Summary
Background
Influenza immunisation during pregnancy is recommended but not widely implemented in some low-income regions. We assessed the safety and efficacy in mothers and infants of year-round maternal influenza immunisation in Nepal, where influenza viruses circulate throughout the year.
Methods
In this phase 4, randomised, placebo-controlled trial, we enrolled two consecutive sequential annual cohorts of pregnant women from the Sarlahi district in southern Nepal. We randomised mothers 1:1 to receive seasonally recommended trivalent inactivated influenza vaccine or saline placebo in blocks of eight, stratified by gestational age at enrolment (17–25 weeks vs 26–34 weeks). Women were eligible if they were married, 15–40 years of age, 17–34 weeks’ gestation at enrolment, and had not previously received any influenza vaccine that season. We collected serum samples before and after immunisation, and cord blood from a subset of women and infants. Staff masked to allocation made home visits every week from enrolment to 6 months after delivery. Midnasal swabs for respiratory virus PCR testing were collected during maternal acute febrile respiratory infections, and from infants with any respiratory symptom. We assessed vaccine immunogenicity, safety, and three primary outcomes: the incidence of maternal influenza-like illness in pregnancy and 0–180 days postpartum, the incidence of low birthweight (<2500 g), and the incidence of laboratory-confirmed infant influenza disease from 0 to 180 days. This trial is registered with ClinicalTrials.gov, number NCT01034254.
Findings
From April 25, 2011, to Sept 9, 2013, we enrolled 3693 women in two cohorts of 2090 (1041 assigned to placebo and 1049 to vaccine) and 1603 (805 assigned to placebo and 798 to vaccine), with 3646 liveborn infants (cohort 1, 999 in placebo group and 1010 in vaccine group; cohort 2, 805 in placebo group and 798 in vaccine group). Immunisation reduced maternal febrile influenza-like illness with an overall efficacy of 19% (95% CI 1 to 34) in the combined cohorts; 9% efficacy (−16 to 29) in the first cohort, and 36% efficacy (9 to 55) in the second cohort. For laboratory-confirmed influenza infections in infants aged 0–6 months, immunisation had an overall efficacy for the combined cohorts of 30% (95% CI 5 to 48); in the first cohort, the efficacy was 16% (−19 to 41), and in the second cohort it was 60% (26 to 88). Maternal immunisation reduced the rates of low birthweight by 15% (95% CI 3–25) in both cohorts combined. The rate of small for gestational age infants was not modified by immunisation. The number of adverse events was similar regardless of immunisation status. Miscarriage occurred in three (0·2%) participants in the placebo group versus five (0·3%) in the vaccine group, stillbirth occurred in 31 (1·7%) versus 33 (1·8%), and congenital defects occurred in 18 (1·0%) versus 20 (1·1%). Five women died in the placebo group and three died in the vaccine group. The number of infant deaths at age 0–6 months was similar in each group (50 in the placebo group and 61 in the vaccine group). No serious adverse events were associated with receipt of immunisation.
Interpretation
Year-round maternal influenza immunisation significantly reduced maternal influenza-like illness, influenza in infants, and low birthweight over the entire course of the study, indicating the strategy could be useful in subtropical regions.
Funding
Bill & Melinda Gates Foundation.
Introduction
Pregnant women with influenza infection have an increased risk of illness and hospital admission compared with non-pregnant women—a risk well documented in the 1918 global influenza pandemic and during the 2009 influenza A H1N1 pandemic and during seasonal influenza.1 Annual influenza immunisation during pregnancy was first recommended by the US Surgeon General in 1960,2 mainly because the risk of influenza disease is high in pregnant women, and many countries have since adopted this recommendation. WHO recommended influenza immunisation during pregnancy in 2012,3 and stated that pregnant women should be the highest priority for annual influenza immunisation, with influenza vaccine available year-round. Influenza immunisation during pregnancy can protect infants through the transfer of maternal antibodies. Infants aged 0–6 months are at increased risk for hospital admission for severe influenza infections compared with older infants,4 and three randomised clinical trials have shown that infants can be protected by maternal immunisation.5–7 Additionally, one study8 showed that intrauterine growth is improved in infants born to women who received influenza vaccine during pregnancy.
The circulation of influenza viruses varies between the northern and southern hemispheres, as well as seasonally within hemispheres and by latitude. Year-round simultaneous circulation of multiple influenza viruses occurs in equatorial tropical regions, which are the source of many new strains that circulate globally.9,10 A review11 of influenza seasonality for 2006–11 in ten tropical and subtropical southeast Asian countries indicated that influenza viruses were present in these regions for most months of most years. An estimated 40% of the world’s population live in tropical zones, yet influenza vaccines are not widely used in this setting. Despite year-round circulation12–14 of influenza in tropical and subtropical regions, we are not aware of previous prospective or controlled randomised studies assessing year-round influenza immunisation from Asia.15
We assessed the effect of year-round influenza immunisation during pregnancy in two consecutive randomised, placebo-controlled trials in pregnant women in subtropical plains of southern Nepal, a region broadly representative of south Asia.
