Abstract
Prognostic scoring systems for primary myelofibrosis (PMF) are not accurate in patients with post-essential thrombocythemia and post-polycythemia vera myelofibrosis (PET-MF; PPV-MF). Given the paucity of data describing the clinical characteristics, disease course and outcomes of these patients, we sought to describe and compare the clinical characteristics and outcomes of 755 patients with PMF, 181 with PPV-MF, and 163 with PET-MF referred to our institution between 1984 and 2013. The median follow-up was 31 months, and 56% (n=616) patients had died. Over an observation period of 3,502 person-years, 11% of patients had progression to AML, with similar rates among groups. The proportion of patients with transfusion dependency (higher in PMF), leukocytosis and systemic symptoms (higher in PPV-MF), and thrombocytopenia (higher in PMF, PPV-MF) differed among groups. Median overall survival (OS) was longest in PET-MF patients (73 mo vs 45 mo (PMF) vs 48 mo (PPV-MF), p<0.001). Stratification of OS by DIPSS was only discriminatory in patients with PMF, and it failed to distinguish higher risk patients with PPV/PET-MF. In multivariate analysis, predictors of inferior OS were higher age, anemia, systemic symptoms, thrombocytopenia, and high peripheral blasts in PMF; age, anemia, and systemic symptoms for PPV-MF; and anemia, peripheral blasts and thrombocytopenia in PET-MF. Although the clinical characteristics of PPV/PET-MF patients are not substantially different from those with PMF, their outcomes differ and prognostic scoring systems for PET/PPV-MF should be improved.
Keywords: post-essential thrombocythemia myelofibrosis, post-polycythemia vera myelofibrosis, primary myelofibrosis, prognosis
1.0 INTRODUCTION
The classical Ph negative chronic myeloproliferative neoplasms (MPN) polycythemia vera (PV) and essential thrombocythemia (ET), though considered relatively benign, share a propensity to progress toward a fibrotic stage (so-called post polycythemia vera myelofibrosis, [PPV-MF] and post essential thrombocythemia myelofibrosis [PET-MF]). PPV- and PET-MF, like primary MF (PMF), are characterized by typical MF features: decreased peripheral blood counts owing to accumulation of reticulin/collagen fibrosis and subsequent bone marrow failure; extramedullary hematopoiesis often accompanied by significant splenomegaly; and debilitating systemic symptoms [1]. PPV-MF and PET-MF are therefore considered as a natural evolution of these neoplasms, with median time to transformation of 7–20 years from PV/ET diagnosis [2–6]. The cumulative incidence of PPV-MF and PET-MF at 15 years has been reported to be between 5–14% for PV and 1.6–9% for ET [2, 7–9]. Although multiple factors have been reported to influence the rate of transformation, leukocytosis >15 × 109 is the most consistently reported factor [10]. Histopathologic findings in the bone marrow of PET/PPV-MF and PMF patients share overlapping features, and clinical characteristics are also very similar, with a typical picture of bone marrow failure, splenomegaly and chronic inflammatory status, leading to worsening quality of life and cachexia.
Few studies have specifically focused on comparing biologic, clinical and prognostic features of PET/PPV-MF patients [11–13] with those of patients with PMF and findings have been conflicting [13–18]. Prognostication in PPV-MF and PET-MF is evolving, and evidence suggests that the International Prognostic Score System (IPSS), an established prognostication tool in PMF, can’t accurately discriminate different risk categories in PET/PPV-MF patients. However, there is a paucity of data describing clinical characteristics, disease course and outcomes of patients with PET/PPV-MF. In clinical practice, PET/PPV-MF patients are managed similarly to those with PMF; however, whether this practice should change is not known. Here, we describe the clinicopathologic characteristics of patients with PET/PPV-MF and compare their clinical, biologic, and prognostic features with those of PMF patients seen at our center.
