Abstract
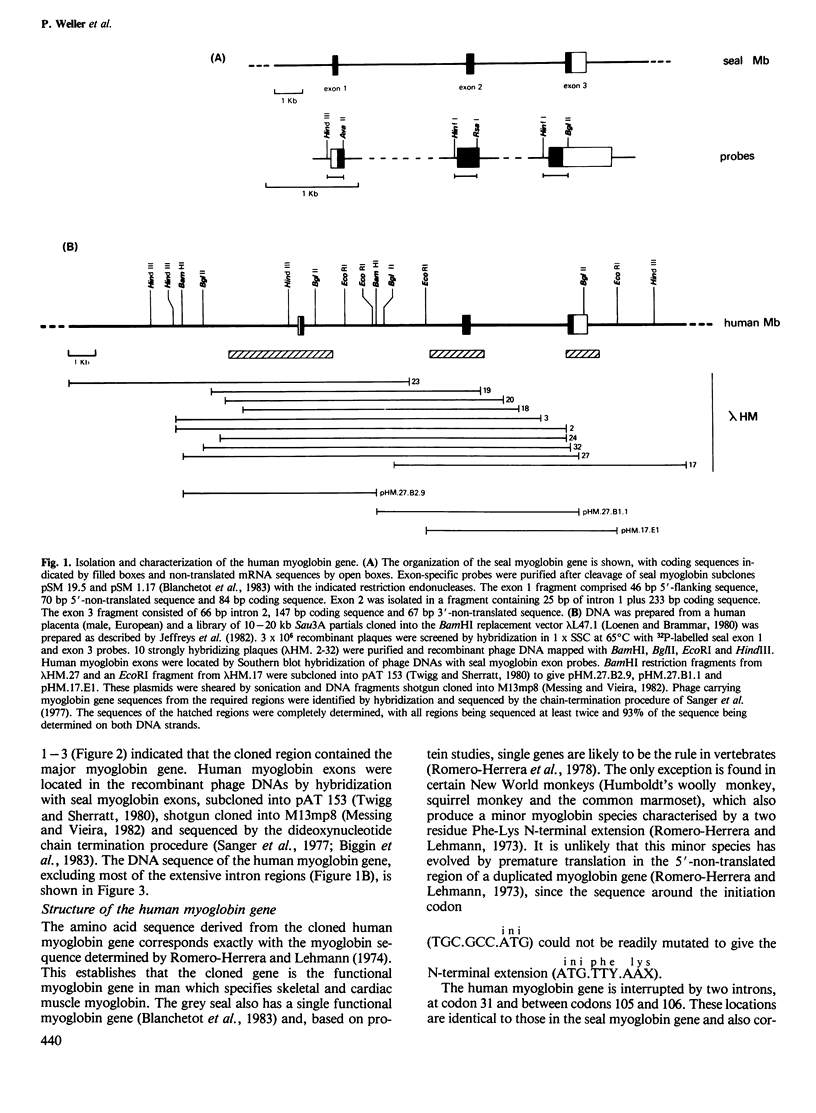
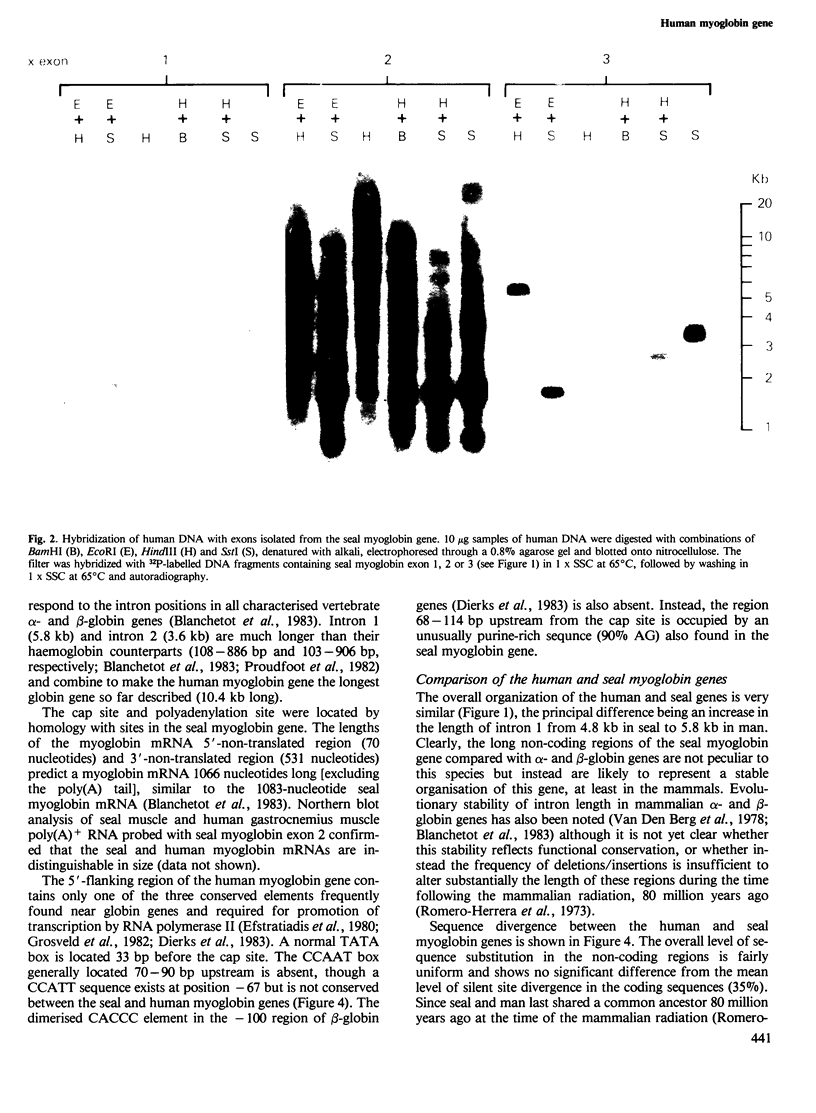
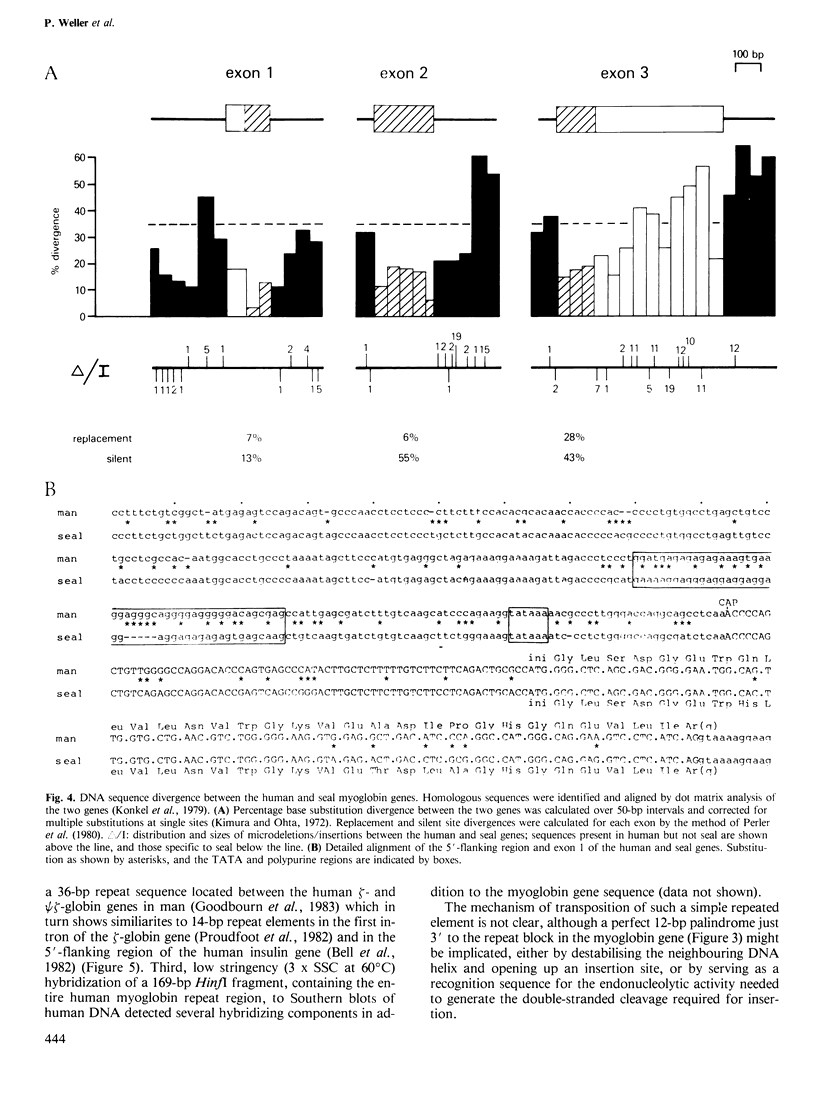
Cross-hybridization of the grey seal myoglobin gene to human DNA detected a single human myoglobin gene plus an extensive family of sequences apparently related to the central exon of this gene. The functional human gene is 10.4 kb long and has a haemoglobin-like three exon/two intron structure with long non-coding regions similar to its seal homologue. At least 300 bp of 5'-flanking region are closely homologous between the two genes, with the exception of a divergent purine-rich region 68-114 bp upstream of the cap site. A diverged tandem repetitive sequence based on (GGAT)165 is located 1100-1750 bp upstream from the gene; internal homology units within this sequence suggest sequence homogenization by gene microconversions. A second 33-bp tandem repeat element in the first intron is flanked by a 9-bp direct repeat, shares homology with other tandem repetitive elements in the human genome and may represent a novel form of transposable element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Selby M. J., Rutter W. J. The highly polymorphic region near the human insulin gene is composed of simple tandemly repeating sequences. Nature. 1982 Jan 7;295(5844):31–35. doi: 10.1038/295031a0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot A., Wilson V., Wood D., Jeffreys A. J. The seal myoglobin gene: an unusually long globin gene. Nature. 1983 Feb 24;301(5902):732–734. doi: 10.1038/301732a0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Jones D. R. The comparative physiology of diving in vertebrates. Adv Comp Physiol Biochem. 1982;8:179–364. doi: 10.1016/b978-0-12-011508-2.50012-5. [DOI] [PubMed] [Google Scholar]
