Abstract
Oversensitivity to uncertain future threat is usefully conceptualized as intolerance of uncertainty (IU). Neuroimaging studies of IU to date have largely focused on its relationship with brain function, but few studies have documented the association between IU and the quantitative properties of brain structure. Here, we examined potential gray and white matter brain structural correlates of IU from 61 healthy participants. Voxel-based morphometric analysis highlighted a robust positive correlation between IU and striatal volume, particularly the putamen. Conversely, tract-based spatial statistical analysis showed no evidence for a relationship between IU and the structural integrity of white matter fiber tracts. Current results converge upon findings from individuals with anxiety disorders such as obsessive-compulsive disorder (OCD) or generalized anxiety disorder (GAD), where abnormally increased IU and striatal volume are consistently reported. They also converge with neurobehavioral data implicating the putamen in predictive coding. Most notably, the relationship between IU and striatal volume is observed at a preclinical level, suggesting that the volumetric properties of the striatum reflect the processing of uncertainty per se as it relates to this dimensional personality characteristic – such a relationship could then potentially contribute to the onset of OCD or GAD, rather than being unique to their pathophysiology.
Keywords: intolerance of uncertainty, striatum, putamen, anxiety, MRI
Uncertainty and ambiguity of potential future threat is central to understanding the generation of anxiety (Grupe & Nitschke, 2013). Neurobiological theories of anxiety traditionally posit several brain regions that constitute the threat processing circuit (LeDoux, 2000) as playing a key role – for example, the amygdala, the medial prefrontal cortex, and the bed nucleus of the stria terminalis. Theoretically and empirically, the neurobiological basis of pathological anxiety has been understood as the structural and functional aberration of these brain regions and circuitries (Kim et al., 2011; Milad & Quirk, 2012). Recently, the striatum has been proposed to serve a role in anxiety alongside the aforementioned brain regions (Lago, Davis, Grillon & Ernst, 2016).
The intolerance of uncertainty is a psychological construct related to anxiety that focuses on how one responds to the uncertainty associated with the potential occurrence of future threat. As per its definition, individuals high in IU display difficulty accepting the possibility of potential negative events in the future (Buhr & Dugas, 2002). Behaviorally, high IU is associated with exaggerated threat generalization (Morriss, Macdonald & van Reekum, 2016) and post-extinction recovery (Dunsmoor, Campese, Ceceli, LeDoux & Phelps, 2015) following fear extinction training in humans. In terms of brain function, increased activity of the bilateral insula during affective ambiguity (Simmons, Matthews, Paulus & Stein, 2008), as well as increased activity of the amygdala in response to cues that predict uncertain outcomes (Schienle, Köchel, Ebner, Reishofer & Schäfer, 2010) was reported for individuals with elevated IU. A recent study by Morriss and colleagues (2015) demonstrated that individuals with high IU displayed sustained activity in the amygdala and the ventromedial prefrontal cortex during the late stages of fear extinction, representing a disproportionate amount of fear expression towards learned threat.
While much of the neuroimaging research on IU has been primarily focused on brain function, brain structural correlates of IU have received little attention. Through the use of structural and diffusion magnetic resonance imaging (MRI) techniques, brain structure can be evaluated in vivo in a number of ways, including the volume of gray and white matter tissue, as well as the structural integrity of white matter tracts. The purpose of this exploratory study was to utilize these methods to investigate potential structural correlates of IU across the whole brain, focusing on psychiatrically healthy individuals. The outcomes of the current study contribute to a clearer understanding of the relationship between IU – a dimensional personality characteristic – and the structural characteristics of the brain in healthy individuals, to better inform future studies that might seek to assess the relationship of IU in individuals with psychopathology.
Method
Participants
Sixty-one healthy Dartmouth College undergraduate students volunteered to participate in the current study (36 females; ages 18–26 years, mean age = 19.1 years). All participants were screened for past or current psychiatric illnesses (Axis I or II) using the Structured Clinical Interview for DSM-IV. No participants had any history of taking psychotropic medications. The study protocol was approved by the Committee for the Protection of Human Subjects at Dartmouth College. Written, informed consent was obtained from the participants prior to the experiment.