2.0 PATIENTS AND METHODS
We retrospectively reviewed the medical records of 1099 patients with MF who were referred to our institution between 1984 and 2013. PMF was diagnosed according to 2008 World Health Organization (WHO) criteria. PET/PPV-MF was diagnosed according to The International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria, which requires a previously documented World Health Organization (WHO)-defined diagnosis of PV or ET and the presence of bone marrow fibrosis grade ≥ 2 (3-point scale) or ≥3 (4-point scale) and two or more additional features: anemia (≥ 2 mg/l decrease from baseline), a leukoerythroblastic peripheral blood smear, splenomegaly, or ≥1 constitutional symptoms, sustained loss of need for phlebotomy and/or cytoreductive therapy for PV or elevated lactate dehydrogenase for ET.[19] Diagnoses of PV or ET were established based on WHO criteria in use at the time of diagnosis. Bone marrow fibrosis grading was assessed according to European Consensus criteria [20]. Molecular testing was performed by real time PCR-based sequencing, using a next generation sequencing platform in our CLIA-certified molecular diagnostic laboratory, as previously reported [21]. All clinical data were collected at the time of referral. Overall survival (OS) was calculated from the date of referral to the date of last follow-up or death, whichever came first. Clinicopathological parameters (categorical and continuous variables) were analyzed by the Fisher’s exact, Kruskal–Wallis or Mann–Whitney U tests, as appropriate. Survival analyses were carried out with the Kaplan–Meier method and compared with the log-rank test. Patients were not censored at the time of SCT (n=99) for the purpose of OS analysis, due to the retrospective study design and because we were not evaluating the impact of therapy. Associations between the clinicopathological factors and survival were assessed by univariate and multivariate analysis. Variables with statistical significance on univariate tests were subsequently analyzed in a multivariate model by using Cox proportional hazard regression with stepwise forward selection. All p-values are two-sided and p < 0.05 was considered to be statistically significant. All statistical computations were performed using SPSS, version 23.0 (Chicago, IL).
3.0 RESULTS
3.1 Demographics and clinic-pathological characteristics
A total of 1,099 patients (755 PMF, 181 PPV-MF, 163 PET-MF) were included in our study (Table 1). Median time to presentation from MF diagnosis was significantly longer in patients with PMF than in those with PPV-MF and PET-MF (4 vs 1 vs 2 months, p<0.001). Of 1,099 patients, 595 (375 PMF, 122 PPV-MF, and 98 PET-MF) presented to our institution within 3 months from MF diagnosis and were previously untreated. Among patients who presented to our institution more than 3 months from diagnosis, the median time to presentation was 21 months (range, 4–382) for PMF (n=380), 17.5 (range, 4–174) for PPV-MF (n=59), and 21 months (range, 4–149) for PET-MF (n=65) (p-value = 0.07).
Table 1.
Demographics and clinical characteristics of all patients
| Characteristics | PMF (N=755) |
PPV-MF (N=181) |
PET-MF (N= 163) |
|---|---|---|---|
| Median age, years (range) | 65 (20–88) | 67 (32–89) | 65 (27–87) |
| Age > 65 years, N (%) | 362 (48) | 102 (56) | 81 (50) |
| Males, N (%) | 488 (65)* | 104 (57.5) | 76 (47) |
| Median WBC, 109/L (range) | 9.5 (0–361) | 14 (1–191)* | 8 (2–61) |
| WBC > 25 × 109/L, N (%) | 133 (18) | 53 (29)* | 16 (10) |
| Median platelets, 109/L (range) | 188 (1–1364) | 213 (6–1958) | 304 (14–2690) |
| Platelets < 100 × 109/L, N (%) | 218 (29) | 44 (24) | 14 (9)* |
| Median hemoglobin, g/dL (range) | 10 (5–19) | 11 (5–18) | 10.5 (4–17) |
| Hgb < 10 g/dL, N (%) | 321 (43) | 68 (38) | 67 (41) |
| Peripheral blasts ≥ 1%, N (%) | 368 (49) | 80 (44) | 73 (45) |
| Splenomegaly, N/known (%) | 416/752 (55) | 143/179 (80)* | 65/163 (40) |
| Symptoms, N (%) | 531 (70) | 146 (81)* | 108 (66) |
| Transfusion dependency, N (%) | 217 (29)* | 30 (17) | 33 (20) |
statistically significant differences (p<0.05) in bold
Sixty-one percent of patients were men (n=668), with a significantly higher proportion (65%) among patients with PMF (p<0.003). The median age at MF diagnosis was 65 years (range, 20–89) and was similar in all groups. Patients with PMF were more frequently transfusion dependent; those with PPV-MF had higher leukocytosis (WBC>25) and systemic symptoms with significant splenomegaly; and those with PET-MF were less likely to be thrombocytopenic (platelets < 100) (Table 1). Other clinical features did not differ among subtypes.
Cytogenetic data with ≥ 10 analyzable metaphases were obtained in 981 (89%) patients, 660 with PMF and 321 with PET/PPV-MF. Overall, 360 (37%) patients had an abnormal karyotype (Abn), and 17% of them had 3 or more abnormalities (complex karyotype, CK, n=62). Abnormal karyotypes present in >10% of patients were single 20q- (n=75, 21%), single 13q- (n=38, 11%), and CK (n=62, 17%). Other abnormalities, such as single +8, +9, single -7/7q-, -5/5q-, or various combinations of two Abn occurred less frequently. Importantly, chromosome (chr) 17 Abn were found only in patients with PMF, while all other Abn were similarly distributed among PMF and PET/PPV-MF (Table 2).
