Abstract
Background
Trauma is the leading cause of mortality under the age of 40. Recent observations on metabolic reprogramming during hypoxia and ischemia indicate that hypoxic mitochondrial uncoupling promotes the generation of succinate, which in turn mediates reperfusion injury and inflammatory sequelae upon reoxygenation. Plasma levels of succinate significantly increase in response to trauma and hemorrhage in experimental models and clinical samples, suggesting that succinate may represent a candidate marker of systemic perfusion in trauma.
Methods
Quantitative mass spectrometry-based metabolomics was used to quantify succinate and lactate in 595 plasma samples from severely injured patients enrolled at the Denver Health Medical Center, a level I Trauma Center in Denver, Colorado.
Results
A total of 95 severely injured patients were sampled for up to 10 time points (595 total samples), from field blood to 7 days post injury. Results indicate that plasma levels of succinate increased up to 25.9 fold in deceased patients versus the median of the surviving patients (p=2.75e-100; Receiver Operating Characteristic area under the curve = 0.911). On the other hand, only 2.4 fold changes increases in lactate were observed (p=5.8e-21; AUC:0.874).
Conclusions
Succinate represents a uniquely sensitive biomarker of post–shock metabolic derangement, and may be an important mediator of sequelae.
Keywords: metabolomics, trauma, lactate, succinate, biomarker
Introduction
Trauma is the leading cause of mortality under the age of 40 and hemorrhage is the most preventable cause of death in civilian and military trauma. However, despite tremendous advances in patient transport in the field, survival within the first hour has changed little over the past 40 years. One of the reasons is that clinical measurements to determine inadequate systemic perfusion and, thus, inform optimal resuscitative intervention, remain elusive. For over half a century there has been an interest in identifying a universal biologic mechanism to explain systemic responses to shock.1 Classic studies have elucidated that progressive hemorrhagic shock is associated with a metabolic switch from aerobic to anaerobic metabolism due to inadequate oxygen delivery with subsequent lactic acidemia.1 Over the years, concepts like base deficit and lactate acidosis have been accepted as markers of occult shock.2 Even though elevated lactate levels have been associated with increased mortality in trauma, unmeasured acidic metabolites quantified by the strong anion gap have been reported to have greater predictive value.3 The introduction and use of advanced metabolomics methods has revealed that additional strong acidic metabolites accumulate in response to shock in experimental models and clinical studies.4
These methods have expanded the concept of hypoxic metabolic reprogramming, highlighting a role for mitochondrial dysfunction in hypoxia as a key driver of metabolic reprogramming in cancer,5 ischemia6 and inflammation/immunomodulation.7 We among others have shown that hemorrhagic hypoxemia promotes increases in circulating levels of citric acid metabolites such as succinate4,8 due to oxygen deprivation driving uncoupling of the electron transport chain.9,10 Increased levels of these weak acid metabolites during ischemia facilitate the generation of minimal levels of high energy phosphate compounds through substrate-level phosphorylation reactions to promote mitochondrial survival. However, carboxylic acid reservoirs – specifically succinate – serve as substrate reservoirs upon reperfusion, when they are rapidly cleared, resulting in the concomitant uncontrolled generation of reactive oxygen species (ROS) and subsequent organ damage.6,9 In this view, succinate would represent an attractive molecular marker of shock due to its specific trend towards increase during hypoxia and rapid clearance after successful resuscitation. In addition, our previous experimental models of severe hemorrhage,4 reveal that hemorrhagic shock results in higher plasma succinate levels that occur within 5min from hemorrhage and more rapidly than lactate,4 a trend that is observed in field trauma patients and exacerbated in emergency department-thoracotomy patients.8 In this light, we hypothesize that plasma succinate levels are a better predictor of mortality than lactate in critically injured patients.
Materials and Methods
Severely injured patients were enrolled at the Denver Health Medical Center, a level I Trauma Center in Denver, Colorado within a framework of a large clinical study on time-course metabolomics of critically-ill patients (COMIRB protocol #: 12-1349 - inclusion criteria: age ≥ 18; acutely injured; SBP<70mmHg or SBP 71–90 mmHg with heart rate>108 beat per minute; exclusion criteria: visibly or verbally reported pregnant women, known prisoners, unsalvageable injuries – defined as asystolic or cardiopulmonary resuscitation prior to randomization; known objection to blood products; patient with opt-out bracelet, necklace or wallet card; family member present at the scene objects to patient’s enrollment in research). A total of 595 longitudinal samples (n = 95) were collected either in the field (<30 min from traumatic injury), at the arrival in the emergency department (ED), and at 2, 4, 6, 12, 24, 48, 72 hours, 5 or 7 days from the injury.