Self-Reported Measures of Anxiety
The Intolerance of Uncertainty Scale (Buhr & Dugas, 2002) was used to assess the degree of IU for each participant. In order to isolate the effects of IU from general anxiety, the State Trait Anxiety Inventory Form Y-2 (STAI-T) was used to measure self-reported levels of trait anxiety (Spielberger, Gorsuch & Lushene, 1988).
Image Acquisition
All MRI data were collected at the Dartmouth Brain Imaging Center using the 3.0 Tesla Philips Intera Achieva Scanner (Philips Medical Systems), equipped with a 32-channel head coil. Each participant’s high-resolution anatomical T1-weighted images were scanned using a magnetization-prepared rapid gradient echo sequence (MPRAGE), with 220 sagittal slices (TE = 3.7 ms, TR = 8.2 ms, FOV = 240 mm, flip angle = 8°, voxel size = 0.9375×0.9375×1 mm). Diffusion-weighted images were acquired using sensitivity encoding (SENSE) parallel imaging with reduction factor of 2 to reduce scan time and geometric distortion in the diffusion-weighted images acquired by the fast MRI pulse sequence, single-shot spin-echo echo planar imaging. Diffusion-weighted images were collected with 60 contiguous 2 mm thick axial slices and 61 noncollinear diffusion gradients (TE = 85 ms, TR = 4008 ms, b-value = 1000 s/mm2, FOV = 224 mm, flip angle = 90°, voxel size = 2×2×2 mm). Diffusion-weighted images from five participants were removed from further analyses due to image quality issues, yielding a total of 56 participants (34 females; ages 18–22 years, mean age = 19 years) with both T1- and diffusion-weighted images.
Voxel-Based Morphometry
T1-weighted images from all 61 participants were submitted to a voxel-based morphometry (VBM; Good et al., 2001) data analysis pipeline, using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) running on the Statistical Parametric Mapping software (SPM8; Wellcome Trust Center for Neuroimaging, London, UK). Images were first segmented into gray matter, white matter, and cerebrospinal fluid. Next, the segmented tissue images were spatially aligned into standard Montreal Neurological Institute (MNI) space (1.5×1.5×1.5 mm) using the diffeomorphic anatomical registration through exponentiated Lie algebra (DARTEL) algorithm implemented in SPM8. Then, in order to acquire volume information for each voxel, gray and white matter images were modulated with the Jacobian determinants derived from the nonlinear spatial alignment procedure. Finally, these modulated gray and white matter images were smoothed using a Gaussian kernel (full-width-half-maximum = 8 mm).
To assess the correlations between IU and gray/white matter volume on a voxel-by-voxel basis, a general linear model (GLM) was constructed at the group level. In the GLM, IU scores were entered as an independent variable, while intracranial volume (ICV), STAI-T, age, and sex were included as covariates of no interest. ICV was estimated by summing the volumes of the gray matter, white matter, and cerebrospinal fluid images derived from the segmentation step. Given the strong correlational nature between IU and STAI-T (r(59) = .466, p = .0002), we decided to isolate regional brain volumes that were correlated with the variance associated with IU that is not shared with trait anxiety. We imposed a significance threshold of p < .05 family-wise error (FWE) corrected for multiple comparisons over the gray and white matter volume across the whole brain, as determined by Monte Carlo simulations (n = 10000) implemented in 3dClustSim (compiled in Jan 2016) within AFNI software (Cox, 1996). The FWE-corrected p < 0.05 threshold was achieved using an uncorrected p < .001, k ≥ 553 voxels (1866 mm3).
Tract-Based Spatial Statistics
Diffusion-weighted images from 56 participants were preprocessed using FSL’s Diffusion Toolbox (FDT; Behrens et al., 2003; Smith et al., 2004), following standard procedures that consist of brain extraction/skull-stripping, and correcting for eddy current/head motion. Head motion was corrected by aligning all diffusion-weighted images to the non-diffusion-weighted (b0) image using affine registration (Jenkinson et al., 2002). The diffusion tensor model was fitted to the data in order to produce individual fractional anisotropy (FA) maps for each participant. FA maps were subjected to a tract-based spatial statistics (TBSS; Smith et al., 2006) procedure – a voxelwise statistical analysis method tailored specifically for diffusion data by incorporating nonlinear registration algorithms and eliminating the need for an arbitrary smoothing extent on the images. In TBSS, individual FA maps were first spatially aligned into a standard FA template in MNI space (1×1×1 mm) using nonlinear registration. Then, a group-mean FA map, averaged across all 56 participants, was generated and subsequently thinned at a threshold of FA > 0.2 to extract the “mean FA skeleton”, which is assumed to represent the center of the white matter fiber tracts common to the group. Next, individual FA maps were projected onto the mean FA skeleton, searching for maximal FA values perpendicular to the skeleton. The resulting data were each individual’s FA skeletons, ready to be submitted to a group-level voxelwise statistical analysis.
