
Kaplan Meier survival curves for kidney failure events in the sub-cohort, by (A) cystatin C-based eGFR, (B) beta-2 microglobulin-based eGFR, and (C) creatinine-based eGFR. The sub-cohort includes 508 participants with 52 kidney failure events. eGFR, estimated glomerular filtration rate.
Table 4.
Association of filtration markers with risk of dialysis-dependent kidney failure (n=280 events) in stable kidney transplant recipients in FAVORIT
| Demographic and Transplant Characteristic Adjusted* | Multivariable Adjusted^ | Multivariable Adjusted^ + eGFRcr | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| eGFRcys | ||||||
| <30 | 15.90 (7.73, 32.68) | <0.001 | 9.49 (4.28, 21.00) | <0.001 | 12.74 (4.88, 33.29) | <0.001 |
| 30 to <45 | 3.76 (1.88, 7.52) | <0.001 | 2.68 (1.20, 5.96) | 0.02 | 3.24 (1.36, 7.71) | 0.008 |
| 45 to <60 | 2.24 (1.05, 4.78) | 0.04 | 1.98 (0.79, 4.92) | 0.14 | 2.18 (0.88, 5.39) | 0.09 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | <0.001 | p-trend | <0.001 | |
| per 10 mL/min/1.73m2 | 0.45 (0.37, 0.55) | <0.001 | 0.50 (0.39, 0.62) | <0.001 | 0.42 (0.32, 0.56) | <0.001 |
| eGFRB2M | ||||||
| <30 | 22.67 (11.78, 43.63) | <0.001 | 15.53 (6.99, 34.51) | <0.001 | 18.62 (7.64, 45.39) | <0.001 |
| 30 to <45 | 5.76 (3.17, 10.48) | <0.001 | 4.59 (2.14, 9.83) | <0.001 | 5.24 (2.29, 12.00) | <0.001 |
| 45 to <60 | 3.10 (1.66, 5.82) | <0.001 | 2.56 (1.18, 5.58) | 0.02 | 2.73 (1.24, 6.01) | 0.01 |
| 60+ | Reference | – | Reference | – | Reference | – |
| p-trend | <0.001 | p-trend | <0.001 | p-trend | <0.001 | |
| per 10 mL/min/1.73m2 | 0.51 (0.44, 0.59) | <0.001 | 0.53 (0.44, 0.64) | <0.001 | 0.49 (0.39, 0.61) | <0.001 |
| eGFRcr | ||||||
| <30 | 3.13 (1.85, 5.28) | <0.001 | 2.09 (1.05, 4.14) | 0.04 | – | – |
| 30 to <45 | 2.01 (1.22, 3.33) | 0.007 | 1.72 (0.90, 3.27) | 0.10 | – | – |
| 45 to <60 | 0.76 (0.43, 1.33) | 0.33 | 0.65 (0.31, 1.36) | 0.25 | – | – |
| 60+ | Reference | – | Reference | – | – | – |
| p-trend | <0.001 | p-trend | 0.001 | – | – | |
| per 10 mL/min/1.73m2 | 0.74 (0.66, 0.83) | <0.001 | 0.80 (0.70, 0.92) | 0.002 | – | – |
Note: Weighted Cox regression models were performed in the dialysis dependent kidney failure analytic sample of 736 participants with 280 events. Results in the shaded area are not presented because models with eGFRcr as the primary exposure cannot be additionally adjusted for eGFRcr.
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage
Adjusted for age, sex, race, country, randomized treatment assignment, donor type, graft vintage, prevalent cardiovascular disease, diabetes, smoking status, LDL cholesterol, HDL cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, body mass index, and natural log transformed urinary albumin to creatinine ratio
Discussion
Lower eGFR based on the low molecular weight serum proteins cystatin C and B2M is strongly associated with cardiovascular events, mortality, and dialysis-dependent kidney failure in stable kidney transplant recipients from the FAVORIT study. These associations persist following adjustment for several established risk factors and were independent of eGFRcr. As strong independent predictors of cardiovascular, mortality, and kidney events these filtration markers are of interest as potential factors that could contribute to risk prediction in this population.