- Czelusniak J., Goodman M., Hewett-Emmett D., Weiss M. L., Venta P. J., Tashian R. E. Phylogenetic origins and adaptive evolution of avian and mammalian haemoglobin genes. Nature. 1982 Jul 15;298(5871):297–300. doi: 10.1038/298297a0. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S. E., Higgs D. R., Clegg J. B., Weatherall D. J. Molecular basis of length polymorphism in the human zeta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5022–5026. doi: 10.1073/pnas.80.16.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Hayashida H., Miyata T. Unusual evolutionary conservation and frequent DNA segment exchange in class I genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 May;80(9):2671–2675. doi: 10.1073/pnas.80.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. E.R., Lehmann H. N-terminal chain elongation as evidence for duplication of myoglobin in three South American Monkeys. FEBS Lett. 1973 Apr 15;31(2):175–180. doi: 10.1016/0014-5793(73)80097-3. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Barrie P. A., Harris S., Fawcett D. H., Nugent Z. J., Boyd A. C. Isolation and sequence analysis of a hybrid delta-globin pseudogene from the brown lemur. J Mol Biol. 1982 Apr 15;156(3):487–503. doi: 10.1016/0022-2836(82)90262-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Harris S. Processes of gene duplication. Nature. 1982 Mar 4;296(5852):9–10. doi: 10.1038/296009a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Wood D., Simons J. P., Kay R. M., Williams J. G. Linkage of adult alpha- and beta-globin genes in X. laevis and gene duplication by tetraploidization. Cell. 1980 Sep;21(2):555–564. doi: 10.1016/0092-8674(80)90493-6. [DOI] [PubMed] [Google Scholar]
- Kimura M., Ota T. On the stochastic model for estimation of mutational distance between homologous proteins. J Mol Evol. 1972 Dec 29;2(1):87–90. doi: 10.1007/BF01653945. [DOI] [PubMed] [Google Scholar]