In order to control for the potential effects of head motion, volume-by-volume head motion was quantified by calculating the root mean square (RMS) deviation of the six motion parameters (three translation and three rotation components), which were derived from the affine registration between each volume to the b0 image, for each pair of consecutive diffusion-weighted brain volumes. The resulting volume-by-volume RMS deviation values were averaged across all images, yielding a summary statistic of head motion for each participant.
For group-level analysis, a GLM was constructed with the IU scores as the main independent variable, and STAI-T, age, sex, and head motion as covariates of no interest. Again, given the strong correlational nature between IU and STAI-T (r(54)= .458, p = .0004), our goal was to isolate the association between white matter tract integrity and IU that could not be explained by trait anxiety. Nonparametric permutation tests (n=10000) were performed to determine FWE-corrected p < .05, using the threshold-free cluster enhancement method implemented in randomise within FSL.
Results
Intolerance of Uncertainty and Striatal Volume
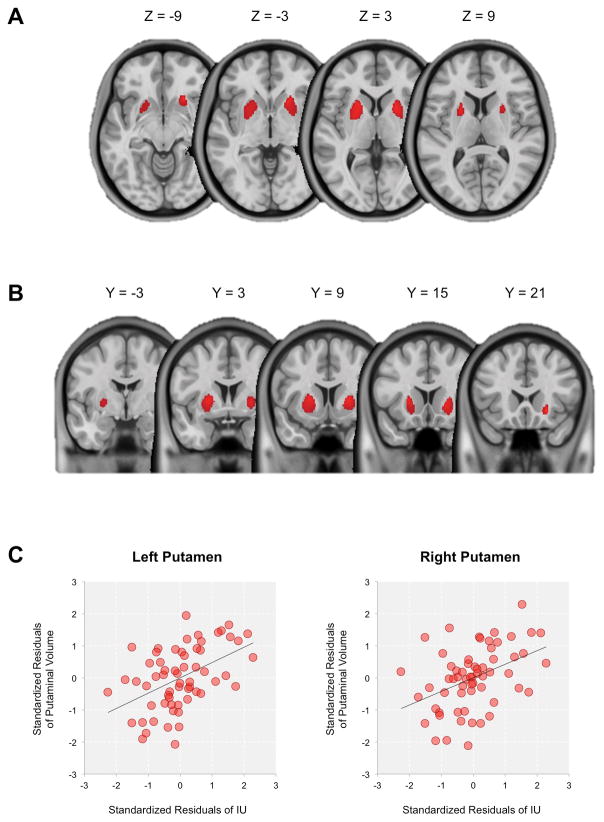
Individual differences in IU scores were significantly positively correlated with the gray matter volume of the bilateral striatum, mapping primarily onto the putamen and to a lesser extent the pallidum (Left: MNI −21, 12, 6, t(55) = 4.54, k = 1073 voxels; Right: MNI 22, 21, −8; t(55) = 4.75, k = 874 voxels; both ps < .05, FWE-corrected; Figure 1). This effect was not explained by trait anxiety, ICV, age, or sex. No other gray or white matter volumes across the whole brain were significantly correlated with IU.
Figure 1.
Whole brain VBM results (FWE-corrected p < .05) depicted on (A) axial and (B) coronal slices of the brain that showed positive correlation with IU scores. (C) Scatterplots displaying correlations between the bilateral putaminal volume and IU scores (represented as standardized residuals after controlling for the effects of ICV, trait anxiety, age, and sex). An anatomical mask of the bilateral putamen from the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) was used to extract average putaminal gray matter volume. We note that scatterplots were presented for visual purposes only. VBM: voxel-based morphometry; IU: intolerance of uncertainty; ICV: intracranial volume.