Table 2.
Clinical characteristics of all patients, molecular data and karyotype
| Pt. Characteristics | PMF, N= 755 | PET/MF, N=163 | PPV-MF, N=181 |
|---|---|---|---|
| JAK2 positive, N (% of eval.) | 369/442 (83) | 65/99 (66) | 157/157 (100) |
| Median JAK2 allele frequency, % (range) | 47 (1–99) | 58 (7–96) | 86 (5–99)* |
| MPL positive, N (%) | 22 (5) | 7 (7) | -- |
| CALR positive, N (%) | 32 (7) | 23 (23) | -- |
| Triple negative, N (%) | 21 (5) | 4 (4) | -- |
| HMR mutations, N (%) | 45/198 (22) | 7/38 (18) | 15/147 (10) |
| DIPSS – low, N (%) | 67 (9) | 9 (6) | 16 (9) |
| Int-1, N (%) | 261 (35) | 68 (42) | 69 (38) |
| Int-2, N (%) | 325 (43) | 80 (49) | 55 (31) |
| High, N (%) | 96 (13) | 23 (14) | 23 (13) |
| Advanced BM fibrosis, N (%) | 588/667 (88) | 150/162 (93) | 145/149 (97)* |
| Karyotype, total eval., N | N = 660 | N= 113 | N=208 |
| Diploid, N (%/) | 429 (65) | 56 (50) | 136 (65) |
| Single Abn, N (%) | 144 (22) | 44 (39) | 31 (15) |
| Single Deletion 13q / 20q, N | 75 | 17 | 21 |
| Deletion 7/7q, 5/5q, 12p-, INV (3), Abn 17, N | 18 | 3 | 2 |
| All Others**, N | 51 | 24 | 8 |
| Double Abn, N (%) | 54 (8) | 5 (4) | 20 (10) |
| Including Abn 5, 7, 12p, 11q23, 17, N | 16 | -- | 3 |
| Including Abn 13q, 20q, N | 10 | 3 | 2 |
| All Others**, N | 28 | 2 | 15 |
| Complex (≥3 Abn), N (%) | 33 (5) | 8 (7) | 21 (10) |
Statistically significant differences (p<0.05) are shown in bold; Advanced bone marrow fibrosis = Grade 2 or higher according to European grading; HMR mutations = ASXL1, IDH1, IDH2, EZH2;
Ab = single or combinations of: INV, DER and TRANSL. of 1, 3, 6, 8, 9, 12, 13, 15, 18 and Y; trisomy of 1, 8, 9, 21, 13; ADD of 21, 2; DEL 6p, 8p, 1p, 13q, 20q, Y; 131 patients with PMF and 39 patients with PET-MF who were negative for JAK2 and MPL, but not tested for CALR are not included in the table.
In total, 698 patients (69%) were tested for all 3 known driver mutations (JAK2 V617F, MPL and CALR). An additional 170 patients (39 PET-MF, 131 PMF) who tested negative for JAK2 and MPL, were not tested for CALR mutations. The distribution of driver mutations was similar among disease subtypes (Table 2). As expected, the majority of patients were JAK2 positive (n=592; 68%) and all PPV-MF patients carried a JAK2 mutation (JAK2V617F mutation in all except 2 who had a JAK2 exon 12 mutation). The JAK2 allele burden was significantly higher in PPV-MF patients than in others (median allele burden 86% for PPV-MF, 47% for PMF, and 58% for PET-MF; p<0.001). High molecular risk mutations (ASXL1, EZH2, IDH1 and IHD2) were tested in 383 (35%) patients (23% of PMF, 26% of PET-MF, 81% of PPV-MF) and were present in total of 67 patients (17%). The frequency of high molecular risk mutations were similar among diagnoses.
3.2 Treatment
During the entire follow-up at our institution, 195 (18%) patients never received any therapy, and of those 130 (67%) were followed > 12 months. The remaining 904 (82%) patients were treated with a median of 2 therapies (range, 1–11), hydroxyurea being the most common (56% of patients, n=506), followed by the JAK2 inhibitor ruxolitinib (32%, n=288), IMIDs (23%, n=206) and other, mostly investigational agents (46%, n=413) (Supplemental Table 1). A total of 145 patients (16%) received more than 3 therapies. Splenectomy was performed after an MF diagnosis in 120 patients (12%) (PMF n=89, 12%; PET-MF n=12, 7%; and PPV-MF n=19). An additional 21 patients had splenectomy at outside institutions prior to progression to PET/PPV- MF (6 ET and 15 PV). Ninety-nine patients (10%) underwent allogeneic stem cell transplantation after a median of 20 months (range, 2–265) from MF diagnosis (PMF n=75, 10%; PPV-MF n=10, 6%, PET-MF n=14, 9%).
