Abstract
Objective
The fornix is a white matter tract carrying the fibers connecting the hippocampus and the hypothalamus, two essential stress-regulatory structures of the brain. We tested the hypothesis that allostatic load (AL), derived from a battery of peripheral biomarkers indexing the cumulative effects of stress, is associated with abnormalities in brain white matter microstructure, especially the fornix; and that higher AL may help explain the white matter abnormalities in schizophrenia.
Methods
We tested AL in 44 schizophrenia patients and 33 healthy controls using thirteen pre-defined biomarkers. Diffusion tensor imaging (DTI) was used to obtain fractional anisotropy (FA) values of the fornix and other white matter tracts.
Results
AL scores were significantly elevated in patients compared to controls (F(3, 77)=7.87, p=0.006). AL was significantly and inversely correlated with FA of fornix in both controls (r=−0.58, p=0.001) and patients (r=−0.36, p=0.023). Several nominally significant (p<0.05 but did not survive Bonferroni correction for multiple comparison) correlations were also observed between AL and FA of other white matter tracts in schizophrenia patients. However, the fornix was the only tract exhibiting a correlation with AL in both groups.
Conclusions
These results provide initial evidence that allostatic processes are linked to fornix microstructure in clinical subjects.
Keywords: schizophrenia, white matter, allostatic load, fornix, stress
Introduction
Growing evidence suggests that white matter dysfunction is part of the pathophysiology in schizophrenia, although the mechanisms remain unclear (1–5). Oligodendrocytes are vulnerable to stress, as chronic glucocorticoid exposure suppresses the proliferation of oligodendrocytes (6) and may delay normal myelination process (7). Animal models demonstrate that chronic stress can increase oxidative damage to lipid membranes and myelin (8–10). Fractional anisotropy (FA) of water diffusion, measured using diffusion tensor imaging (DTI), reflects the “integrity” of the white matter microstructure. Decreased FA of major white matter tracts is a replicable imaging finding in schizophrenia (11–15). Reduced FA has been linked to elevated cortisol in individuals exposed to early adverse events (16–19). Chronic stress may cumulatively affect white matter over time and as a consequence contribute to abnormal white matter structural connectivity (20). This study aimed to test the hypothesis that elevated levels of chronic stress may be an important mechanism contributing to white matter abnormalities in humans in general and in patients with schizophrenia in particular.
Stress triggers physiological and behavioral changes to optimize short-term response, and can also induce longer-term adaptive shifts in biological ‘set-points,’ a process termed ‘allostasis’ (21–22). Chronic stressors or mal-adaptive responses to stress can push cardiovascular, metabolic, immune and neuroendocrine system set-points beyond healthy homeostatic limits, and these changes are conceptualized as ‘allostatic load’ (AL) (21–23). Elevated AL is hypothesized to reflect the ‘wear and tear’ effects of chronic stress on the body and the brain (24). AL was found to be significantly elevated in the initial testing of this concept in patients with schizophrenia (25).
While the research on AL in schizophrenia patients is new, a number of studies have considered the relationship between allostatic processes and depression (26), PTSD (27), and bipolar disorder (28–29). However, few studies have directly examined the frequently hypothesized AL effect on brain structure (28, 30–31) in patients with psychiatric conditions. We previously found that prolonged, but not acute, cortisol reactivity to psychological stress was associated with reduced white matter DTI-FA, particularly at the fornix, in schizophrenia (32). This finding appears consistent with McEwen’s working hypothesis of AL: hippocampal structural plasticity would be most vulnerable to damage from repeated or chronic stress (30–31). This hypothesis is well-supported in animal models using chronic stress manipulations (24, 30–31). However, empirical evidence on how AL may affect hippocampal circuitry in humans is needed.
The fornix is the principal white matter tract that connects the hippocampus to subcortical areas including the hypothalamus, medial septum, nucleus of the diagonal band, and dorsal raphe nucleus (33) and also to cortical areas including prefrontal and cingulate cortices (34–35). The hippocampal formation and these subcortical areas regulate cardiovascular, immune and endocrine responses to stress and environmental challenges (36–37). Furthermore, one study found high AL was related to reduced white matter, but not gray matter volume, in older adults (average 72.5 years of age), highlighting the importance of focusing on how AL is related to white matter (38). There are no published studies examining the effect of AL on DTI-based white matter assessments. Therefore, we tested the hypothesis that higher AL would be related to lower cerebral white matter microstructure, in particular the fornix. Furthermore, we were interested in exploring the question of whether AL contributes to FA deficits in schizophrenia.