Due to the potential shortcomings of using residuals from linear regressions in subsequent statistical tests (Freckleton, 2002), additional post hoc analyses were performed on the current data. To avoid using residuals as data, a hierarchical regression analysis was used to test for the relationship between IU scores and striatal volume, while accounting for the effects of age, sex, ICV, and trait anxiety in the model. For this supplemental analysis, putaminal volume was extracted using an anatomical mask (Automated Anatomical Labeling atlas: Tzourio-Mazoyer et al., 2002). Consistent with the initial results, hierarchical regression confirmed that adding IU scores in the second step significantly improved the model from the first step that included age, sex, ICV, and trait anxiety, for the bilateral putamen: left putamen, first step: R2 = .217, 95% confidence interval [CI] [.02, .35], F(4, 56) = 3.883, p = .007, second step: ΔR2 = .179, 95% CI [.06, .40], ΔF(1, 55) = 16.267, p = .00017; right putamen, first step: R2 = .2, 95% CI [.01, .33], F(4, 56) = 3.504, p = .013, second step: ΔR2 = .144, 95% CI [.03, .35], ΔF(1, 55) = 12.03, p = .001.
Intolerance of Uncertainty and the Structural Integrity of White Matter Fiber Tracts
No significant correlations between IU and any white matter tract integrity, as indexed by fractional anisotropy, were observed. We confirmed that all participants displayed an acceptable degree of head motion (average range: .2–.58 mm), and it did not correlate with IU or STAI-T scores.
Discussion
Here we demonstrate that across the whole brain, the volume of the striatum, particularly the putamen, was positively correlated with individual differences in the intolerance of uncertainty. We found no evidence for other gray matter structures or white matter tissue to be associated with IU. The present results extend the findings from a recent large-scale meta-analysis of functional neuroimaging studies that suggest the striatum is involved in a wide range of psychological processes (Pauli et al., 2016). Importantly, our data argue that structural alterations of the striatum are linked to normal variations in a dimensional personality characteristic (i.e., IU) that has ties with certain anxiety disorders (OCD, GAD), rather than the pathophysiology of these disorders per se.
The current data can be better understood by considering respective findings from the OCD and GAD literature. Individuals affected by either OCD or GAD commonly display symptoms that are marked by uncontrollable repetitive thoughts and actions (i.e., worrying/obsessing), mainly pertaining to negative emotions (Comer, Kendall, Franklin, Hudson & Pimentel, 2004). It follows then that excessive IU has been associated with the psychopathology of both OCD and GAD (see Grupe & Nitschke, 2013 for review).
Previous structural brain imaging studies examining gray matter volumes in patients with OCD and GAD have consistently found increased volume in the striatum, in particular the putamen (Hilbert et al., 2015; Radua, van den Heuvel, Surguladze & Mataix-Cols, 2010), implying a promising neural candidate for a shared neural basis common to both psychiatric disorders. Of particular relevance to the current study, Hilbert and colleagues (2015) reported that increased striatal volumes not only differentiated healthy individuals from those with GAD at a categorical level, but also on a dimensional level, as self-reported measure of IU positively correlated with putaminal volume when both groups were pooled into a single study sample. Our findings contribute to this work by demonstrating that increased striatal volumes as a function of elevated IU can be observed within healthy individuals. Thus, a diagnosis of OCD or GAD is not a prerequisite for an enlarged striatum. Rather, variations in IU within the normal range are sufficient to detect an increase in the volume of the striatum. In light of this, the current data can be interpreted such that increased striatal volumes, which are commonly found in OCD and GAD, may originate from a variation in a dimensional personality characteristic – elevated IU – and not necessarily a neural outcome or a byproduct of the disorders. We note that the current findings were derived from young adults in their late teens or early twenties. Since the neurodevelopment of the striatum is known to extend into young adulthood (Wierenga et al., 2014), interpretation of the present results should be made within this context.