3.3 Survival outcomes
3.3.1 Overall survival
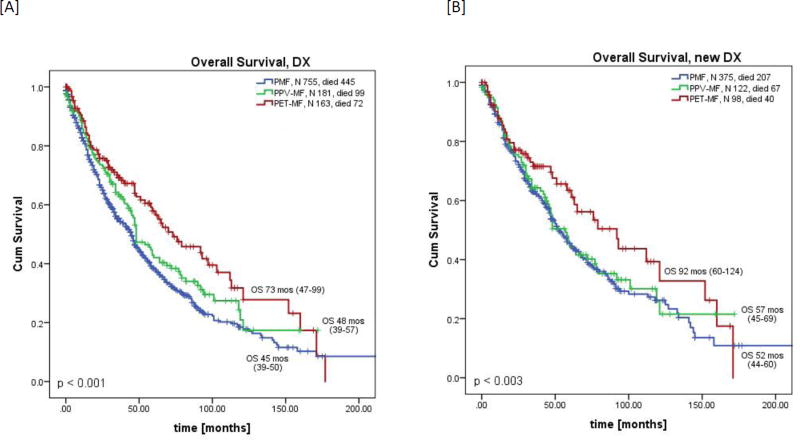
The overall median follow-up time from presentation to our institution was 31 months (range, 0.2–251) and was similar among all groups. Median overall survival (OS) was significantly longer among patients with PET-MF than that of those with PMF and PPV-MF (median 73 vs 45 vs 48 months; p<0.001, Figure 1A). Three year OS rates were 69% vs 63% vs 55% for PET-MF, PPV-MF and PMF, respectively. To eliminate referral bias, we also conducted a sub-analysis including only those patients who were referred to MDACC < 3 months from diagnosis and were previously untreated (considered newly diagnosed; n=595, 375 PMF, 122 PPV-MF and 98 PET-MF). Among this subgroup, OS trends were similar to those of the whole group, with PET-MF patients having the longest OS (PET-MF, 92 mo vs PPV-MF, 57 mo vs PMF 52 months; p=0.003, Figure 1B).
Figure 1.
Overall survival by diagnosis from [A] the date of presentation [all patients] or [B] the date of diagnosis [newly diagnosed]
3.3.2 Driver mutations and overall survival
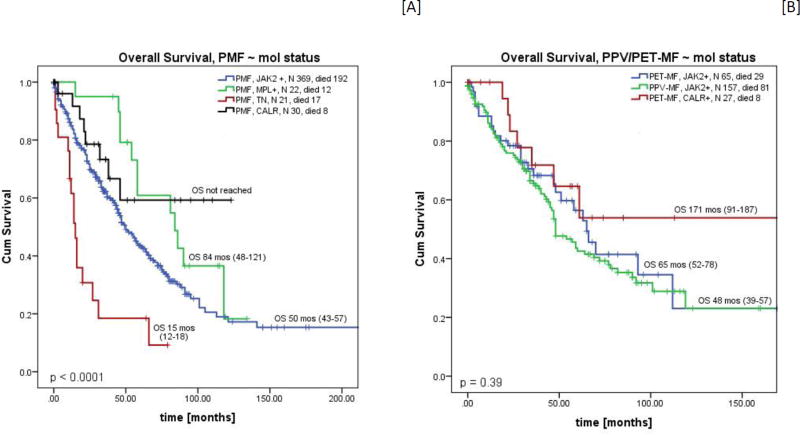
Overall survival was the longest in patients with a CALR mutation, with OS of 171 months for PET-MF and not reached for PMF. Patients with PMF who were triple negative had the shortest OS (median 15 months), and PET-MF/PPV-MF patients with a JAK2 mutation had OS of 65 and 48 months, respectively. We did not have enough PET-MF triple negative patients (n=4) to evaluate for representative OS (Figure 2A & B). Similar results were observed in a sub-analysis including only newly diagnosed patients (time to referral < 3 months, Supplemental Table 2). Patients harboring the same driver mutations (CALR, JAK2) had similar OS regardless of diagnosis (CALR mutated PET/MF vs PMF - 171 months vs NR, p=0.919; and JAK2 mutated PET/MF vs PPV/MF vs PMF, 65 vs 48 vs 50 months, p=0.37). Due to the small number of patients with MPL mutation or those who were triple negative (TN) within each diagnosis, comparison of OS in these patients was not performed.
Figure 2.