Methods
Participants
This study included 44 patients with schizophrenia (including n=10 with schizoaffective disorder; they are together called schizophrenia hereafter) who were recruited from outpatient clinics of the Maryland Psychiatric Research Center and neighboring outpatient clinics. Healthy control participants (n=33) were recruited using local media advertisements and frequency-matched on age and sex with the patients (Table 1). The Structured Clinical Interview for DSM-IV was performed on all individuals (39). Control participants had no current Axis I diagnosis, although individuals with a history of past mood or anxiety disorders were not excluded to better match schizophrenia/schizoaffective patients on non-psychosis pathology (30% of the controls had previous mood or anxiety disorders based on Structured Clinical Interview lifetime record). Psychiatric symptoms were assessed with the Brief Psychiatric Rating Scale (BRPS); the psychosis subscale was used to examine positive symptoms of psychosis specifically (40). Other than 4 patients who were unmedicated at time of study, all patients were taking antipsychotics, including 34 on atypical, 4 on typical, and 2 taking both an atypical and a typical antipsychotic. Exclusion criteria for both groups included major neurological conditions, history of head trauma with clinical sequelae, intellectual disability, and substance abuse or dependence within the past 6 months (except nicotine). To reduce variance from cyclic hormonal effects, females were tested for AL measures within the first 10 days of their menstrual cycle. The data were collected from August 2013 to February 2016. Participants gave written informed consent as approved by the University of Maryland IRB.
Table 1.
Clinical information.
| Schizophrenia (n=44) | Healthy control (n=33) | F or χ2 statistic | p-value | |
|---|---|---|---|---|
| Age (years) | 32.7±12.6 | 35.3±14.2 | 0.72 | 0.40 |
| Sex (M/F) | 28/16 | 19/14 | 0.29 | 0.59 |
| Smoker/Nonsmoker | 14/30 | 7/26 | 1.07 | 0.30 |
| Race (White/African-American/Asian) | 20/21/3 | 21/9/3 | 3.32 | 0.19 |
| BPRS | 39.2±11.0 | n/a | n/a | n/a |
| Age of onset (years) | 20.1±5.3 | n/a | n/a | n/a |
| Subjects with Depression and/or Anxiety (%) | 15 (34%) | 10 (30%) | 0.37 | 0.54 |
| Metabolic Syndrome (%) | 11 (25%) | 5 (15%) | 1.11 | 0.29 |
| Allostatic Load | 4.42±2.76 | 3.28±2.22 | 7.87 | 0.006 |
Data are presented as mean±s.d.
BPRS: Brief psychiatric rating scale. Statistics for allostatic load include age and sex as covariates.
Allostatic load assessment
The measure of AL was an index of 13 biomarkers, including resting systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate; body-mass index (BMI) and waist-hip ratio; blood levels of high-density lipoprotein (HDL) cholesterol, total cholesterol, glycated hemoglobin (HbA1c), C-reactive protein (CRP), and dehydroepiandrosterone (DHEA); and 12-hour overnight levels of urine epinephrine, norepinephrine, and cortisol. To collect the urine, participants were given a plastic container, a cooler and an ice pack. Subjects were instructed to discard the first urination at 2000 h, and then to collect all subsequent urine until 0800 h the following morning, keeping the container in the cooler with the ice pack. Participants were asked to refrain from any food or drink besides water and regular medications from midnight prior to blood collection. Blood and urine samples were sent to a CLIA-certified commercial laboratory for analysis. Urine cortisol, epinephrine and norepinephrine values were adjusted for creatine levels. Anthropometric measures were conducted with participants wearing light clothing. Blood pressure and heart rate was measured after at least 10 minutes of rest. Fasting blood samples were collected between 0900 h and 1100 h in all participants. AL data for 26 patients and 17 controls in this study were reported in a previous publication that addressed the initial question of whether AL is elevated in schizophrenia (25).
Adherent to previously reported methodology for calculation of AL index (41), we identified the 25th and 75th percentile values of each of the 13 biomarkers for the control sample distribution. Participants who had a biomarker value greater or equal to 75th percentile (or less than or equal to the 25th percentile for HDL and DHEA) received a score of 1 for that specific biomarker; cutoff values are reported in Table 2. Participants taking one or more hypoglycemic agent were automatically given a score of 1 for HbA1c; participants taking one or more antihypertensive medication were given a score of 1 for SBP; and participants taking one or more lipid-lowering medication were given a score of 1 for total cholesterol. The sum of biomarker values was computed, and imputed based on a 13 point scale in case of missing data points, such that the AL index score could range from 0 to 13. We explored alternative means of calculating AL index, including using clinically meaningful cutoffs instead of percentiles from our sample, and using averaged Z scores but found minimal differences between these methods of calculating AL index as already suggested by previous studies (41).
