Abstract
Islet transplantation offers a minimally-invasive approach for beta cell replacement in diabetic patients with hypoglycemic unawareness. Attempts at insulin independence may require multiple islet re-infusions from distinct donors, thus increasing the risk for allogeneic sensitization. Currently, solid organ pancreas transplant is the only remaining surgical option following failed islet transplantation in the US. However, the immunologic impact of repeated exposure to donor antigens on subsequent pancreas transplantation is unclear. Here we describe a case series of seven patients undergoing solid organ pancreas transplant following islet graft failure, with long-term follow-up of pancreatic graft survival and renal function. Despite highly variable panel-reactive antibody (PRA) levels prior to pancreas transplant (mean 27±35%), all seven patients achieved stable and durable insulin independence with a mean follow-up of 6.7 years. Mean hemoglobin A1c (HgbA1c) values improved significantly from post-islet, pre-pancreas levels (mean 8.1±1.5%) to post-pancreas levels (mean 5.3±0.1%; p=0.0022). Three patients experienced acute rejection episodes successfully managed with thymoglobulin and methylprednisolone, and none of these pre-uremic type 1 diabetic recipients developed Stage 4 or 5 chronic kidney disease postoperatively. These results support pancreas-after-islet (PAI) transplantation with aggressive immunosuppression and protocol biopsies as a viable strategy to restore insulin independence after islet graft failure.
Introduction
Current therapeutic options for beta cell replacement to treat Type 1 diabetes mellitus in non-uremic patients include solid organ pancreas transplantation and islet transplantation. Islet transplantation has shown great progress since its inception in the 1960s, with rates of insulin independence now exceeding 50% at 5 years in select patients. These advancements in islet transplantation provide an alternative route for beta cell replacement which avoids the morbidity of open abdominal surgery (1–7). However, when islet allografts fail after an extended period of insulin independence, repeat islet infusions can result in sensitization, exacerbated by multiple infusions from distinct deceased donors. Since the majority of current experimental protocols for islet transplantation exclude patients with failed alloislet transplants, solitary pancreas transplantation is the best remaining option to return to an insulin independent state.
Although prior islet transplantation and sensitization increases risk for subsequent islet graft failure, its effects on subsequent pancreas transplant are unknown. In a prior study of sensitized pancreas transplant recipients, sensitization did not decrease graft survival following pancreas transplant (8). A 9-year follow up of 167 pancreas recipients showed that the presence of donor-specific antibodies (DSA) increased the number of acute rejection episodes without a significant difference in graft or patient survival when compared to patients without DSA (8). However, pancreas transplants in the pre-uremic recipient (PTA) are at higher risk of rejection with poorer overall long-term outcomes as compared to simultaneous pancreas-kidney transplants (SPK) (9). Even with minimal sensitization, patients with failed islet allografts who desperately desire a return to insulin independence have been largely excluded from pancreas transplant due to their immunologic risk. The increasing frequency of pancreas after islet (PAI) transplantation was recently reported using registry data (10) and in a case report of two patients (11). Due to the limiting constraints of registry data, PAI has not been studied for the effect of prior sensitization on graft or patient outcomes. In this report, we identified a population of seven consecutive patients who received a solitary pancreas transplant after failed islet transplantation, and followed long-term graft survival, insulin independence, and renal function.
Materials and Methods
Seven consecutive PAI patients receiving care at our institution from 2007–2016 were included in this study. For inclusion, patients were older than 18 years of age with a prior history of non-uremic type 1 diabetes and hypoglycemic unawareness, and had previously received islet transplants with loss of C-peptide production prior to pancreas transplant. Six of the seven pancreas transplants were performed at our institution; one transplant was performed at another institution with subsequent follow-up care maintained at our institution. Records were retrospectively reviewed for data acquisition, and patient demographic data are included in Table 1. Statistical analysis was performed using paired Student’s T-test, with values expressed as median plus range or mean +/− standard deviation.
Table 1.