Overall our findings are consistent with results for cystatin C and B2M observed in non-transplant populations(14–21) and extend the current literature of the strong risk factor associations of these filtration markers with important outcomes into the kidney transplant population. Few studies have evaluated risk associated with cystatin C and B2M in kidney transplant recipients.(22–25) In small studies (sample size from 79 to 129) higher cystatin C levels were associated with graft loss(24, 25) and higher serum B2M levels were also associated with eGFRcr decline at 1 and 2 years follow-up.(22) In the largest study to date, Astor and colleagues reported that higher serum B2M levels at discharge were associated with both mortality and graft loss and with associations of greater magnitude when compared to serum creatinine in a cohort of 2190 primary kidney transplant recipients from a single center in the United States.(23) Our findings extend the current literature in kidney transplant recipients by confirming that cystatin C and B2M are strongly associated with mortality and dialysis-dependent kidney failure in a diverse cohort of stable kidney transplant recipients from the United States, Canada, and Brazil, in addition to our novel findings related to increased risk of cardiovascular events.
It is difficult to compare the magnitude of the risk associations that we observed for eGFRcys and eGFRB2M with those for eGFRcr. The HRs for eGFRcys and eGFRB2M with cardiovascular events, mortality and dialysis-dependent kidney failure are larger than those observed for eGFRcr in the present case-cohort analysis as well as those observed in a previous study in the full FAVORIT cohort.(8) In the previous study, the HRs for eGFRcr with cardiovascular disease and all-cause mortality were modestly stronger than in this study and statistically significant, although current estimates fall within prior 95% CIs with substantial overlap. These differences may be related to the sampling design in the case-cohort study, which may reduce the relative efficiency of the eGFRcr analyses compared to the full cohort. A different reference group was also selected in the previous study (eGFRcr 60–75mL/min/1.73m2) whereas we combined the two highest categories of 60–75 and 75+ for more stable estimates due to our smaller size. Given the null findings in the 75+ compared to the reference group 60–75mL/min/1.73m2 in the previous study it is less likely that this difference explains the eGFRcr associations observed here. Our interpretation is that the risk associations of eGFRcys and eGFRB2M with these outcomes are likely as strong, and possibly stronger, than for eGFRcr.
Risk associations of eGFRcys, eGFRB2M, and eGFRcr with important adverse outcomes reflect risk related both to GFR and to non-GFR determinants of the serum levels of the filtration markers. For creatinine, muscle mass is the main non-GFR determinant, and age, sex, and race are incorporated into eGFRcr as surrogates in populations where major deviations in muscle mass are not expected. Factors that reduce muscle mass, such as chronic illness and glucocorticosteroid use, may lead to overestimation of GFR and consequent attenuation of risk associations with eGFRcr.
The non-GFR determinants of cystatin C and B2M are not as well understood. Cystatin C is a 120 amino acid serine protease inhibitor that serves many biologic functions. In non-transplant populations, factors associated with non-GFR determinants of cystatin C include larger body size, inflammation, male sex, and smoking.(35–38) Results from in vitro studies indicate that cystatin C is generated by immune cells and adipose tissue, consistent with variation in generation of cystatin C independent of GFR.(39, 40) As in the non-transplant population, obesity and inflammation may affect serum levels of cystatin C in kidney transplant recipients and contribute to increased risk of adverse outcomes independent of decreased GFR. In kidney transplant recipients, recent work suggests that multiple cardiovascular risk factors are associated with eGFRcys independent of measured GFR, including higher age, diabetes, smoking, CVD, dialysis, higher BMI, higher serum triglycerides, higher urine protein, and higher hemoglobin A1c; representing a larger number of non-GFR determinants associated with cystatin C when compared to creatinine.(41) This suggests that the differential risk associations observed in our study may reflect risk related to non-GFR determinants of cystatin C rather than kidney function.