Table 2.
Individual values for the thirteen measures made up of the allostatic load index. Threshold: the cut off value beyond which the item was scored a 1 for calculation of AL.
| Biomarker | HC (N=33) | SZ (N=44) | Threshold | F-value | p-value |
|---|---|---|---|---|---|
| Cardiovascular | |||||
| Resting SBP (mmHg) | 115.57±2.94 | 112.53±1.81 | >= 128.00 | 1.11 | 0.29 |
| Resting DBP (mmHg) | 71.21±1.60 | 70.34±1.41 | >= 77.50 | 0.16 | 0.68 |
| Resting heart rate (beats per minute) | 67.93±1.77 | 75.89±2.38 | >= 76.50 | 6.35 | 0.01 |
| Metabolic – lipids | |||||
| BMI (kg/m2) | 27.67±0.94 | 28.27±0.94 | >= 30.03 | 0.19 | 0.66 |
| Waist-Hip Ratio | 0.88±0.01 | 0.91±0.03 | >= 0.93 | 1.04 | 0.31 |
| HDL Cholesterol (mg/dL) | 55.12±2.94 | 55.40±4.66 | <= 42.00 | 0.00 | 0.96 |
| Total Cholesterol (mg/dL) | 182.35±7.06 | 178.27±5.43 | >= 217.00 | 0.22 | 0.64 |
| Metabolic - glucose metabolism | |||||
| Glycosylated hemoglobin (HbA1c) | 5.50±0.04 | 5.58±0.08 | >= 5.67 | 0.90 | 0.44 |
| Inflammation | |||||
| CRP (mg/L) | 1.65±0.37 | 3.70±0.77 | >= 2.32 | 4.62 | 0.03 |
| Sympathetic Nervous System | |||||
| Urine Epinephrine (ug/g creatine) | 3.76±0.47 | 4.97±0.58 | >= 4.72 | 2.34 | 0.13 |
| Urine Norepinephreine (ug/g creatine) | 22.08±2.33 | 33.73±3.12 | >= 28.66 | 5.31 | 0.02 |
| HPA Axis | |||||
| Urine Cortisol (ug/g creatine) | 17.88±3.39 | 12.84±1.42 | >= 18.25 | 2.25 | 0.14 |
| Blood DHEA (ug/dL) | 237.79±26.14 | 272.82±21.84 | <= 119.40 | 1.06 | 0.31 |
The measures comprising the AL index have considerable overlap with measures used to define metabolic syndrome, a condition that contributes to the development of cardiovascular disease (42). To determine if any relationship between AL and white matter was driven primarily by presence of metabolic syndrome we used our data to categorize individuals as likely having metabolic syndrome or not. We defined metabolic syndrome based on a modification of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) definition (43) as follows: individuals were defined as having metabolic syndrome if they had at least 2 of the following: 1) HgA1c > 6.5 (considered a useful threshold for diagnosis of diabetes) (44), or taking hypoglycemic medication; 2) waist circumference >40 inches for males or >35 inches for females; 3) HDL < 40 for males, or < 50 for females; 4) SBP > 130, DBP > 85, or taking hypotensive medication. Note that because we did not have triglyceride levels available we dropped this criterion, and substituted HgA1c as a proxy measure of insulin resistance due to lack of fasting glucose data.
Imaging
Diffusion tensor data were collected at the University of Maryland Center for Brain Imaging Research using a Siemens 3T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. DTI data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence with a spatial resolution of 1.7×1.7×3.0 mm. The sequence parameters were: TE/TR=87/8000ms, FOV=200mm, axial slice orientation with 50 slices and no gaps, five b=0 images and 64 isotropically distributed diffusion weighted directions with b= 700 s/mm2. These parameters maximized the contrast to noise ratio for FA measurements (45). ENIGMA-DTI pipeline (https://www.nitrc.org/projects/enigma_dti) was used for tract-based analysis of diffusion anisotropy (46). First, fractional anisotropy (FA) images were created by fitting the diffusion tensor to the motion and eddy current diffusion data. RMSDIFF (47) was used to estimate the root mean square (RMS) movement distance between diffusion sensitized and b=0 images. All data passed QA control of <3mm accumulated motion during the scan. There was no difference in the average motion per TR between patients and controls (0.42±0.21 vs. 0.43±0.20, for patients and controls, respectively). In the next step, all FA images were spatially normalized to the Johns Hopkins University (JHU) (48) and then nonlinearly aligned to a group-wise, minimal-deformation target (MDT) brain using the FLIRT method (46,49). The group’s MDT brain was identified by warping all individual brain images in the group to each (50). Next, individual FA images were averaged to produce a group-average anisotropy image. This image was used to create a group-wise skeleton of white matter tracts. The skeletonization procedure was a morphological operation, which extracts the medial axis of an object. Finally, FA images were thresholded at FA=0.20 level to eliminate non-white matter voxels, and FA values were projected onto the group-wise skeleton of white matter structures. This step accounts for residual misalignment among individual white matter tracts. FA values were assigned to each point along a skeleton using the peak value found within a designated range perpendicular to the skeleton. This processing was performed under two constraints. A distance map was used to establish search borders for individual tracts. The borders were created by equally dividing the distance between two nearby tracts. Secondly, a multiplicative 20mm full width at half-max Gaussian weighting was applied during the search to limit maximum projection distance from the skeleton. The average FA values along the spatial course of the fornix (column and body of fornix) and eleven other major white matter tracts were calculated. As this study had no a priori hypothesis with regard to hemispheric lateralization, we averaged the FA values for the individual left and right tracts as in our previous studies (32). Whole brain averaged FA was also explored. DTI data was collected on 39 schizophrenia patients and 30 control participants.