Whole blood samples were collected in sodium citrate tubes and immediately centrifuged at 1,000g for 10 min at 4°C and 12,600g for 6 min at 4°C to sort plasma from blood components, prior to storage at −80°C and subsequent extraction for metabolomics analyses. Briefly, 10 μl of plasma were extracted with 240 μl ice cold methanol:acetonitrile: water (5:3:2), containing 5 mM of 13C1-lactate and 1 μM of 13C4-succinate as internal standards, respectively. Samples were vortexed for 30 min at 4°C prior to centrifugation at 10,000 g for 10 min at 4°C. Supernatants were subjected to ultra-high performance liquid chromatography coupled with high resolution mass spectrometry (Vanquish-Q Exactive – Thermo Fisher, Bremen, Germany). Briefly, 20 μl of samples were injected into a Kinetex C18 column (150 x 2.1 mm, 1.7 μM, Phenomenex – Torrance, CA) at 250 μL per min (phase A: water+0.1% formic acid; phase B: acetonitrile+0.1% formic acid). The mass spectrometer scanned in Full MS mode (2 μscans) at 70,000 resolution in the 60–900 m/z range in negative ion mode. Eluate was subjected to electrospray ionization (ESI).4,11 Metabolite assignments were determined as reported.4,11 Absolute quantitation was determined by integrating peak areas of the light and heavy isotopologues for lactate and succinate (matched, in each sample tested), according to the formula:
with a Dilution factorLight of 10 μl in 240μl (= 25 fold dilution). Absolute levels of lactate were determined by first subtracting the naturally occurring 13C1-lactate (3.3% abundance of the parent 12C-lactate) from the observed 13C1-lactate signal.
Technical stability was assessed by confirming <5% coefficient of variations (standard deviation/mean) for measurements of lactate and succinate in technical mixtures (5μl mixes of all tested samples) run every fifteen injections. Extensive description and analytical validation of the method has been recently described.11
Statistical analyses (including T-test of lactate and succinate measurements) were performed through GraphPad Prism 5.0 and Metaboanalyst 3.0.12 Power analysis on preliminary data from our group8 were performed as previously reported12,13, indicating that a sample size of 200 would be sufficient to ensure >80% probability of false discovery rate <0.1 for the observed significant changes on the metabolites of interest. Receiver Operating Characteristic (ROC) curves were calculated as previously reported.13,14
Results and Discussion
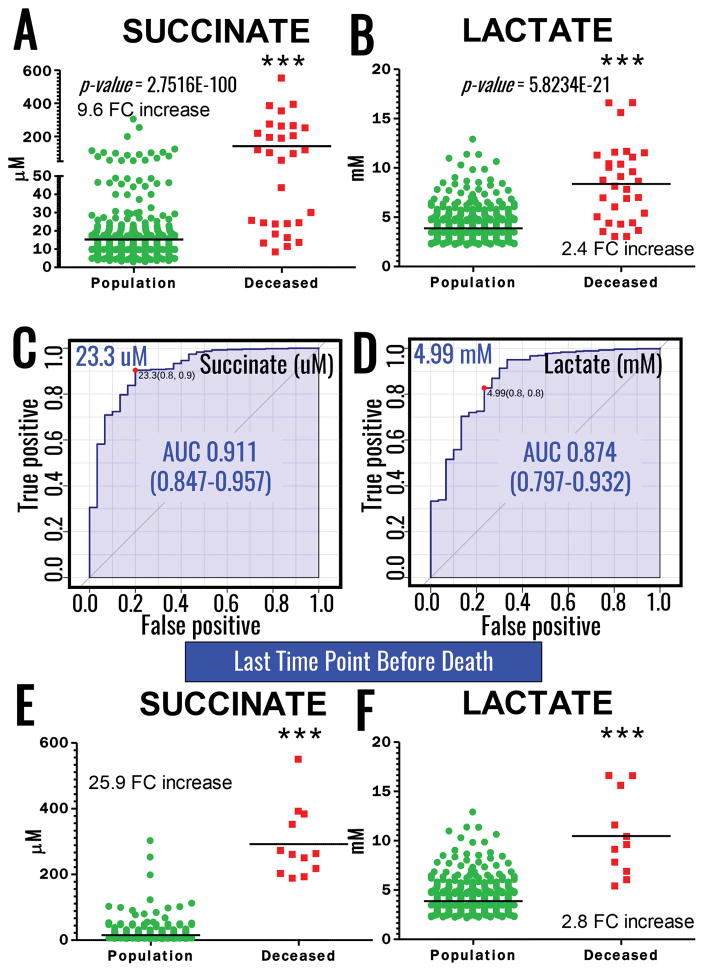
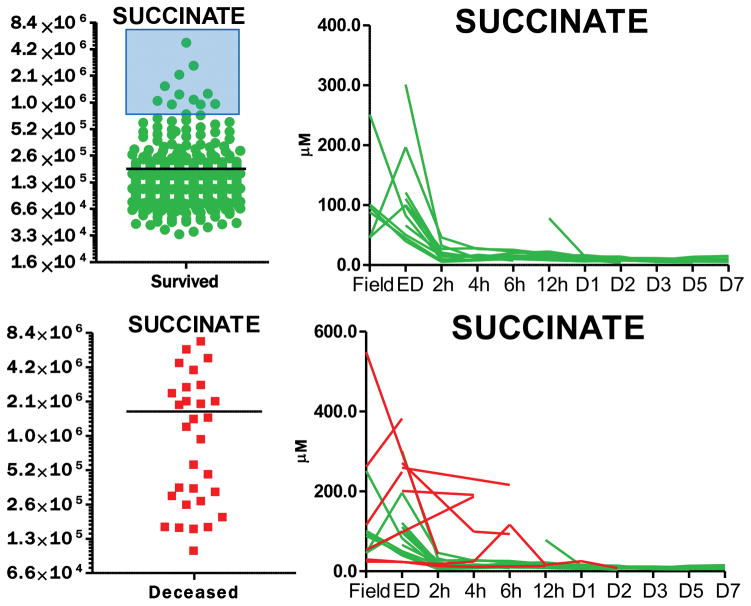
Of a total of 95 patients enrolled in this study, twelve patients died during the study, 9 of which in the ED, and one each within two, twelve or 48h hours from the initial injury. Paired absolute quantitative values for succinate and lactate in each patient at any tested time point are provided in Supplementary Table 1. Median plasma lactate levels in the total population were 3.4 ± 1.6 mM, while the levels significantly increased to a median of 8.3 ± 3.8 mM in the available samples from deceased patients (2.4 fold change increase, p < 0.0001). Similarly, plasma succinate levels increased 9.6 fold (p<0.0001) in deceased patients (96.1 ± 144.2 μM) in comparison to the total population (10.1 ± 22.7 μM – Figure 1. A–B). ROC curves (calculated with Metaboanalyst 3.0)12 indicated increased area under the curve (AUC) for succinate (0.91 for values higher than 23.3 μM) in comparison to lactate (0.87 for values higher than 4.99 mM) as a predictor of mortality (Figure 1. C–D). Owing to the preliminary nature of this study, results would suggest that plasma succinate is at least as good a predictor of mortality as plasma lactate in the civilian critically ill population, though further validation studies are necessary to determine whether the observed increases in AUC of ROC for succinate vs lactate are significant. Of note, normal blood succinate levels in control subjects are 8.8 +/− 2.7 uM (Human Metabolome DataBase - http://www.hmdb.ca/metabolites/HMDB00254), while plasma succinate levels in healthy controls are 3.7 + 1.8 uM (in house measurements – n=20) or in between 1–5 uM, as reported by others.15,16 Combined biomarker analysis did not improve AUC values. Focusing on the last time point available upon resuscitation for all patients vs deceased patients highlighted an even more significant distinction between plasma levels of succinate and lactate between the two groups, both metabolites increasing either 2.8 or 25.9 fold, respectively, in deceased patients at the last available time point vs the equivalent time point in the rest of the patient population (Figure 1. E). String plot visualization of the data further underscores how patients displaying high levels of succinate are significantly more likely to die than patients with high levels of lactate (Figure 1. F). Finally, a subset of patients survived with high initial (field) levels of succinate (>23 μM), when succinate levels were reduced by the second available time point (Figure 2). On the other hand, succinate levels in patients who died where not normalized by early resuscitation and instead increased in four of 8 patients for whom post-field time points were available (Figure 2).
Figure 1.
Quantification curves for succinate (A) and lactate (B), and Receiving Operator Characteristic (ROC, C and D, respectively) determined upon correlation to mortality in a cohort of 95 critically injured patients enrolled at the Denver Health – University of Colorado Hospitals (595 samples were assayed). Plasma succinate and lactate levels higher than 23.3 uM and 4.99 mM were good predictors of mortality in the tested cohort. Quantification of matched succinate (E) and lactate (F) in the last available time point before death or discharge from the hospital. String plot (right panel) shows the correlation between mortality and low/high levels of succinate and lactate (top and bottom, respectively) in the tested cohort. String thickness is proportional to the fold changes vs median value of the tested metabolite across the whole population, clearly indicating significantly higher increases in succinate in patients with poor outcome in comparison to the survivors.
Figure 2. Time course analysis.
reveals that early elevated levels of plasma succinate in a subset of patients (<10) who eventually survived were effectively corrected by early (field or ED) resuscitation. Longitudinal time points were assayed through ultra-high performance liquid chromatography and mass spectrometry, from field blood to emergency department, and at 2, 4, 6, 12, 24, 48, 72, 120 and 168h from the admission (total: 595 samples). A total of 12 patients died, 9 of which in the ED and one each before 2, 12 or 48h from the initial injury.