- Kimura M. Was globin evolution very rapid in its early stages?: a dubious case against the rate-constancy hypothesis. J Mol Evol. 1981;17(2):110–113. doi: 10.1007/BF01732682. [DOI] [PubMed] [Google Scholar]
- Kohne D. E. Evolution of higher-organism DNA. Q Rev Biophys. 1970 Aug;3(3):327–375. doi: 10.1017/s0033583500004765. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Kreitman M. Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature. 1983 Aug 4;304(5925):412–417. doi: 10.1038/304412a0. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Longo L. D., Koos B. J., Power G. G. Fetal myoglobin: quantitative determination and importance for oxygenation. Am J Physiol. 1973 May;224(5):1032–1036. doi: 10.1152/ajplegacy.1973.224.5.1032. [DOI] [PubMed] [Google Scholar]
- Maeda N., Bliska J. B., Smithies O. Recombination and balanced chromosome polymorphism suggested by DNA sequences 5' to the human delta-globin gene. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5012–5016. doi: 10.1073/pnas.80.16.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. L., Zimmer E. A., Davidson W. S., Wilson A. C., Kan Y. W. The untranslated regions of beta-globin mRNA evolve at a functional rate in higher primates. Cell. 1981 Sep;25(3):737–741. doi: 10.1016/0092-8674(81)90181-1. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Flavell R. A. The DNA sequence of the 5' flanking region of the human beta-globin gene: evolutionary conservation and polymorphic differences. Nucleic Acids Res. 1982 Mar 25;10(6):2109–2120. doi: 10.1093/nar/10.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Genetic recombination: strand transfer and mismatch repair. Annu Rev Biochem. 1978;47:847–880. doi: 10.1146/annurev.bi.47.070178.004215. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. Molecular evolution of myoglobin and the fossil record: a phylogenetic synthesis. Nature. 1973 Dec 14;246(5433):389–395. doi: 10.1038/246389a0. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. On the evolution of myoglobin. Philos Trans R Soc Lond B Biol Sci. 1978 May 9;283(995):61–163. doi: 10.1098/rstb.1978.0018. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H. The amino acid sequence of human myoglobin and its minor fractions. Proc R Soc Lond B Biol Sci. 1974 Jul 9;186(1084):249–279. doi: 10.1098/rspb.1974.0048. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Yoshida K., Ren C. S., Fujinaga K., Rajagopalan S., Chinnadurai G. Hinf family: a novel repeated DNA family of the human genome. Nature. 1983 Apr 14;302(5909):587–590. doi: 10.1038/302587a0. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipler T. D., Edwards Y. H., Hopkinson D. A. Developmental changes in the protein profiles of human cardiac and skeletal muscle. Ann Hum Genet. 1978 May;41(4):409–418. doi: 10.1111/j.1469-1809.1978.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Wilson V., Jeffreys A. J., Barrie P. A., Boseley P. G., Slocombe P. M., Easton A., Burke D. C. A comparison of vertebrate interferon gene families detected by hybridization with human interferon DNA. J Mol Biol. 1983 Jun 5;166(4):457–475. doi: 10.1016/s0022-2836(83)80281-2. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B. Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol Rev. 1970 Oct;50(4):559–636. doi: 10.1152/physrev.1970.50.4.559. [DOI] [PubMed] [Google Scholar]
- Wood D., Blanchetot A., Jeffreys A. J. Molecular cloning of seal myoglobin mRNA. Nucleic Acids Res. 1982 Nov 25;10(22):7133–7144. doi: 10.1093/nar/10.22.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg J., van Ooyen A., Mantei N., Schamböck A., Grosveld G., Flavell R. A., Weissmann C. Comparison of cloned rabbit and mouse beta-globin genes showing strong evolutionary divergence of two homologous pairs of introns. Nature. 1978 Nov 2;276(5683):37–44. doi: 10.1038/276037a0. [DOI] [PubMed] [Google Scholar]