Statistical analysis
Patient-control group difference on AL was compared using univariate ANOVAs with age and sex as covariates. To explore the relationship of AL to white matter microstructure, Pearson’s correlation coefficients were calculated for the relationship of AL and FA of the 12 major white matter tracts. Only tracts that showed a significant correlation with AL at a Bonferroni-corrected threshold of p<0.004 were examined in further analyses. Further analyses included 1) linear regression to examine the influence of AL on white matter FA covarying for age, sex, metabolic syndrome, and diagnosis; 2) repeating this linear regression in patient and control samples independently; 3) for patients, further Pearson’s correlation and linear regression tests to examine the influence of antipsychotic medication and symptoms on AL.
Results
Allostatic Load and the Fornix
Univariate ANOVA results showed that AL index scores were significantly higher in patients compared to controls (F(3, 77)=7.87, p=0.006) after covarying out age and sex effects. Whole brain tract average FA was significantly lower in patients compared to controls (F(3,69)=5.13, p=.027), but none of the 12 major white matter tracts was significantly different between patients and controls after Bonferroni correction for multiple comparisons (all p>0.05/12=0.004).
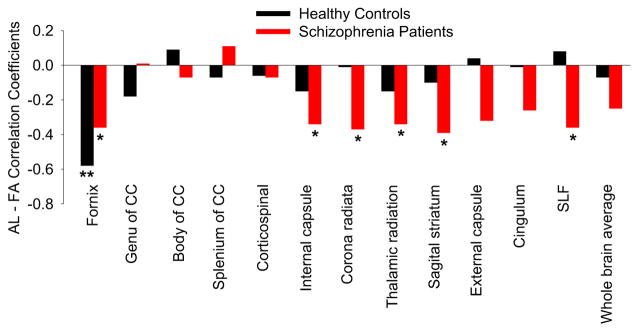
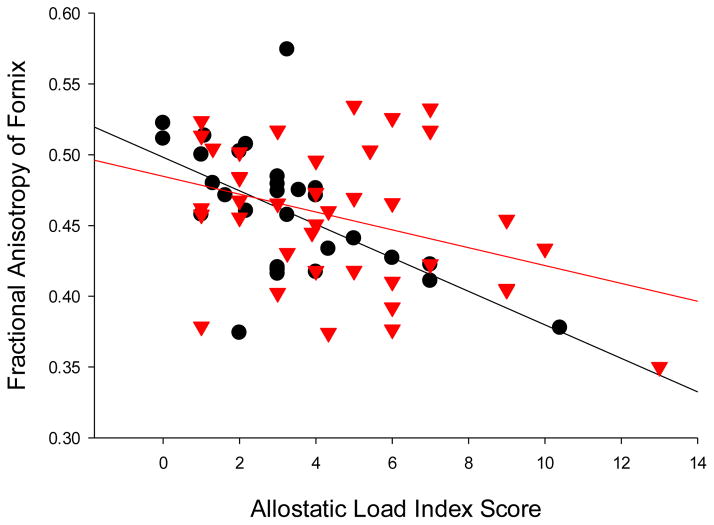
The relationships between AL and FA of the 12 major white tracts were examined in the whole sample (Figure 1). Only the correlation between AL and FA of the fornix was significant and passed the threshold for Bonferroni correction (r=−0.43, p<0.001). Trend level (i.e., 0.004<p<0.05) associations were observed between AL and FA of corona radiata (r=−.24, p=.046) and sagittal striatum (r=−.25, p=.036). A linear regression analysis using FA of fornix as the dependent variable and age, sex, metabolic syndrome, diagnosis and AL as predictors found that AL was still a negative predictor of fornix FA (β=−0.40, p=0.016), suggesting a similar AL association with fornix FA even after metabolic syndrome was covariated out. The correlations between fornix FA and AL were significant and in the same direction in both controls (r=−0.58, p=0.001) and patients (r=−0.36, p=0.023) (Figure 2). When adjusting for history of mood disorders, the results did not change for controls (r=−0.59, p=0.001) and patients (r=−0.36, p=0.026).