Overall survival according to molecular status in [A] PMF and [B] PET/PPV-MF.* Molecular groups with fewer than 10 cases are not shown [PET-MF TN, n=4, median OS = 73 months, and PET-MF MPL, n=7, median OS = 121 months (range, 22–220)].
3.3.3 DIPSS/IPSS score and overall survival
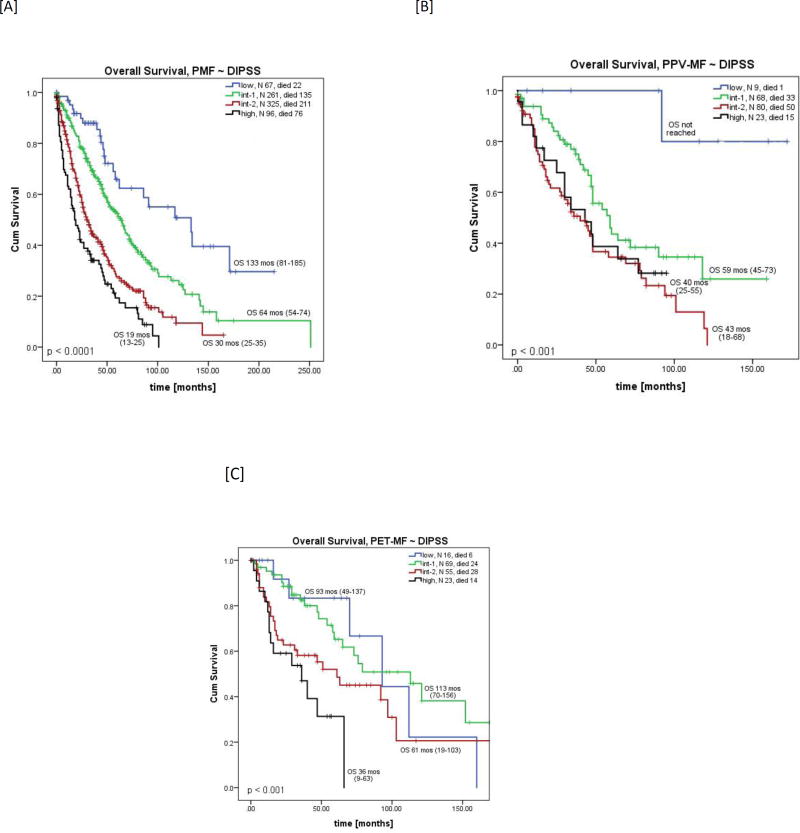
When patients were stratified according to their DIPSS score, only those with PMF had distinct survival curves for each risk group (Table 3, Figure 3A). Among PPV-MF patients, those classified as int-2 or high-risk had similar OS (Figure 3B). Among those with PET-MF, the low and int-1 risk as well as the int-2 and high risk survival curves overlapped (Figure 3C). An OS analysis stratified by IPSS for patients with newly diagnosed MF confirmed these results (Supplemental Figure 1A–C).
Table 3.
Factors associated with worse overall survival according to univariate and multivariate analysis
| Variables | PMF | PPV-MF | PET-MF |
|---|---|---|---|
| Univariate analysis | p-value, HR [95 % CI] | p-value, HR [95 % CI] | p-value, HR [95 % CI] |
| Age>65 years | <0.001, 1.88 [1.5–2.3] | 0.005, 1.82 [1.20–2.77] | -- |
| Hemoglobin below 10 | 0.03, 1.2 [1.02–1.5] | 0.007, 1.75 [1.17–2.63] | 0.007, 1.9 [1.19–3.10] |
| Platelets below 100 | <0.001, 1.4 [1.1–1.7] | 0.003, 1.9 [1.24–2.94] | <0.001, 6.75 [3.33–13.7] |
| Peripheral blasts >1% | 0.002, 1.4 [1.1–1.6] | 0.07, NA | <0.001, 3.3 [2.0–5.4] |
| Symptoms | <0.001, 1.6 [1.3–2.03] | 0.005, 2.28 [1.29–4.06] | 0.013, 1.8 [1.13–2.88] |
| Abn Unfavorable CG* | 0.012; 1.3 [1.07–1.67] | -- | 0.04, 1.81 [1.03–3.21] |
| Multivariate analysis | |||
| Age>65 years | <0.001, 1.87 [1.5–2.3] | 0.046, 1.58 [1.01–2.49] | -- |
| Hemoglobin below 10 | 0.031, 1.27 [1.02–1.58] | 0.008, 1.81 [1.17–2.78] | -- |
| Platelets below 100 | 0.032, 1.27 [1.02–1.59] | -- | <0.001, 4.63 [2.18–6.80] |
| Peripheral blasts >1% | 0.004, 1.35 [1.10–1.65] | -- | <0.001, 2.83 [1.64–4.88] |
| Presence of symptoms | <0.001, 1.53 [1.20–1.95] | 0.003, 2.56 [1.37–4.79] | -- |
All variables are categorical, symptoms are defined according to IWG criteria;
Abn unfavorable cytogenetic = abnormalities other than single deletion 13q, or 20q, and abnormality of chromosome 9;
not statistically significant.