B2M is a 100-amino acid protein that is a component of class I major histocompatibility molecules and is present on the surface of nucleated cells. B2M is higher in some lymphoproliferative and plasma cell disorders and has been used as a biomarker in these conditions.(42, 43) Previous studies have investigated serum B2M in kidney transplant recipients and maintenance hemodialysis patients. In kidney transplant recipients, serum B2M has been studied as a marker of allograft rejection(23) and cytomegalovirus infection(44), but results are confounded by its strong relationship to GFR. In patients treated with maintenance hemodialysis, higher serum B2M has been associated with increased all-cause and infectious mortality risk.(45, 46) While non-GFR determinants of B2M have not been evaluated in kidney transplant recipients, in maintenance hemodialysis patients factors associated with higher levels of pre-dialysis serum B2M included lower age, black race, diabetes, lower BMI, longer dialysis duration, lower residual kidney function, and lower dialysis B2M clearance.(45) In non-transplant and non-dialysis populations, factors most strongly associated with non-GFR determinants of B2M include higher urine protein, smoking, lower serum albumin, diabetes, and higher C-reactive protein, with weaker associations observed for lower age, male sex, non-black race, and higher body mass index.(38) It is possible that some of the mechanisms underlying these associations also contribute to the increased risk observed with B2M levels independent of GFR in kidney transplant recipients.
Our study has several limitations. Our study sample was drawn from a selected sample of stable kidney transplant recipients with decreased kidney function and elevated serum homocysteine and may not be representative of the general kidney transplant population. The case-cohort design utilized a 15% random sample to estimate the exposure distribution in the overall study. Although demographic characteristics in the random sub-cohort are similar to those observed in previous analyses in the overall cohort, we did observe that serum creatinine was higher in participants selected in the sub-cohort compared to those who were not included in the sub-cohort from the overall population (Table S3) and we cannot rule out the impact of sub-cohort selection on the differences in eGFRcr with outcomes observed in the overall cohort, where eGFRcr was statistically associated with cardiovascular disease and all-cause mortality.(8) We also observed a non-significant increased incidence of dialysis dependent kidney failure in participants selected in the sub-cohort compared to those who were not included in the sub-cohort (Table S3), which may impact the generalizability of the findings to the overall kidney transplant population. Cystatin C and B2M were measured once in previously frozen baseline serum samples. Serum levels may vary over time, but we anticipate that variability would be non-differential with respect to outcome assessment and would likely bias our results towards the null. The B2M assay method performed at Brigham and Women’s Hospital has not been directly compared to the method used at the University of Minnesota, where the CKD-EPI eGFRB2M equation was developed. We did not assess all potential factors associated with non-GFR determinants of filtration markers, such as CRP, which contribute to the association of filtration markers with outcomes. Finally, eGFRcys and eGFRB2M equations were not developed in the kidney transplant population, which may lead to bias and imprecision in estimates based on different filtration markers; however this effect is likely non-differential with respect to outcome assessment, potentially biasing associations towards the null.
This study has a number of strengths. Our study sample was drawn from a multi-national, well-characterized cohort of stable kidney transplant recipients with long-term follow-up for adjudicated cardiovascular events, all-cause mortality, and dialysis-dependent kidney failure. The case-cohort study design is an efficient methodological approach for studying associations of novel factors with risk for multiple outcomes within a large existing cohort.
In conclusion, we found that the eGFR based on the low molecular weight serum protein filtration markers cystatin C and B2M are independent predictors of cardiovascular events, mortality, and dialysis-dependent kidney failure in stable kidney transplant recipients from the FAVORIT trial. Further work is needed to characterize non-GFR determinants of cystatin C and B2M and to evaluate the impact of LMW serum protein filtration markers on risk prediction beyond creatinine in kidney transplant recipients.