Figure 1.
Relationship between allostatic load (AL) scores and fractional anisotropy (FA) of all twelve major tracts and whole brain average. ** Significant at p < 0.01. * Nominally significant at p < 0.05). CC: corpus callosum. SLF: superior longitudinal fasciculus.
Figure 2.
Scatter plot of relationship between allostatic load scores and fractional anisotropy of fornix in controls (black circles) (r=−0.58, p=0.001) and schizophrenia patients (red triangles) (r=−0.36, p=0.023).
Other Clinical Covariates
Because many demographic and lifestyle factors may affect AL, a univariate analysis of the entire sample was performed to test the effects of age, sex, diagnosis, smoking status, and diagnosis and smoking interaction on AL. This model revealed an effect of diagnosis on AL (F=9.4, p=0.003) and a trend diagnosis × smoking interaction (F=3.9, p=0.054). Age was a significant covariate (p<0.001), but sex was not (p=0.10). Smokers had higher AL than nonsmokers in patients (mean±sd: 6.0±3.6 versus 3.9±2.2, respectively, F=6.6, p=0.015) but not in controls (p=0.45). In comparison, using the same model but replacing AL with fornix FA, there was no significant diagnosis × smoking interaction for fornix FA (p=0.91). Age of onset was ascertained in 33 of 44 patients; age of onset was significantly correlated with AL such that earlier age of onset was associated with higher AL (partial r=−0.44, p=0.009). Current antipsychotic medication dose chlorpromazine equivalent (CPZ) was significantly correlated with AL (r=0.33, p=0.042) although it was not significant after correcting for age (p=0.12). A partial correlation correcting for CPZ shows the relationship between AL and fornix FA is marginally affected by CPZ (r=−.30, p=.065). After covarying out race, the correlation between fornix FA and AL were similar in both controls (r=−0.60; p=0.001) and patients (r=−0.36, p=0.025). AL was not significantly associated with BRPS total or psychosis subscale scores (all p>0.25).
Discussion
In this study we found that high level of AL was associated with reduced FA of the fornix in both patient and healthy control groups. Among patients, AL was also related to reduced FA of a number of white matter tracts. These results support the possibility that uncompensated allostatic processes may be related to compromised integrity of the major hippocampal white matter tract fornix regardless of diagnosis, while providing preliminary evidence that pathophysiological consequences of AL may contribute to more diffuse white matter deficits in schizophrenia.
The hippocampus has been theorized to be the primary brain structure affected by AL (30–31). Extensive evidence from animal models has demonstrated the negative impact of chronic stress on hippocampal neurons, volume, neurogenesis and dendritic remodeling, through mechanisms including elevated glucocorticoids (51–53), increased excitatory amino acids (54) and free radicals (55), epigenetic changes (56), and pro-inflammatory cytokines (57). However, previous studies have not directly examined the potential impact of AL on the white matter tracts connecting hippocampus with other brain areas. The fornix is the principal tract linking hippocampus with subcortical areas, specifically the medial septum, nucleus of the diagonal band, supramammillary nucleus, lateral hypothalamus, dorsal raphe nucleus, and the thalamic nucleus reuniens, all of which send projections through the fornix to terminate in the hippocampus and the adjacent parahippocampal region (33). The fornix also contains hippocampal fibers projecting to cortical regions, including the prefrontal cortex (34,58). The functions of these regions may implicate the mechanisms underlying the observed relationship between AL and fornix FA. For example, the diagonal band - medial septum system integrates neuroendocrine output and central blood pressure control (36, 59–61). Neuroendocrine and blood pressure measures are components of the AL index. Another example is the dorsal raphe nucleus, which provides major serotonergic projections to brain areas controlling behavioral and neuroendocrine responses to stress (62–64). Therefore, the AL - fornix relationship may not be simply hippocampal in origin and may also be related to other limbic subcortical regions connected through the fornix.