Figure 3.
Overall survival by DIPSS score in patients with [A] PMF, [B] PPV-MF, and [C] PET-MF.
3.3.4 Progression to AML
During an observation period of 3,502 person-years, 108 (11%) patients had progression to AML after a median time from presentation of 25 months (range, 0.2–175), with an incidence rate of 3.05 cases per 100 person-years. The incidence of AML was similar among PMF (n=78, 10% of all), PET-MF (n=16, 8%) and PPV-MF (n=14, 10%) patients. Among 99 patients with progression to AML who had corresponding cytogenetic data, 44% had an Abn karyotype, and the proportions were similar among patients with PMF and PET/PPV-MF.
3.3.5 Cause of death
During the entire observation period, 56% (n=616) of patients died. Fewer patients with PET-MF died than those with PMF and PPV-MF (59% PMF, n=445, 55% PPV-MF, n=99; and 45% PET-MF, n=72; p<0.05). Causes of death were known in 53% of patients (n=327), and included progression of MF/AML in 45% (n=147), infection in 14% (n=47), multi-organ failure in 18% (n=58), and complications after SCT, secondary malignancy, or other medical conditions (<10% each) (Supplemental Table 3).
3.4 Prognostic factors
To identify prognostic factors for each disease subtype, we performed univariate analysis. Hemoglobin < 10 g/dl, platelets < 100 × 109/L, peripheral blasts ≥ 1%, and symptoms were associated with significantly shorter survival in all groups. Age > 65 years was a prognostic factor for PMF and PPV-MF patients, while transfusion dependency was prognostic for PMF and PET-MF patients only. Abnormal unfavorable karyotype vs others (diploid, single deletion 13q, 20q and abnormalities of chromosome 9) was a significant prognostic factor for PMF and PET-MF patients, but not for PPV-MF. In multivariate logistic regression analysis, age > 65 years, hemoglobin < 10 g/dl, platelets < 100 × 109/L, peripheral blasts ≥ 1% and symptoms, but not unfavorable karyotype, remained significant for inferior OS in PMF (Table 3). For PPV-MF, only age > 65 years, hemoglobin <10 g/dl, and symptoms remained significant. For PET-MF, hemoglobin < 10 g/dl, platelets < 100 × 109/L, peripheral blasts ≥ 1% l retained significance.
4.0 DISCUSSION
In this study, we compared clinical characteristics and outcomes of 1,099 patients with PPV-MF, PET-MF, and PMF from a single institution. Some important differences in overall survival and prognostic factors were identified. We found that age > 65 years, hemoglobin < 10 g/dL and poor performance status with significant symptoms were the most detrimental for OS of patients with PPV-MF, while hemoglobin < 10 g/dL, peripheral blood blasts ≥ 1% and platelets < 100 × 109/L were prognostic factors for those with PET-MF. Our results are similar to those reported by others. Hernandez-Boluda et al [22] identified age > 65, hemoglobin < 10 g/dL, increased peripheral blood blasts, and treatment with hydroxyurea at the time of transformation as an independent factors for predicting OS in PPV/PET-MF. Passamonti et al[10] also reported that hemoglobin < 10 g/dL, platelet count < 100 × 109/l, and leukocyte count > 30 × 109/l are independent risk factors for shorter survival of PPV-MF patients, with a 4.2-fold increased risk of death when any of these risk factors are present.
Similar to what was reported by Rotunno et al [23] we found leukocytosis and systemic symptoms with organomegaly more frequently in patients with PPV-MF and thrombocytopenia less frequently in patients with PET-MF, both likely explainable by the phenotype of the antecedent MPN disorder. In contrast, we did not observe more anemia in patients with PET-MF.
The frequencies of cytogenetic abnormalities were similar among disease subtypes, with the exception that only patients with PMF had chr 17 Abn. This is in contrast to results published by Boiocchi et al[15] who reported that patients with PPV-MF tend to have a higher frequency of cytogenetic abnormalities, with a larger number of abnormalities and often complex karyotype. The authors postulated that this was a consequence of slowly progressive clonal disease. Our findings that chr 17 Abn, which confer an unfavorable prognosis across most of hematologic malignancies, were only present in PMF, and the similar frequencies of other abnormalities among disease types suggest the opposite.