Demographic data for pancreas after islet (PAI) recipients prior to pancreas transplantation. Edmonton protocol consists of sirolimus, everolimus, and daclizumab (16).
| Patient | Gender | Age at T1DM Diagnosis | Age at First Islet Txp | No. of Islet Infusions | Islet Transplant Immunosuppression | Duration of Insulin Independence (in mos.) | PRA Following Islet Txp | Maintenance Immunosuppression following Islet Failure |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 11 | 24 | 1 | Edmonton Protocol | 0 | 91% | None |
| 2 | Female | 23 | 32 | 2 | Edmonton Protocol | 2 | 11% | Tacrolimus, sirolimus |
| 0 | 11% | |||||||
| 3 | Female | 6 | 40 | 3 | Edmonton Protocol | 0 | 0% | Tacrolimus, sirolimus, prednisone |
| 0 | 0% | |||||||
| 0 | 0% | |||||||
| 4 | Female | 3 | 48 | 1 | Belatacept-based Protocol | 20.7 | 13% | Tacrolimus, mycophenolate |
| 5 | Male | 2 | 38 | 1 | No immunosuppression | 0 | 10% | None |
| 6 | Female | 20 | 40 | 2 | Efalizumab-based Protocol | 3.5 | 2% | Sirolimus, mycophenolate |
| 17.8 | 2% | |||||||
| 7 | Male | 11 | 43 | 2 | Belatacept-based Protocol | 16 | 0% | Belatacept, sirolimus, mycophenolate |
| 11 | 2% |
Islet Transplantation
Human islets were isolated, cultured, and percutaneously infused into the portal system as previously described (12–15). All patients had received one to three islet transplants (mean 1.7±0.8; Table 1). Immunosuppressive protocols during islet transplantation varied, with patients either receiving glucocorticoid-free Edmonton protocol immunosuppression (16), belatacept-based immunosuppression (17), or an efalizumab-based immunosuppression regimen (17, 18). One patient (Patient 5) underwent an infusion of fetal tissue as a commercial therapeutic for beta cell replacement, and did not receive immunosuppression following his infusion. To prevent sensitization, five out of seven patients were maintained on low-dose immunosuppression following islet failure until time of PAI transplant (Table 1).
Pancreas Transplantation
All donors and recipients were ABO compatible, and were confirmed to have a negative virtual and/or physical crossmatch without DSAs prior to transplantation. Donor pancreata were prepared for transplant using a donor iliac artery Y-graft to the donor splenic and superior mesenteric arteries, as described previously (7, 19). Heterotopic solid organ pancreas transplants were performed via midline laparotomy, with arterial anastomosis to the right iliac artery and systemic venous drainage by anastomosis to the recipient iliac vein. Enteric drainage of pancreatic exocrine secretions was established by side-to-side anastomosis of the graft duodenum to the recipient ileum. All patients received 4–5 days of prophylactic anticoagulation postoperatively.
Immunosuppressive Therapy
For PAI transplant immunosuppression, induction therapy consisted of anti-thymocyte globulin (thymoglobulin, 6mg/kg) and methylprednisolone. Standard four-drug maintenance therapy included low dose tacrolimus (TAC, trough 5–7ng/ml), mycophenolic acid (MPA, 360mg–720mg twice daily), and prednisone 5 mg daily. Low-dose mammalian target of rapamycin (mTOR) inhibition with sirolimus or everolimus (trough 5–7 ng/ml) was added one month following transplant, with subsequent reduction of MPA dosage to 360mg orally twice daily. The strategy for long term maintenance was quadruple therapy, with low levels of TAC, MPA, and mTOR inhibitors to avoid the toxicities of these agents. If patients developed oral ulcers, significant proteinuria (urine protein-to-creatinine ratio > 1g/g) or other toxicities associated with mTOR inhibitors, this drug was discontinued and MPA was increased to full dose. Tacrolimus was targeted to lower trough levels in all recipients to minimize nephrotoxicity in this pre-uremic population. At one year following transplantation, maintenance of immunosuppression is listed in Table 2.
Table 2.
Demographic data for PAI recipients following pancreas transplantation. Standard four-drug PAI immunosuppression includes tacrolimus, mycophenolate, prednisone, and mTOR inhibitor. Withdrawal of immunosuppressive agents indicated above.