Supplementary Material
Table S1: Comparison of characteristics of FAVORIT participants selected for the 15% random sub-cohort for the case-cohort study to those eligible but not selected for the 15% random sub-cohort.
Table S2: Baseline characteristics of the FAVORIT sub-cohort, stratified by baseline beta-2 microglobulin-based estimated glomerular filtration rate (eGFRB2M).
Table S3: Baseline characteristics of the FAVORIT sub-cohort, stratified by baseline creatinine -based estimated glomerular filtration rate (eGFRcr).
Acknowledgments
The FAVORIT Trial was supported by a cooperative agreement from the NIDDK (U01 DK 061700).
Abbreviations
- B2M
beta-2-microglobulin
- CI
confidence interval
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CVD
cardiovascular disease
- eGFRcys
estimated glomerular filtration rate from cystatin
- eGFRB2M
estimated glomerular filtration rate from beta-2-microglobulin
- eGFRcr
estimated glomerular filtration rate from creatinine
- ESRD
end-stage renal disease
- HR
hazard ratio
- LMW
low molecular weight
- n or N
number in group
- P
probability
- yr
year
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Jarolim has received research support through his institution from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Beckman Coulter, Daiichi-Sankyo, Inc., GlaxoSmithKline, Merck & Co., Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation, and consulting fees from Roche Diagnostics Corporation Dr. Pfeffer reports (1) Research Grant Support: Amgen, Celladon, Novartis, Sanofi; (2) Consultant: Bayer, Boehringer Ingelheim, DalCor, Genzyme, Gilead, GlaxoSmithKline, Janssen, Lilly, Medicines Company, Merck, Novartis, Novo Nordisk, Relypsa, Salix, Sanofi, Teva, Thrasos and Vericel; (3) Stock Options: DalCor ; (4) Other: The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of MI with Novartis. Dr. Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably and unconditionally assigned to Rockford College. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Results from this study were presented in abstract form at the American Heart Association EPI/Lifestyle Scientific Sessions 2016 in Phoenix, AZ, March 1–4, 2016.
The authors thank Andrew Simon, ScM for assistance in manuscript preparation.
Reference List
- 1.Chronic Kidney Disease Prognosis Consortium. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. Epub 2010/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52. doi: 10.1038/ki.2010.536. Epub 2011/02/11. [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney international. 2011;80(1):93–104. doi: 10.1038/ki.2010.531. Epub 2011/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331–40. doi: 10.1038/ki.2010.550. Epub 2011/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LY. Early renal insufficiency and hospitalized heart disease after renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14(9):2358–65. doi: 10.1097/01.asn.0000083008.25305.67. Epub 2003/08/26. [DOI] [PubMed] [Google Scholar]
- 6.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75(8):1291–5. doi: 10.1097/01.TP.0000061602.03327.E2. Epub 2003/04/30. [DOI] [PubMed] [Google Scholar]
- 7.Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(2):338–53. doi: 10.1111/j.1600-6143.2009.02949.x. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 8.Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(9):2437–45. doi: 10.1111/j.1600-6143.2012.04101.x. Epub 2012/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jardine AG, Fellstrom B, Logan JO, Cole E, Nyberg G, Gronhagen-Riska C, et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis. 2005;46(3):529–36. doi: 10.1053/j.ajkd.2005.05.014. Epub 2005/09/01. [DOI] [PubMed] [Google Scholar]
- 10.Vanrenterghem YF, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, et al. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85(2):209–16. doi: 10.1097/TP.0b013e318160254f. Epub 2008/01/24. [DOI] [PubMed] [Google Scholar]
- 11.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney international. 2002;62(1):311–8. doi: 10.1046/j.1523-1755.2002.00424.x. Epub 2002/06/26. [DOI] [PubMed] [Google Scholar]