Regarding the frequency of driver mutations among disease groups, we found the distribution of CALR, JAK2V617F, MPLW515 mutations to be similar among PMF and PET-MF patients[17]. Median JAK2 allele burden was highest in patients with PPV-MF (p<0.001), but it did not have any impact on OS, in line with a previous report[13]. Similar to previous reports in PMF patients, patients with the CALR mutation constitute a group with a more favorable prognosis than those with JAK2 or MPL mutations, and patients who are negative for the 3 driver mutations (so called “triple negative”) have the worst prognosis among PMF patients. For reasons that are unclear, we observed a higher percentage of JAK2 (PMF 83%, PET-MF 65%) and lower percentage of CALR mutated patients (PMF 7%, PET-MF 23%) across all diagnoses compared with those previously published by Rumi et al for patients with PMF[17] (JAK2 65%, CALR 23%) or Rotunno et al for PET-MF[23] (JAK2 49% and CALR 34%). We also observed shorter OS than what has been published by the previous authors in all patients across all diagnostic entities. This observation was more prominent among JAK2-positive patients, even after adjustment for only newly diagnosed patients. This observation may be due in part to the fact that our institution is a referral center, where we see a higher proportion of advanced cases (48–56% present with int-2 or high-risk DIPSS score and 65–80% present with int-2 or high-risk IPSS score).
Because analyses of mutations beyond the 3 driver mutations were not available in all patients and because the proportions of patients tested within each diagnosis were not equal, we can’t make any conclusions regarding the type, frequency and prognostic impact of so called “high molecular risk” (ASXL1, EZH2, SRSF2, IDH1, and IDH2) mutations in our population. A recently published study [23], suggests that the mutational profile of patients with PPV/PET-MF is different from those with PMF, and their prognostic relevance is not known. Therefore, improved molecular profiling of these patients to better understand the relevance of their genetic background to disease behavior is needed, and studies are currently underway at our institution.
Eleven percent of all patients progression to blast phase (AML), and the incidence of transformation did not differ by disease entity, which is in contrast to the study from Rotunno et al., showing that patients with PPV-MF had a higher rate of progression to AML that those with PMF.
In our study, we show that patients with PET-MF have better OS than those with PMF and PPV-MF. As reported previously,[10] we found that PPV-MF and PMF patients have similar OS. We also confirmed previous reports [22, 24] that the current prognostic scoring systems, such as IPSS and DIPSS, which were developed for PMF, are not effective for prognostic stratification of patients with PET/PPV-MF, and thus, should not be used for medical decision-making in these patients. Better identification of prognostic factors for patients with secondary MF is strongly needed to guide appropriate treatment. Results from our multivariate analysis suggest that anemia with hemoglobin < 10 g/dL and poor performance status with significant symptoms were independent risk factors in patients with PPV-MF, and platelets < 100 × 109/L and blasts ≥ 1% were independent risk factors in patients with PET-MF. These risk factors should be tested in a larger cohort of patients for validation.
A major limitation of our study is that it is based on retrospective data from a single center. Therefore, the data should be interpreted with caution. A prospective multicenter, observational study should be performed in the future to provide a better understanding of the biology and clinical behavior of these diseases. Future studies specifically focused on PET/PPV-MF patients are crucial for developing more accurate predictors of survival with potential clinical implications.
Supplementary Material
Highlights.
Retrospective comparison of 1099 patients with primary and PET/PPV-MF myelofibrosis.
Patients with PET-MF have longer overall survival than those with PPV-MF and PMF.
Current prognostic scoring systems are not valid for prognostication of PET/PPV-MF.
More accurate prognostic factors for PET/PPV-MF are needed.
Acknowledgments
This work was supported in part by a Cancer Center Support Grant to The University of Texas MD Anderson Cancer Center (P30 CA016672) from the US National Cancer Institute.