| Patient | Age at PAI Txp | Date of PAI Transplant | Time Between Last Islet Txp and PAI Txp (in year) | PRA at PAI Txp (%) | Post-PAI Immunosuppression | Follow Up (mos.) |
|---|---|---|---|---|---|---|
| 1 | 27 | 10/4/2006 | 3.2 | 91% | Standard (prednisone withdrawal) | 118 |
| 2 | 35 | 8/27/2007 | 1.5 | 11% | Standard (mTOR withdrawal) | 108 |
| 3 | 45 | 1/2/2008 | 4.4 | 0% | Standard (mTOR withdrawal) | 103 |
| 4 | 51 | 8/26/2010 | 3.2 | 10% | Standard (mTOR withdrawal) | 71 |
| 5 | 46 | 10/4/2010 | 7.6 | 10% | Standard | 70 |
| 6 | 44 | 3/26/2011 | 3.0 | 62% | Standard | 64 |
| 7 | 48 | 5/10/2014 | 2.9 | 2% | Standard | 26 |
Graft Surveillance and Management of Rejection
Protocol pancreas biopsies were performed on all patients undergoing PAI at our institution, targeted between 2 and 6 months postoperatively. One patient (patient #4) had a delayed protocol biopsy due to recovery from an unrelated extremity operation, while a second patient (patient #5) had an inaccessible graft by percutaneous access on first attempt; repeat attempt 6 months later was successful. For-cause pancreas biopsies were performed for clinical and/or laboratory findings concerning for rejection, including elevation of serum amylase or lipase, graft pain, fever of unclear etiology, and/or elevated serum glucose. Patients who experienced acute rejection were managed with thymoglobulin (3mg/kg for Grade 1 rejection, 6mg/kg for Grade 2 or Grade 3 rejection) and methylprednisolone (125mg × 2 days, with rapid taper to baseline prednisone dose). If trough levels of tacrolimus or sirolimus were below targeted levels, doses were adjusted accordingly.
Post-Transplant Prophylaxis and Follow-Up
All PAI transplant recipients received a standardized prophylactic antimicrobial regimen early after transplant. All recipients were treated with piperacillin/tazobactam for five days postoperatively, until donor duodenal cultures were finalized. If donor cultures returned positive, antibiotics were focused according to sensitivities and continued to complete a 10 day course. Fluconazole was administered for fungal prophylaxis, with 400mg administered intravenously at time of transplant, followed by 200mg IV for five days postoperatively. If donor cultures returned positive for yeast, fluconazole was continued for a 30 day course. Patients continued fungal prophylaxis orally for two months following transplant. Sulfamethoxazole/trimethoprim was administered daily for one month, then three times weekly for Pneumocystis pneumonia (PCP) prophylaxis. Patients were followed regularly in clinic for the first six months with twice weekly laboratory checks; subsequently patients returned to clinic every six months with monthly laboratory studies.
Results
Patients’ Characteristics
Seven patients met inclusion criteria for PAI transplantation, five female and two male patients ranging in age from 27 to 51 years old at the time of PAI transplant. All patients were pre-uremic type 1 diabetics with a prior history of hypoglycemic unawareness. Patients were diagnosed with type 1 diabetes at a mean of 10.8 years of age (range 2–23 years), and underwent their first islet transplant at an average age of 37.8 years of age (range 24–48 years). Each patient underwent between one and three islet infusions, with an average of two islet infusions per patient. One patient (Patient 3) underwent 3 islet infusions spaced 2 months apart, and three patients (Patient 2, 6, and 7) had an average of 20.7±8 months between first and second islet infusion. Patient characteristics are summarized in Tables 1 and 2. All patients had negative c-peptide production at time of PAI transplant, with preoperative glycemic control assessed by Hemoglobin A1c ranging from 5.6% to 9.9% (mean 8.1%±1.5%). Six out of seven patients had prior sensitization at time of pancreas transplant with PRA ranging from 0% to 91% (mean 27±35%). Preoperative baseline creatinine measured from 0.7 mg/dL to 1.25 mg/dL (mean 1.0±0.2 mg/dL) with glomerular filtration rate (GFR) from 66 mL/min/1.73m2 to 113 mL/min/1.73m2 (mean 81.7±17 mL/min/1.73m2).