Significant positive correlations (r= 0.642, p<0.0001) were observed between succinate and lactate levels in all the tested samples (Supplementary Figure 1). In all tested samples from patients who died (total samples available from these patients = 30), succinate and lactate levels were lower than the threshold for mortality calculated here in 4 and 7 cases, respectively. Of note, in the case of succinate the four samples were collected from a patient (no. X-025 in Supplementary Table 1) who died at 12h from hospitalization because of complications from end stage liver disease and not because of trauma with hemorrhage – as gleaned by follow up analyses. This is suggestive of the high specificity of succinate as a marker for death by trauma/hemorrhage. On the other hand, 46 instances were observed where lactate > 5 mM threshold with succinate <23.3 uM and the patients survived. Though further verification and validation in prospective cohorts will be necessary, our results suggest that succinate, a previously recognized marker of hypoxic metabolic reprogramming in cancer,5 ischemia,6 inflammation,7 and immunomodulation9 may represent a valuable early metabolic marker of the severity of shock. Quantitative analytical methods are already available for the high-throughput (<3min) analysis of plasma succinate,4,11 a workflow that can provide simultaneous quantitative measurements of lactate, succinate and theoretically up to >500 metabolites in 10–15 min from sample collection to the result.11 This analytical strategy may become a point of care tool in the ED to potentially inform, in the near future, resuscitative strategies in critically injured patients.
Supplementary Material
Acknowledgments
Funding and AcknowledgmentsAD received funds from the National Blood Foundation and the Boettcher Webb-Waring Early Career Award (2017). This study is supported by the US Army Medical Research Acquisition Act of the Department of Defense under Contract Award Number W81XWH1220028 and the National Institutes of Health (P50 GM049222, T32 GM008315, and UMHL120877). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Disclosure of Conflicts of interest The Authors disclose no conflicts of interest.
The study was approved by the University of Colorado and Denver Health Ethic Committees and the Combined Multi-Institutional Internal Review Board (COMIRB protocol #: 12-1349).
Level of evidence: Prognostic study, Level III
Authors’ contributions
AD, JAR, MJW performed metabolomics analysis. AD prepared figures. AD wrote the first draft of the manuscript. HBM, AG, JC contributed to sample collection. EEM, AB, CCS and KCH designed and supervised the study. All the authors critically contributed to the finalization of the manuscript.
References
- 1.Cuthbertson D. Post-shock metabolic response. The Lancet. 1942;239(6189):433–437. [Google Scholar]
- 2.Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185(5):485–491. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32(5):1120–1124. doi: 10.1097/01.ccm.0000125517.28517.74. [DOI] [PubMed] [Google Scholar]
- 4.D’Alessandro A, Moore HB, Moore EE, Wither M, Nemkov T, Gonzalez E, Slaughter A, Fragoso M, Hansen KC, Silliman CC, et al. Early hemorrhage triggers metabolic responses that build up during prolonged shock. Am J Physiol Regul Integr Comp Physiol. 2015;308(12):R1034–1044. doi: 10.1152/ajpregu.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4(6):437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 6.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peltz ED, D’Alessandro A, Moore EE, Chin T, Silliman CC, Sauaia A, Hansen KC, Banerjee A. Pathologic metabolism: an exploratory study of the plasma metabolome of critical injury. J Trauma Acute Care Surg. 2015;78(4):742–751. doi: 10.1097/TA.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. 2016;1857(8):1086–1101. doi: 10.1016/j.bbabio.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter AL, D’Alessandro A, Moore EE, Banerjee A, Silliman CC, Hansen KC, Reisz JA, Fragoso M, Wither MJ, Bacon AW, et al. Glutamine metabolism drives succinate accumulation in plasma and the lung during hemorrhagic shock. J Trauma Acute Care Surg. 2016;81(6):1012–1019. doi: 10.1097/TA.0000000000001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemkov T, Hansen KC, D’Alessandro A. A Three-Minute Method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31(8):663–673. doi: 10.1002/rcm.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr Protoc Bioinformatics. 2016;55:14.10.1–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57(2):325–336. doi: 10.1111/trf.13892. [DOI] [PubMed] [Google Scholar]
- 14.Paglia G, D’Alessandro A, Rolfsson Ó, Sigurjónsson ÓE, Bordbar A, Palsson S, Nemkov T, Hansen KC, Gudmundsson S, Palsson BO. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128(13):e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 15.Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y, Tones MA. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens. 2007;20(11):1209–1215. doi: 10.1016/j.amjhyper.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts WL. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem. 2001;47(11):1993–2002. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.