Funding Source: This work was supported in part by a Cancer Center Support Grant to MD Anderson Cancer Center (P30 CA016672) from the National Cancer Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cervantes F, Pereira A. Prognostication in primary myelofibrosis. Curr Hematol Malig Rep. 2012;7:43–9. doi: 10.1007/s11899-011-0102-1. [DOI] [PubMed] [Google Scholar]
- 2.Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al. Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med. 2004;117:755–61. doi: 10.1016/j.amjmed.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Passamonti F, Rumi E, Arcaini L, Boveri E, Elena C, Pietra D, et al. Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica. 2008;93:1645–51. doi: 10.3324/haematol.13346. [DOI] [PubMed] [Google Scholar]
- 4.Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124:2507–13. doi: 10.1182/blood-2014-05-579136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiladjian JJ, Gardin C, Renoux M, Bruno F, Bernard JF. Long-term outcomes of polycythemia vera patients treated with pipobroman as initial therapy. Hematol J. 2003;4:198–207. doi: 10.1038/sj.thj.6200250. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Larran A, Cervantes F, Bellosillo B, Giralt M, Julia A, Hernandez-Boluda JC, et al. Essential thrombocythemia in young individuals: frequency and risk factors for vascular events and evolution to myelofibrosis in 126 patients. Leukemia. 2007;21:1218–23. doi: 10.1038/sj.leu.2404693. [DOI] [PubMed] [Google Scholar]
- 7.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32. doi: 10.1200/JCO.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 8.Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–66. doi: 10.4065/81.2.159. [DOI] [PubMed] [Google Scholar]
- 9.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–84. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- 10.Passamonti F, Rumi E, Caramella M, Elena C, Arcaini L, Boveri E, et al. A dynamic prognostic model to predict survival in post-polycythemia vera myelofibrosis. Blood. 2008;111:3383–7. doi: 10.1182/blood-2007-11-121434. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Larran A, Bellosillo B, Martinez-Aviles L, Saumell S, Salar A, Abella E, et al. Postpolycythaemic myelofibrosis: frequency and risk factors for this complication in 116 patients. Br J Haematol. 2009;146:504–9. doi: 10.1111/j.1365-2141.2009.07804.x. [DOI] [PubMed] [Google Scholar]
- 12.Cervantes F, Alvarez-Larran A, Talarn C, Gomez M, Montserrat E. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability, presenting characteristics and evolution in a series of 195 patients. Br J Haematol. 2002;118:786–90. doi: 10.1046/j.1365-2141.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- 13.Guglielmelli P, Barosi G, Pieri L, Antonioli E, Bosi A, Vannucchi AM. JAK2V617F mutational status and allele burden have little influence on clinical phenotype and prognosis in patients with postpolycythemia vera and post-essential thrombocythemia myelofibrosis. Haematologica. 2009;94:144–6. doi: 10.3324/haematol.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sangle N, Cook J, Perkins S, Teman CJ, Bahler D, Hickman K, et al. Myelofibrotic transformations of polycythemia vera and essential thrombocythemia are morphologically, biologically, and prognostically indistinguishable from primary myelofibrosis. Appl Immunohistochem Mol Morphol. 2014;22:663–8. doi: 10.1097/PAI.0000000000000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boiocchi L, Mathew S, Gianelli U, Iurlo A, Radice T, Barouk-Fox S, et al. Morphologic and cytogenetic differences between post-polycythemic myelofibrosis and primary myelofibrosis in fibrotic stage. Mod Pathol. 2013;26:1577–85. doi: 10.1038/modpathol.2013.109. [DOI] [PubMed] [Google Scholar]
- 16.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–9. doi: 10.1038/leu.2013.119. [DOI] [PubMed] [Google Scholar]
- 17.Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124:1062–9. doi: 10.1182/blood-2014-05-578435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tefferi A, Guglielmelli P, Lasho TL, Rotunno G, Finke C, Mannarelli C, et al. CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28:1494–500. doi: 10.1038/leu.2014.57. [DOI] [PubMed] [Google Scholar]
- 19.Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437–8. doi: 10.1038/sj.leu.2404914. [DOI] [PubMed] [Google Scholar]
- 20.Gianelli U, Vener C, Bossi A, Cortinovis I, Iurlo A, Fracchiolla NS, et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod Pathol. 2012;25:1193–202. doi: 10.1038/modpathol.2012.87. [DOI] [PubMed] [Google Scholar]
- 21.Luthra R, Patel KP, Reddy NG, Haghshenas V, Routbort MJ, Harmon MA, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99:465–73. doi: 10.3324/haematol.2013.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Boluda JC, Pereira A, Gomez M, Boque C, Ferrer-Marin F, Raya JM, et al. The International Prognostic Scoring System does not accurately discriminate different risk categories in patients with post-essential thrombocythemia and post-polycythemia vera myelofibrosis. Haematologica. 2014;99:e55–7. doi: 10.3324/haematol.2013.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotunno G, Pacilli A, Artusi V, Rumi E, Maffioli M, Delaini F, et al. Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thrombocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am J Hematol. 2016;91:681–6. doi: 10.1002/ajh.24377. [DOI] [PubMed] [Google Scholar]
- 24.Gowin K, Coakley M, Kosiorek H, Mesa R. Discrepancies of applying primary myelofibrosis prognostic scores for patients with post polycythemia vera/essential thrombocytosis myelofibrosis. Haematologica. 2016;101:e405–e6. doi: 10.3324/haematol.2016.149013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.