Post-transplant Graft and Renal Function
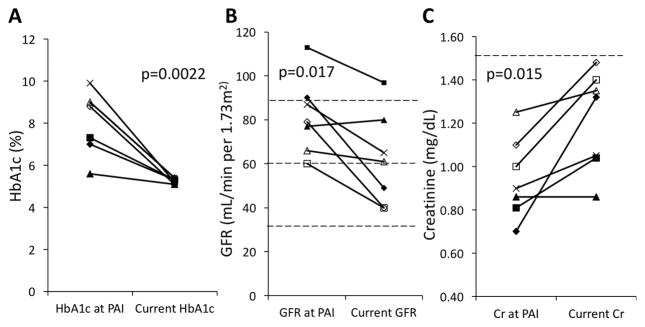
Patients underwent pancreas transplant following failed islet transplantation between October 2006 and May 2014, with follow-up ranging from 26 to 118 months. No patients were lost to follow-up. All patients were monitored regularly for self-identified episodes of symptomatic hypoglycemia and HgbA1c levels. Figure 1 shows postoperative pancreatic and renal outcomes. At time of most recent follow-up, Hemoglobin A1c (HgbA1c) values ranged from 5.1% to 5.4% (mean 5.3±0.1%). From time of transplant, the mean improvement in HgbA1c was 2.8±1.5% (p=0.0022), with all patients remaining insulin independent with normal HgbA1c (Fig. 1A–B). With exception of the immediate post-transplant period, all patients remained entirely insulin independent for the duration of follow-up. Three out of seven patients were found to have proteinuria by urine protein-to-creatinine ratio; one patient had one isolated episode of proteinuria (patient #6, 348mg/g), while two patients had sustained proteinuria (patients #1 and #3; peak 950 and 246mg/g, respectively). Despite a mean decrease in GFR of 20.0±16 mL/min/1.73m2 (p=0.017; Fig. 1B) and an overall increase in serum creatinine of 0.3±0.2 mg/dL (p=0.015; Fig. 1C), no patients progressed to Stage 4 or 5 chronic kidney disease, and all patients maintained a serum creatinine below 1.5 mg/dL.
Figure 1.
Postoperative clinical outcomes following PAI. A) Comparison of HbA1c (%) from time of PAI transplant to most recent laboratory testing; B) Comparison of glomerular filtration rate (GFR; mL/min per 1.73m2) from time of PAI transplant to most recent laboratory testing; and C) Comparison of serum creatinine (mg/dL) from time of PAI transplant to most recent laboratory testing. PAI, pancreas after islet.
Graft Survival and Rejection Episodes
All patients underwent protocol pancreas biopsies within the first year of transplant. Three of seven patients were diagnosed with one episode of rejection, while four patients had no rejection on protocol biopsy or clinical follow-up (Table 3). Of the three patients who experienced rejection episodes, two were diagnosed by protocol biopsy in the absence of clinical signs or symptoms. One patient (patient #4) showed grade 1 acute cellular rejection (ACR) on histology, and one patient (patient #2) revealed grade 3 ACR on histology. Rejection episodes were treated with thymoglobulin (3mg/kg for Grade 1 and 6mg/kg for grade 3 ACR) and methylprednisolone. No additional biopsies were performed, and these patients remained free of clinical rejection for the remainder of the study period. One patient (patient #5) experienced clinical rejection, as identified by fever, graft pain, and elevated serum amylase to 666 U/L. This single episode of early rejection occurred two weeks after transplant, and the rejection episode was successfully managed with methylprednisolone. Based on the patient’s clinical syndrome and exclusion of other causes on work-up, no biopsy was performed at that time. A delayed protocol biopsy performed at six months post-transplant showed no signs of histologic rejection, and the patient remained without further episodes of clinical rejection. One patient (patient #1) underwent two for-cause biopsies at an outside institution based on suspicion of rejection; both of these biopsies confirmed normal pancreas histology without evidence of rejection.
Table 3.
Summary of postoperative surveillance, rejection episodes, and non-immunologic complications (where applicable). Txp: transplant; Bx: biopsy; POD: postoperative day; ACR: acute clinical rejection.