- 12.Fellstrom B, Holdaas H, Jardine AG, Nyberg G, Gronhagen-Riska C, Madsen S, et al. Risk factors for reaching renal endpoints in the assessment of Lescol in renal transplantation (ALERT) trial. Transplantation. 2005;79(2):205–12. doi: 10.1097/01.tp.0000147338.34323.12. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 13.Kasiske BL, Israni AK, Snyder JJ, Skeans MA. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. 2011;57(3):466–75. doi: 10.1053/j.ajkd.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369(10):932–43. doi: 10.1056/NEJMoa1214234. Epub 2013/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59(5):653–62. doi: 10.1053/j.ajkd.2011.11.042. Epub 2012/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster MC, Inker LA, Levey AS, Selvin E, Eckfeldt J, Juraschek SP, et al. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis. 2013;62(1):42–51. doi: 10.1053/j.ajkd.2013.01.016. Epub 2013/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster MC, Inker LA, Hsu CY, Eckfeldt JH, Levey AS, Pavkov ME, et al. Filtration Markers as Predictors of ESRD and Mortality in Southwestern American Indians With Type 2 Diabetes. Am J Kidney Dis. 2015;66(1):75–83. doi: 10.1053/j.ajkd.2015.01.013. Epub 2015/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster MC, Coresh J, Hsu CY, Xie D, Levey AS, Nelson RG, et al. Serum beta-Trace Protein and beta-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.01.015. Epub 2016/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavsar NA, Appel LJ, Kusek JW, Contreras G, Bakris G, Coresh J, et al. Comparison of measured GFR, serum creatinine, cystatin C, and beta-trace protein to predict ESRD in African Americans with hypertensive CKD. Am J Kidney Dis. 2011;58(6):886–93. doi: 10.1053/j.ajkd.2011.07.018. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tangri N, Inker LA, Tighiouart H, Sorensen E, Menon V, Beck G, et al. Filtration markers may have prognostic value independent of glomerular filtration rate. J Am Soc Nephrol. 2012;23(2):351–9. doi: 10.1681/ASN.2011070663. Epub 2011/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker LA, Coresh J, Sang Y, Hsu CY, Foster MC, Eckfeldt JH, et al. Filtration Markers as Predictors of ESRD and Mortality: Individual Participant Data Meta-Analysis. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(1):69–78. doi: 10.2215/CJN.03660316. Epub 2017/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trailin AV, Pleten MV, Ostapenko TI, Iefimenko NF, Nikonenko OS. High Serum Level of beta2-Microglobulin in Late Posttransplant Period Predicts Subsequent Decline in Kidney Allograft Function: A Preliminary Study. Disease markers. 2015;2015:562580. doi: 10.1155/2015/562580. Epub 2015/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astor BC, Muth B, Kaufman DB, Pirsch JD, Michael Hofmann R, Djamali A. Serum beta2-microglobulin at discharge predicts mortality and graft loss following kidney transplantation. Kidney international. 2013;84(4):810–7. doi: 10.1038/ki.2013.172. Epub 2013/05/10. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigo E, Ruiz JC, Fernandez-Fresnedo G, Fernandez MD, Pinera C, Palomar R, et al. Cystatin C and albuminuria as predictors of long-term allograft outcomes in kidney transplant recipients. Clinical transplantation. 2013;27(2):E177–83. doi: 10.1111/ctr.12082. Epub 2013/02/05. [DOI] [PubMed] [Google Scholar]
- 25.Lezaic V, Dajak M, Radivojevic D, Ristic S, Marinkovic J. Cystatin C and serum creatinine as predictors of kidney graft outcome. International urology and nephrology. 2014;46(7):1447–54. doi: 10.1007/s11255-013-0624-7. Epub 2013/12/18. [DOI] [PubMed] [Google Scholar]
- 26.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. American heart journal. 2006;152(3):448 e1–7. doi: 10.1016/j.ahj.2006.03.004. Epub 2006/08/23. [DOI] [PubMed] [Google Scholar]