Methods
Study design and participants
Detailed methods for these clinical trials have been published.16 We did two sequential randomised, placebo-controlled, masked trials to assess the efficacy of influenza immunisation for pregnant women on respiratory illness and laboratory-confirmed influenza infection among the women and their newborn babies up to 6 months after birth. Both trials were population-based and done in nine village development committees in the rural Sarlahi district in southern Nepal.
Annual cohorts of pregnant women were eligible if they were married, 15–40 years of age, 17–34 weeks’ gestation at enrolment, and had not previously received any influenza vaccine that season. Women were excluded if they had already participated in an influenza study, did not intend to deliver their child in the study area, or were allergic to any vaccine component. Babies born more than 2 weeks after maternal immunisation were included in the analysis. Active surveillance for pregnancy was done every 5 weeks in study households. Only one pregnancy per woman was enrolled.
Verbal informed consent was obtained at the time of recruitment for pregnancy surveillance and reconfirmed at the time of enrolment or vaccine (placebo) receipt. This protocol was reviewed and approved by the institutional review boards of the Cincinnati Children’s Medical Center (Cincinnati, OH, USA), Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA), the Institute of Medicine at Tribhuvan University (Kathmandu, Nepal), and the Nepal Health Research Council (Kathmandu, Nepal). Institutional review boards at Seattle Children’s Hospital (Seattle, WA, USA), the University of Washington (Washington, DC, USA), and George Washington University (Washington, DC, USA) granted oversight to the institutional review board at Johns Hopkins Bloomberg School of Public Health.
An independent data and safety monitoring board met to review the study protocol before the start of the study, once approximately midway through the first trial, and after both trials were complete. They also reviewed protocol deviations and adverse event summaries throughout the studies.
Randomisation and masking
Women were individually randomised (1:1) in each cohort to receive the local current trivalent inactivated influenza vaccine or saline placebo. Six lots of two WHO-recommended formulations of influenza vaccines with northern and southern strains were used. Between April 25, 2011, and October 15, 2012, vaccine 1 containing A H3N2 Perth, A H1N1 California, and B Brisbane (V) was used in cohort 1 and part of cohort 2; between October 16, 2012, to September 9, 2013, vaccine 2 containing A H3N2 Victoria, A H1N1 California, and B Wisconsin (Y) was used in cohort 2 (appendix). We randomised mothers to receive vaccine or saline placebo in blocks of eight, stratified by gestational age at time of enrolment (17–25 weeks vs 26–34 weeks) using sealed envelopes with sequential participant number on the outside and allocation code within.16 In the first cohort, women were enrolled and vaccinated as soon as their pregnancies were identified and they were between 17 and 34 weeks’ gestation. In the second cohort, when women were identified as pregnant they were randomly allocated a week of gestation from 17 to 34 weeks to enrol and receive the vaccine or placebo. The allocation for timing of vaccine or placebo receipt was done by drawing one of 18 blocks from a hat, each labelled from 17–34 at the time the women were identified as pregnant. Unmasked study staff (comprised of nurses and auxiliary nurse midwives who did not participate in outcome assessments) administered vaccine or placebo. All other study staff, investigators, and participants were masked. At the conclusion of the overall study, all women randomised to placebo were offered the seasonal influenza vaccine.
Procedures
We used an initial household census to identify married women between 15 years and 40 years of age and to collect basic information on the demographic and socioeconomic characteristics of the women and their households. Families who consented were visited at home every 5 weeks for pregnancy surveillance. Pregnancy was confirmed with a urine pregnancy test (EasyTest, Visiomed, Paris, France) done at the household. All pregnant women received an enrolment interview and examination that included a reproductive history, tobacco and alcohol use, and plans for delivery. We measured height, weight, temperature, blood pressure, and pulse. We used monthly visits throughout pregnancy to ascertain pregnancy-associated morbidity and for repeat measurements of weight, temperature, and blood pressure. We measured birthweight at a home visit as soon as possible after birth. For all deaths of enrolled mothers and infants, verbal autopsies were done about 1 month after the death report.
We assessed morbidity through weekly home visits from enrolment to 180 days post partum. Trained field staff visited the home and asked about symptoms and signs in the mother, and later, the infant, for each day in the preceding week. For mothers, we collected data for influenza-like illness, including self-reported fever, persistent cough, sore throat, nasal congestion, and myalgia. For infants, we recorded the maternal report of high fever, cough, difficult or rapid breathing, wheeze, and otorrhoea. We also recorded whether any outside care was sought, including visits to a physician or hospital.
The intervention in both trials was immunisation during pregnancy at 17–34 weeks’ gestation using the current trivalent, inactivated influenza Vaxigrip vaccine (Sanofi-Aventis Pharma, Mumbai, India) containing WHO-recommended antigens for the time of enrolment for each woman in the vaccine group.17 The placebo was sterile saline.
A midnasal swab was collected from women who reported fever plus one additional sign (persistent cough, sore throat, nasal congestion, or myalgia) on any of the past 7 days.18 A midnasal swab was also collected from infants whose mothers reported they had any one of fever, cough, wheeze, difficulty breathing, or otorrhoea on any of the preceding 7 days. In a subsample of 82 women and infants, a second midnasal swab was collected and analysed for viral culture, and frozen.