Reports of additional brain alterations observed in patient groups (Radua et al., 2010) lend themselves to the interesting speculation that the striatal alterations might be specific to IU, with the additional pathology necessary to observe the diagnosable condition. A whole brain inspection of white matter tissue, including the volume and the structural integrity of fiber tracts, did not reveal any significant associations with IU. Considering the abnormalities of various white matter tracts in OCD (see Koch, Reess, Rus, Zimmer & Zaudig, 2014 for review) and frontolimbic pathways in GAD patients (Tromp et al., 2012), alterations in white matter tissue, unlike striatal volume, may be more directly linked to the pathophysiology of the disorders, and less related to normal variations in IU.
Further insight into the presently observed IU-striatum relationship is provided by the nonhuman animal literature. Neurophysiological studies have demonstrated that the striatum is sensitive to the predictability of reward outcomes during learning tasks (Tremblay, Hollerman & Schultz, 1998). To put it another way, the striatum encodes how predictable and expected a reward is – a higher form of reward processing compared to simply responding to a reward (Schultz, Tremblay & Hollerman, 2000). Given that an important component of IU is a desire for predictability (Berenbaum, Bredemeier & Thompson, 2008), our findings offer a neuroanatomical link related to a need for structure, that will in turn decrease anxiety related to the anticipation and prediction of environmental outcomes.
Acknowledgments
This work was supported by the National Institute of Mental Health under Grant R01 MH080716 to Paul J. Whalen.
References
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, … Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance Medicine. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Bredemeier K, Thompson RJ. Intolerance of uncertainty: Exploring its dimensionality and associations with need for cognitive closure, psychopathology, and personality. Journal of Anxiety Disorders. 2008;22:117–125. doi: 10.1016/j.janxdis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Buhr K, Dugas MJ. The intolerance of uncertainty scale: psychometric properties of the English version. Behavioral Research and Therapy. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Comer JS, Kendall PC, Franklin ME, Hudson JL, Pimentel SS. Obsessing/worrying about the overlap between obsessive-compulsive disorder and generalized anxiety disorder in youth. Clinical Psychology Review. 2004;24:663–683. doi: 10.1016/j.cpr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Campese VD, Ceceli AO, LeDoux JE, Phelps EA. Novelty-facilitated extinction: Providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biological Psychiatry. 2015;78:203–209. doi: 10.1016/j.biopsych.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freckleton RP. On the misuse of residuals in ecology: regression of residuals vs. multiple regression. Journal of Animal Ecology. 2002;71:542–545. [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert K, Pine DS, Muehlhan M, Lueken U, Steudte-Schmiedgen S, Beesdo-Baum K. Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization. Psychiatry Research: Neuroimaging. 2015;234:314–320. doi: 10.1016/j.pscychresns.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AM, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Reess TJ, Rus OG, Zimmer C, Zaudig M. Diffusion tensor imaging (DTI) studies in patients with obsessive-compulsive disorder (OCD): a review. Journal of Psychiatric Research. 2014;54:26–35. doi: 10.1016/j.jpsychires.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Lago T, Davis A, Grillon C, Ernst M. Striatum on the anxiety map: Small detours into adolescents. Brain Research. 2016 doi: 10.1016/j.brainres.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss J, Christakou A, van Reekum CM. Intolerance of uncertainty predicts fear extinction in amygdala-ventromedial prefrontal cortical circuitry. Biology of Mood and Anxiety Disorders. 2015;5:4. doi: 10.1186/s13587-015-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss J, Macdonald B, van Reekum CM. What is going on around here? Intolerance of uncertainty predicts threat generalization. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, O’Reilly RC, Yarkoni T, Wager TD. Regional specialization within the human striatum for diverse psychological functions. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:1907–1912. doi: 10.1073/pnas.1507610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs. other anxiety disorders. Archives of General Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- Schienle A, Köchel A, Ebner F, Reishofer G, Schäfer A. Neural correlates of intolerance of uncertainty. Neuroscience Letters. 2010;479:272–276. doi: 10.1016/j.neulet.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neuroscience Letters. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, … Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, … Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI-Manual for the State Trait Anxiety Inventory. 3. Palo Alto, CA: Consulting Psychologists Press; 1988. [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. Journal of Neurophysiology. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- Tromp DPM, Grupe DW, Oathes DJ, McFarlin DR, Hernandez PJ, Kral TR, … Nitschke JB. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Archives of General Psychiatry. 2012;69:925–934. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Human Brain Mapping. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]