- 27.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011;123(16):1763–70. doi: 10.1161/CIRCULATIONAHA.110.000588. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N, Carpenter MA, Weiner DE, Levey AS, Pfeffer M, Kusek JW, et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol. 2016;27(7):2109–21. doi: 10.1681/ASN.2015030292. Epub 2015/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarolim P, Claggett BL, Conrad MJ, Carpenter MA, Ivanova A, Bostom AG, et al. B-Type Natriuretic Peptide and Cardiac Troponin I Are Associated With Adverse Outcomes in Stable Kidney Transplant Recipients. Transplantation. 2016 doi: 10.1097/TP.0000000000001080. Epub 2016/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ix JH, Katz R, Bansal N, Foster M, Weiner DE, Tracy R, et al. Urine Fibrosis Markers and Risk of Allograft Failure in Kidney Transplant Recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.10.019. Epub 2016/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. Epub 2009/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. Epub 2012/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inker LA, Tighiouart H, Coresh J, Foster MC, Anderson AH, Beck GJ, et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–8. doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–72. doi: 10.1016/s0895-4356(99)00102-x. Epub 1999/12/02. [DOI] [PubMed] [Google Scholar]
- 35.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney international. 2009;75(6):652–60. doi: 10.1038/ki.2008.638. Epub 2009/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney international. 2004;65(4):1416–21. doi: 10.1111/j.1523-1755.2004.00517.x. Epub 2004/04/17. [DOI] [PubMed] [Google Scholar]
- 37.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney international. 2013;83(6):1169–76. doi: 10.1038/ki.2013.7. Epub 2013/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Foster MC, Tighiouart H, Anderson AH, Beck GJ, Contreras G, et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. doi: 10.1053/j.ajkd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–52. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 40.Lafarge JC, Naour N, Clement K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie. 2010;92(11):1580–6. doi: 10.1016/j.biochi.2010.04.011. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 41.Keddis MT, Amer H, Voskoboev N, Kremers WK, Rule AD, Lieske JC. Creatinine-Based and Cystatin C-Based GFR Estimating Equations and Their Non-GFR Determinants in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2016;11(9):1640–9. doi: 10.2215/CJN.11741115. Epub 2016/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schardijn GH, Statius van Eps LW. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney international. 1987;32(5):635–41. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]
- 43.Ploegh HL, Orr HT, Strominger JL. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981;24(2):287–99. doi: 10.1016/0092-8674(81)90318-4. Epub 1981/05/01. [DOI] [PubMed] [Google Scholar]
- 44.Matos AC, Durao MS, Jr, Pacheco-Silva A. Serial beta-2 microglobulin measurement as an auxilliary method in the early diagnosis of cytomegalovirus infection in renal transplant patients. Transplantation proceedings. 2004;36(4):894–5. doi: 10.1016/j.transproceed.2004.03.110. Epub 2004/06/15. [DOI] [PubMed] [Google Scholar]
- 45.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17(2):546–55. doi: 10.1681/ASN.2005020132. Epub 2005/12/31. [DOI] [PubMed] [Google Scholar]
- 46.Cheung AK, Greene T, Leypoldt JK, Yan G, Allon M, Delmez J, et al. Association between serum 2-microglobulin level and infectious mortality in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(1):69–77. doi: 10.2215/CJN.02340607. Epub 2007/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of characteristics of FAVORIT participants selected for the 15% random sub-cohort for the case-cohort study to those eligible but not selected for the 15% random sub-cohort.
Table S2: Baseline characteristics of the FAVORIT sub-cohort, stratified by baseline beta-2 microglobulin-based estimated glomerular filtration rate (eGFRB2M).
Table S3: Baseline characteristics of the FAVORIT sub-cohort, stratified by baseline creatinine -based estimated glomerular filtration rate (eGFRcr).