| Patient | Date of PAI Txp | Bx Date (POD) | Rejection | Type of Rejection | Treatment | Other Complications | Management |
|---|---|---|---|---|---|---|---|
| 1 | 10/4/06 | 12/28/06 (86); 2/14/07 (134) | No | Small bowel obstruction (x2) | Lysis of adhesions (x2) | ||
| 2 | 8/27/07 | 10/10/07 (45) | Yes | Grade 3 ACR | Thymoglobulin, methylprednisolone | Anemia, oral ulcers | Discontinuation of mTOR inhibitor |
| 3 | 1/2/08 | 3/13/08 (72) | No | Small bowel obstruction; Recurrent urinary tract infection | Lysis of adhesions; Antibiotic therapy, discontinuation of mTOR inhibitor | ||
| 4 | 8/26/10 | 6/8/11 (287) | Yes | Grade 1 ACR | Thymoglobulin, methylprednisolone | Peripancreatic abscess; Gastrointestinal distress, insomnia | Percutaneous drainage, Antibiotic therapy; discontinuation of mTOR inhibitor |
| 5 | 10/4/10 | 6/29/11 (269) | Yes | Clinical | Methylprednisolone | None | N/A |
| 6 | 3/26/11 | 8/28/11 (156) | No | Facial squamous cell carcinoma | Local resection | ||
| 7 | 5/10/14 | 6/28/14 (49) | No | Bronchitis | Antibiotic therapy |
Post-transplant Complications
Non-immunologic complications following pancreas transplant are listed in Table 3. Nine total complications in six patients occurred during the postoperative period. These included anemia and oral ulcers (patient #2); one patient with recurrent urinary tract infections requiring antibiotic therapy (patient #3), a peripancreatic abscess successfully managed with percutaneous drainage and antibiotic therapy (patient #4); a facial squamous cell cancer requiring surgical resection (patient #6), and an episode of bronchitis requiring antibiotic therapy (patient #7). In addition, two patients experienced three small-bowel obstructions (patients #1 and #3) requiring lysis of adhesions; patient #1 had recurrent intra-abdominal adhesive disease requiring two lysis of adhesions, while patient #3 experienced a small bowel obstruction secondary to adhesive disease and an internal hernia at the anastomosis. Three patients required discontinuation of mTOR inhibitor based on side effects, including anemia and oral ulcers (patient #2); recurrent infections (patient #3); and insomnia and gastrointestinal distress (patient #4). All complications have resolved and all patients remain in routine follow-up.
Discussion
This is the largest case series describing patients who have undergone solid organ pancreas transplant following failed islet transplants. Islet transplantation has been associated with allogeneic sensitization following islet failure in some recipients, although repeat islet infusion from additional donors has not necessarily lead to further sensitization risks (20). The results of this case series suggest that PAI transplantation is a viable strategy to achieve long-term insulin independence in non-uremic Type 1 diabetic patients. Our rates of graft rejection episodes are comparable to other published reports of solitary pancreas transplants and simultaneous kidney-pancreas (SPK) transplants (21, 22). Importantly, all seven patients demonstrated preservation of insulin independence, with no deterioration of graft function as a result of early subclinical or clinical rejection episodes. These results are especially promising as these patients have maintained long term insulin independence irrespective of their pre-transplant PRA positivity. Although elevated PRA remains a marker of sensitization, an elevated PRA resulting from islet transplantation should not be a contraindication to subsequent pancreas transplant. These findings require further validation in larger follow-up studies, but our results suggest that PAI is an excellent modality to achieve insulin independence in the management of Type 1 diabetes following a failed islet transplant. Moreover, repeat islet infusions are not supported by most third party payers in the US, making solitary pancreas transplants the only option to return to a state of insulin independence following a failed islet transplant.
Of the three rejection episodes in our cohort, two were subclinical and identified on routine protocol biopsy alone; only one patient experienced a single episode of clinical rejection. We believe that early protocol biopsy is an essential tool for rapid detection and treatment of subclinical rejection in sensitized patients undergoing PAI transplant. Increased surveillance by protocol biopsy limits damage to the allograft when subclinical rejection does occur, helping to promote favorable long-term graft outcomes through early intervention. In cases where histologic rejection is identified, our protocol is to treat for rejection without repeat biopsy to confirm resolution of rejection, as the benefits of further monitoring must be balanced against the risk of traumatic allograft injury (23). Although not performed on the seven patients included in this study, we now routinely evaluate pancreas recipients for presence of autoantibodies on a prospective basis, to further monitor for risk for immune-mediated graft injury.