The pattern of AL association with white matter was different in healthy controls vs. schizophrenia patients. We speculate that the fornix is likely more sensitive to AL and this could explain an AL - fornix correlation observed even in healthy controls. It is possible that healthy controls otherwise may not have a predisposed vulnerability to white matter damage, limiting the relationship between AL and FA to the fornix. In schizophrenia patients, due to genetic and developmental vulnerability inherent to the disease, AL effects on their brains may be more diffuse. An even more eminent question is on the causal relationship between AL and fornix. The initial formulation of the AL theory assumes that changes in the hippocampus are caused by stress and elevated AL, which implies an AL → fornix causal interpretation (30–31). The cross sectional design of this study limits our ability to affirm causality. However, there may be reason to consider the opposite causal pathway of fornix → AL. For example, one could speculate that the relationship between AL and fornix could also be driven by a feed-forward mechanism in which individuals with any insult to the fornix through genetic, developmental, and/or environmental vulnerability may have more maladaptive responses to stress and as a result they would develop increased AL. Furthermore, the directionality between AL and the fornix may not necessarily be uni-directional but likely dynamic where increased AL affects the fornix and in turn, abnormal fornix exacerbates AL. Future studies using animal models of psychosis, or perhaps longitudinal studies in humans, may help to determine the pattern of causality regarding the relationship between AL and white matter changes.
Besides affecting the white matter axons through the neuronal origins, stress may also directly affect white matter. Evidence supporting a role of chronic stress on white matter impairment has been found in both animal and human studies. At a cellular level, chronic elevation of glucocorticoids may inhibit functions of glial cells critical for maintaining normal white matter development and function (65). Deficits in white matter microstructure are evident in adolescent monkeys who had experienced maltreatment and high levels of cortisol in infancy (66). Cushing’s disease and its chronic hypercortisolism are related to widespread reductions in FA of white matter tracts (67). In another study, subjective psychological distress reported by middle-aged women predicted the number of white matter lesions 32 years later (68).
The findings in the study are potentially confounded by the effects of antipsychotic drugs, which are well known to have metabolic and cardiovascular effects, including effects on AL components such as cholesterol, HgA1c, and heart rate (69–70). However, findings in the fornix were replicated in controls as well as patients, suggesting that findings in the patients were unlikely all due to medication effect. The concept of metabolic syndrome partially overlaps with AL, although in our linear regression analyses the effect of AL on fornix FA remained after a proxy metabolic syndrome index was covariated out. However, due to limitations in the available data we used a modified definition of metabolic syndrome. Another limitation of this study is a modest sample size. As such, we did not examine how individual measures within the AL index may be related to white matter differently because the current sample lacked the power to examine 13 AL component measures across 12 white matter tracts.
This initial study of AL effect on DTI-based white matter microstructure measures showed that AL is related to fornix microstructure in two independent cohorts, consistent with the hypothesis that the hippocampal structures are most likely to be affected by AL. However, AL may have a more diffuse relationship with cerebral white matter in patients with schizophrenia. Although additional longitudinal human studies and animal research are needed to identify causal mechanisms underlying this relationship, AL may provide a new framework to understand white matter impairment in schizophrenia, and how somatic health and brain white matter health may be closely linked.
Acknowledgments
We thank the participants in this study. This work was supported by the National Institutes of Health (grant numbers R01MH112180, U01MH108148, R01EB015611, P50MH103222, T32MH067533, K23MH112010, a State of Maryland contract (M00B6400091), and a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation.
Abbreviations
- AL
allostatic load
- DTI
diffusion-tensor imaging
- FA
fractional anisotropy
Footnotes
Conflict of Interest Statement
Dr. Hong has received or is planning to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho Pharmaceutical, Heptares, and Pfizer. All other authors declare no financial interests that could represent a conflict of interest.