Preservation of renal function is another priority in the pre-uremic diabetic population. To minimize nephrotoxicity, we employ a four-drug immunosuppression strategy to minimize calcineurin inhibitor (CNI) exposure with a goal tacrolimus trough level of 5–7ng/ml. Prior to transplant, five out of seven patients had pre-existing Stage 2 chronic kidney disease, stressing the importance of renal preservation. Overall, we observed a mild but statistically significant change in renal function over the course of the study, with a mean increase in serum creatinine of 0.3±0.2 mg/dL (p=0.015) and a mean decrease in GFR of 20.0±16 mL/min/1.73m2 (p=0.017). Three patients progressed to Stage 3 chronic kidney disease and four patients maintained their pre-transplant renal function, while all patients maintained serum creatinine levels below 1.5 mg/dL. Although normoglycemia following successful PAI transplant prevents the progression of diabetic glomerulopathy (24), the slight deterioration of renal function seen in this cohort emphasizes that CNI minimization remains the greatest modifiable risk factor to protect renal function following PAI transplant. We feel that maintaining consistently low CNI levels without compromising the low rejection rates is critical to successful PAI transplantation. Along these lines, we have an ongoing study protocol to determine the efficacy of costimulation blockade as a non-nephrotoxic immunosuppressive agent to permit lower doses of calcineurin inhibitors. When episodes of acute kidney injury did occur postoperatively, the majority were associated with supratherapeutic tacrolimus trough levels, stressing the importance of CNI minimization to preserve long-term renal function in this pre-uremic transplant population.
This study is limited by its small sample size and the inherently observational nature of a case series. In this observational study, DSAs were not routinely monitored postoperatively, which would further characterize the immunologic response to PAI transplant. Gruessner et. al. first reported the prevalence of this new pathway to insulin independence based on a review of International Pancreas Transplant Registry (IPTR) and United Network for Organ Sharing (UNOS) data (10). While the results of their review further support the efficacy of PAI transplantation, wider applicability of that data set is limited by the heterogeneity of maintenance immunosuppression and rejection treatment protocols for pancreas transplant. The aggressive immunosuppressive strategy and monitoring protocol used in this single center series resulted in long-term (6.7 year) insulin independence in this immunologically challenging group of sensitized pre-uremic pancreas transplant recipients. The high frequency of post-operative complications associated with pancreas transplantation reflect the technical challenges of this procedure. Nonetheless, this is the best strategy to return to the state of insulin independence enjoyed by these islet recipients prior to loss of islet function.
In conclusion, this study highlights PAI transplantation as a successful strategy to achieve long-term insulin independence in non-uremic Type 1 diabetic patients despite the presence of sensitization from prior failed islet allografts. The use of a multi-drug immunosuppression regimen which minimizes CNI dosage remains essential to preservation of renal function in this non-uremic population. Protocol biopsy should be performed in all patients to ensure long-term graft survival, although sensitization from failed islet transplantation should not be considered a contraindication to subsequent pancreas transplantation.
Acknowledgments
SW was supported by a NIAID Training Grant from the National Institutes of Health under an award to the University of California, San Francisco (T32AI125222).
Abbreviations
- ACR
acute cellular rejection
- PRA
panel reactive antibody
- CNI
calcineurin inhibitor
- DSA
donor specific antibody
- GFR
glomerular filtration rate
- HgbA1c
hemoglobin A1c
- MPA
mycophenolic acid
- mTOR
mammalian target of rapamycin
- PAI
pancreas after islet
- SPK
simultaneous pancreas-kidney transplant
- TAC
tacrolimus
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Al-Adra DP, Gill RS, Imes S, O’Gorman D, Kin T, Axford SJ, et al. Single-donor islet transplantation and long-term insulin independence in select patients with type 1 diabetes mellitus. Transplantation. 2014;98(9):1007–12. doi: 10.1097/TP.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 2.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(6):1576–83. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lablanche S, Borot S, Wojtusciszyn A, Bayle F, Tetaz R, Badet L, et al. Five-Year Metabolic, Functional, and Safety Results of Patients With Type 1 Diabetes Transplanted With Allogenic Islets Within the Swiss-French GRAGIL Network. Diabetes care. 2015;38(9):1714–22. doi: 10.2337/dc15-0094. [DOI] [PubMed] [Google Scholar]