References
- 1.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 2.Du F, Cooper AJ, Thida T, Shinn AK, Cohen BM, Ongur D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol Psychiatry. 2008;74:451–457. doi: 10.1016/j.biopsych.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochunov P, Glahn DC, Rowland LM, Olvera RL, Winkler A, Yang YH, Sampath H, Carpenter WT, Duggirala R, Curran J, Blangero J, Hong LE. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry. 2013;73:482–491. doi: 10.1016/j.biopsych.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochunov P, Chiappelli J, Wright SN, Rowland LM, Patel B, Wijtenburg SA, Nugent K, McMahon RP, Carpenter WT, Muellerklein F, Sampath H, Hong LE. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Res. 2014;223:148–156. doi: 10.1016/j.pscychresns.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright SN, Hong LE, Winkler AM, Chiappelli J, Nugent K, Muellerklein F, Du X, Rowland LM, Wang DJ, Kochunov P. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp. 2015;36:3793–804. doi: 10.1002/hbm.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–31. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Harper C, Evans S, Newnham J, Dunlop S. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Dev Neurosci. 2001;19:415–25. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 8.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 9.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacol. 2001;24:420–9. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 10.Schiavone S, Jaquet V, Sorce S, Dubois-Dauphin M, Hultqvist M, Bäckdahl L, Holmdahl R, Colaianna M, Cuomo V, Trabace L, Krause KH. NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl Psychiatry. 2012;2:e111. doi: 10.1038/tp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Friedman JI, Tang C, Carpenter D, Buchsbaum M, Schmeidler J, Flanagan L, Golembo S, Kanellopoulou I, Ng J, Hof PR, Harvey PD, Tsopelas ND, Stewart D, Davis KL. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- 13.Kubicki M, Shenton ME, Maciejewski PK, Pelavin PE, Hawley KJ, Ballinger T, Swisher T, Jabbar GA, Thermenos HW, Keshavan MS, Seidman LJ, Delisi LE. Decreased axial diffusivity within language connections: a possible biomarker of schizophrenia risk. Schizophr Res. 2013;148:67–73. doi: 10.1016/j.schres.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 15.Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–28. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 17.Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 18.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–34. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frodl T, Carballedo A, Fagan AJ, Lisiecka D, Ferguson Y, Meaney JF. Effects of early-life adversity on white matter diffusivity changes in patients at risk for major depression. Journal of Psychiatry and Neuroscience. 2012;37:37. doi: 10.1503/jpn.110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar N, Sibille E. A putative functional role for oligodendrocytes in mood regulation. Transl Psychiatry. 2012;2:e109. doi: 10.1038/tp.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterling P, Eyer J. Allostasis. A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition, and Health. Wiley; Chichester, UK: 1988. pp. 629–649. [Google Scholar]
- 22.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 23.Kyrou I, Chrousos GP, Tsigos C. Stress, Visceral Obesity, and Metabolic Complications. Ann NY Acad Sci. 2006;1083:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 25.Nugent KL, Chiappelli J, Rowland LM, Hong LE. Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology. 2015;60:120–9. doi: 10.1016/j.psyneuen.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen BS. Mood Disorders and allostatic load. Biol Psychiatry. 2003;54:200–7. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 27.McFarlane A. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9:3–10. doi: 10.1002/j.2051-5545.2010.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, Kauer-Sant’anna M, Grassi-Oliveira R, Post RM. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–99. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Vieta E, Popovic D, Rosa AR, Solé B, Grande I, Frey BN, Martinez-Aran A, Sanchez-Moreno J, Balanzá-Martínez V, Tabarés-Seisdedos R, Kapczinski F. The clinical implications of cognitive impairment and allostatic load in bipolar disorder. Eur Psychiatry. 2013;28:21–9. doi: 10.1016/j.eurpsy.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann NY Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 31.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–39. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 32.Nugent KL, Chiappelli J, Sampath H, Rowland LM, Thangavelu K, Davis B, Du X, Muellerklein F, Daughters S, Kochunov P, Hong LE. Cortisol reactivity to stress and its association with white matter integrity in adults with schizophrenia. Psychosom Med. 2015;77:733–42. doi: 10.1097/PSY.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders RC, Aggleton JP. Origin and topography of fibers contributing to the fornix in macaque monkeys. Hippocampus. 2007;17:396–411. doi: 10.1002/hipo.20276. [DOI] [PubMed] [Google Scholar]
- 34.Poletti CE, Creswell G. Fornix system efferent projections in the squirrel monkey: An experimental degeneration study. J Comp Neurol. 1977;175:101–128. doi: 10.1002/cne.901750107. [DOI] [PubMed] [Google Scholar]
- 35.Aggleton JP, Wright NF, Rosene DL, Saunders RC. Complementary patterns of direct amygdala and hippocampal projections to the macaque prefrontal cortex. Cereb Cortex. 2015;25:4351–73. doi: 10.1093/cercor/bhv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelsema AJ, Calaresu FR. Chemical microstimulation of the septal area lowers arterial pressure in the rat. Am J Physiol. 1987;252(4 Pt 2):R760–7. doi: 10.1152/ajpregu.1987.252.4.R760. [DOI] [PubMed] [Google Scholar]
- 37.Bratt AM, Kelley SP, Knowles JP, Barrett J, Davis K, Davis M, Mittleman G. Long term modulation of the HPA axis by the hippocampus. Behavioral, biochemical and immunological endpoints in rats exposed to chronic mild stress. Psychoneuroendocrinology. 2001;26:121–45. doi: 10.1016/s0306-4530(00)00033-0. [DOI] [PubMed] [Google Scholar]
- 38.Booth T, Royle NA, Corley J, Gow AJ, Valdés Hernández Mdel C, Muñoz Maniega S, Ritchie SJ, Bastin ME, Starr JM, Wardlaw JM, Deary IJ. Association of allostatic load with brain structure and cognitive ability in later life. Neurobiol Aging. 2015;36:1390–9. doi: 10.1016/j.neurobiolaging.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.First M, Spitzer R, Williams J, Gibbon M. Biometrics research department. New York State Psychiatric Institute; Structured clinical interview for DSM-IV-patient edition (SCID-P) [Google Scholar]
- 40.Overall JE, Gorham DR. The Brief psychiatric rating scale. Psychological Reports. 1962;10:790–812. [Google Scholar]
- 41.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98:4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Medicine. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 44.The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009 Jul;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kochunov P, Williamson D, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33:9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 49.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 50.Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P. Regional spatial normalization: toward an optimal target. J Comput Assist Tomogr. 2001;25:805–816. doi: 10.1097/00004728-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 51.Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–31. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs E, Flügge G. Stress, glucocorticoids and structural plasticity of the hippocampus. Neurosci Biobehav Rev. 1998;23:295–300. doi: 10.1016/s0149-7634(98)00031-1. [DOI] [PubMed] [Google Scholar]
- 53.Sapolsky R. A possible mechanism for glucocorticoid toxicity in the hippocampus: increased vulnerability of neurons to metabolic insults. J Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20:755–63. doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palumbo ML, Fosser NS, Rios H, Zorrilla Zubilete MA, Guelman LR, Cremaschi GA, Genaro AM. Loss of hippocampal neuronal nitric oxide synthase contributes to the stress-related deficit in learning and memory. J Neurochem. 2007;102:261–74. doi: 10.1111/j.1471-4159.2007.04528.x. [DOI] [PubMed] [Google Scholar]
- 56.Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nisticò R, McEwen BS. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA. 2015;112:14960–5. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goshen I, Kreisel T, Ben-Menachem-Zidon O. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 58.Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1997;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 59.Kirouac GJ, Pittman QJ. Identification of barosensitive neurons in the mediobasal forebrain using juxtacellular labeling. Am J Physiol. 1999;276:R1766–71. doi: 10.1152/ajpregu.1999.276.6.R1766. [DOI] [PubMed] [Google Scholar]
- 60.Tavares RF, de Aguiar Corrêa FM. Pressor effects of L-glutamate injected into the diagonal band of Broca of unanesthetized rats. Brain Res. 2003;959:312–9. doi: 10.1016/s0006-8993(02)03769-1. [DOI] [PubMed] [Google Scholar]
- 61.Crestani CC, Tavares RF, Alves FH, Resstel LB, Correa FM. Diagonal band of Broca modulates the cardiac component of the baroreflex in unanesthetized rats. Neuroscience Letters. 2008;448:189–193. doi: 10.1016/j.neulet.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 62.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 64.Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–95. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- 65.Jauregui-Huerta F, Ruvalcaba-Delgadillo Y, Gonzalez-Castañeda R, Garcia-Estrada J, Gonzalez-Perez O, Luquin S. Responses of glial cells to stress and glucocorticoids. Curr Immunol Rev. 2010;6:195–204. doi: 10.2174/157339510791823790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howell BR, McCormack KM, Grand AP, Sawyer NP, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biol Mood Anxiety Disord. 2013;3:21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Werff SJ, Andela CD, Nienke Pannekoek J, Meijer OC, van Buchem MA, Rombouts SA, van der Mast RC, Biermasz NR, Pereira AM, van der Wee NJ. Widespread reductions of white matter integrity in patients with long-term remission of Cushing’s disease. Neuroimage Clin. 2014;4:659–67. doi: 10.1016/j.nicl.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansson L, Skoog I, Gustafson DR, Olesen PJ, Waern M, Bengtsson C, Björkelund C, Pantoni L, Simoni M, Lissner L, Guo X. Midlife psychological distress associated with late-life brain atrophy and white matter lesions: a 32-year population study of women. Psychosom Med. 2012;74:120–5. doi: 10.1097/PSY.0b013e318246eb10. [DOI] [PubMed] [Google Scholar]
- 69.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, Lieberman JA CAFE Investigators. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 70.See RE, Fido AA, Maurice M, Ibrahim MM, Salama GM. Risperidone-induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Biol Psychiatry. 1999;45:1653–6. doi: 10.1016/s0006-3223(98)00199-1. [DOI] [PubMed] [Google Scholar]