- 4.Vantyghem MC, Defrance F, Quintin D, Leroy C, Raverdi V, Prevost G, et al. Treating diabetes with islet transplantation: lessons from the past decade in Lille. Diabetes & metabolism. 2014;40(2):108–19. doi: 10.1016/j.diabet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Qi M, Kinzer K, Danielson KK, Martellotto J, Barbaro B, Wang Y, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta diabetologica. 2014;51(5):833–43. doi: 10.1007/s00592-014-0627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepper AR, Gala-Lopez B, Ziff O, Shapiro AJ. Current status of clinical islet transplantation. World journal of transplantation. 2013;3(4):48–53. doi: 10.5500/wjt.v3.i4.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moassesfar S, Masharani U, Frassetto LA, Szot GL, Tavakol M, Stock PG, et al. A Comparative Analysis of the Safety, Efficacy, and Cost of Islet Versus Pancreas Transplantation in Nonuremic Patients With Type 1 Diabetes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(2):518–26. doi: 10.1111/ajt.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantarovich D, De Amicis S, Akl A, Devys A, Vistoli F, Karam G, et al. Posttransplant donor-specific anti-HLA antibodies negatively impact pancreas transplantation outcome. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(12):2737–46. doi: 10.1111/j.1600-6143.2011.03729.x. [DOI] [PubMed] [Google Scholar]
- 9.Kandaswamy R, Skeans MA, Gustafson SK, Carrico RJ, Tyler KH, Israni AK, et al. OPTN/SRTR 2013 Annual Data Report: pancreas. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(Suppl 2):1–20. doi: 10.1111/ajt.13196. [DOI] [PubMed] [Google Scholar]
- 10.Gruessner RW, Gruessner AC. Pancreas After Islet Transplantation: A First Report of the International Pancreas Transplant Registry. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016;16(2):688–93. doi: 10.1111/ajt.13468. [DOI] [PubMed] [Google Scholar]
- 11.Andres A, Livingstone S, Kin T, Campbell PM, Senior PA, Kneteman NM, et al. Islet-after-failed-pancreas and pancreas-after-failed islet transplantation: two complementary rescue strategies to control diabetes. Islets. 2016:0. doi: 10.1080/19382014.2015.1126036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posselt AM, Szot GL, Frassetto LA, Masharani U, Stock PG. Clinical islet transplantation at the University of California, San Francisco. Clin Transpl. 2010:235–43. [PubMed] [Google Scholar]
- 13.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–20. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 14.Szot GL, Lee MR, Tavakol MM, Lang J, Dekovic F, Kerlan RK, et al. Successful clinical islet isolation using a GMP-manufactured collagenase and neutral protease. Transplantation. 2009;88(6):753–6. doi: 10.1097/TP.0b013e3181b443ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen RJ, Ryan EA, O’Kelly K, Lakey JR, McCarthy MC, Paty BW, et al. Percutaneous transhepatic pancreatic islet cell transplantation in type 1 diabetes mellitus: radiologic aspects. Radiology. 2003;229(1):165–70. doi: 10.1148/radiol.2291021632. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England journal of medicine. 2000;343(4):230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 17.Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010;90(12):1595–601. doi: 10.1097/TP.0b013e3181fe1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posselt AM, Bellin MD, Tavakol M, Szot GL, Frassetto LA, Masharani U, et al. Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(8):1870–80. doi: 10.1111/j.1600-6143.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipshutz GS, Wilkinson AH. Pancreas-kidney and pancreas transplantation for the treatment of diabetes mellitus. Endocrinol Metab Clin North Am. 2007;36(4):1015–38. x. doi: 10.1016/j.ecl.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Naziruddin B, Wease S, Stablein D, Barton FB, Berney T, Rickels MR, et al. HLA class I sensitization in islet transplant recipients: report from the Collaborative Islet Transplant Registry. Cell transplantation. 2012;21(5):901–8. doi: 10.3727/096368911X612468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers J, Farney AC, Orlando G, Iskandar SS, Doares W, Gautreaux MD, et al. Pancreas transplantation: The Wake Forest experience in the new millennium. World J Diabetes. 2014;5(6):951–61. doi: 10.4239/wjd.v5.i6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niederhaus SV, Leverson GE, Lorentzen DF, Robillard DJ, Sollinger HW, Pirsch JD, et al. Acute cellular and antibody-mediated rejection of the pancreas allograft: incidence, risk factors and outcomes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):2945–55. doi: 10.1111/ajt.12443. [DOI] [PubMed] [Google Scholar]
- 23.Margreiter C, Pratschke J, Margreiter R. Immunological monitoring after pancreas transplantation. Curr Opin Organ Transplant. 2013;18(1):71–5. doi: 10.1097/MOT.0b013e32835c51b5. [DOI] [PubMed] [Google Scholar]
- 24.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. The New England journal of medicine. 1998;339(2):69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]