In another subsample of 208 women–infant pairs, blood was collected from the mother at enrolment and at approximately 1 week and 3 months post partum and cord blood was collected from the infant at the time of delivery.
We assessed laboratory-proven influenza by testing nasal swabs by real-time RT-PCR targeting the influenza A and B matrix genes. Specimens positive for influenza A were subtyped for seasonal H1N1, H3N2, and the novel 2009 pandemic influenza A H1N1 subtypes using real time RT-PCR targeting the specific subtype haemagglutinin genes.19,20 Influenza-positive nasal swab samples with a sufficient viral load (determined by PCR cycle threshold), were strain-typed using an RT-PCR and a mass spectrometry method called Plex-ID (Abbott Diagnostics, Maidenhead, UK).21
Viral culture was done on frozen stored samples by cell culture using standard WHO methods.22 Antibodies to influenza antigens contained in the vaccines were tested by haemagglutination inhibition assay of samples by a previously described method (appendix).
Data were collected on paper forms, checked for completeness in the field, and transferred to the data centre in Kathmandu for data entry and management. Episodes of laboratory-confirmed influenza were constructed using the serial data collected weekly for all individuals in the trial and nasal swab test results. For women, we defined influenza-like illness as reported fever plus either cough or sore throat on at least 1 day. We defined laboratory-confirmed influenza in mothers as an illness episode with fever plus one or more of cough, sore throat, nasal congestion or runny nose, or myalgia concurrent with a PCR-positive nasal swab for influenza. Among infants, we defined influenza-like illness as maternal report of fever plus cough, difficulty breathing, or ear discharge on at least 1 day. We defined infant laboratory-confirmed influenza as an illness episode with any one of fever, cough, wheeze, ear discharge, or difficulty breathing concurrent with a nasal swab that was PCR positive for influenza. For each outcome, we required at least 7 symptom-free days after an episode before a mother or a child could experience another episode.
Outcomes
Primary outcomes for the trials were incidence of laboratory-confirmed influenza illness in the infant from birth to 180 days, incidence of influenza-like illness in the mothers during pregnancy and to 180 days post partum, and incidence of low birthweight. The secondary outcomes were maternal laboratory-confirmed influenza, infant rate of influenza-like illness, incidence of preterm birth and of small for gestational age infants, and mean gestational age (appendix). We will report the association between gestational age at immunisation and maternal and infant outcomes in a forthcoming publication.
Statistical analysis
We analysed data using Stata version 13 and SAS version 9. We present the data for each annual cohort and the combined cohorts. We present the intention-to-treat analysis, although this is equivalent to per protocol, because all women received the assigned vaccine.
The sample size was calculated separately for each cohort’s results to stand alone. We considered all three primary outcomes equivalent in importance and we corrected for type 1 error for these multiple primary endpoints. We assumed a total type 1 error of 5% with a sample size corresponding to a type 1 error of 0·017 for each primary outcome. An expected baseline rate of 21·6 cases per 100 person-years of laboratory-confirmed influenza illness in infants was based on a Bangladesh maternal immunisation trial.5 With 1850 livebirths (925 in each group), a 50% reduction could be detected with 90% power. For influenza-like illness during pregnancy and through 6 months post partum, we assumed a baseline rate of 34 cases per 100 person-years, based on previous studies in this region.23 Assuming 1850 pregnancies, a 33% relative reduction in incidence was detectable with 90% power. We used our previous data for pregnancy accrual to assess how large a geographical area we needed to vaccinate 1850 women in 1 year. Cohort 1 contained more than 1850 pregnancies because we enrolled prevalent as well as incident pregnancies. We enrolled fewer than 1850 pregnancies in cohort 2 because we included only incident pregnancies and this was the number of pregnancies we had accrued after enrolling for 1 year in the same geographical area.
We calculated incidence rates using the number of cases of laboratory-confirmed influenza divided by the number of days at risk for influenza by treatment group and compared groups using the risk ratio and 95% CIs from mixed effects binomial regression models with log link to account for correlation between repeated morbidity measures. We excluded days with symptoms, as well as 7 subsequent days, from days at risk. We estimated vaccine efficacy as the proportionate reduction in laboratory-confirmed influenza incidence for the vaccine group compared with the placebo group.
Birthweight was analysed if obtained less than 72 h after birth. We defined small for gestational age as having a birthweight of less than the 10th percentile of a birthweight standard or reference at a specific gestational age. We defined the cutoffs using both the US birthweight reference,24 and the international Intergrowth-21 standard.25 We calculated the differences in mean birthweights and gestational ages between treatment groups using linear regression. We compared prevalence of low birthweight (<2500 g), preterm birth (<37 completed weeks’ gestation), and small for gestational age by treatment group using risk ratios (RR) and 95% CIs from Poisson regression models with robust variance.26,27
In addition to the incidence density approach described above, we also calculated the attack rate, defined as the proportion of enrolled participants who had at least one positive episode, as is typical for influenza vaccine trials—this approach did not include multiple episodes within the same person.
This trial is registered with ClinicalTrials.gov, number NCT01034254.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
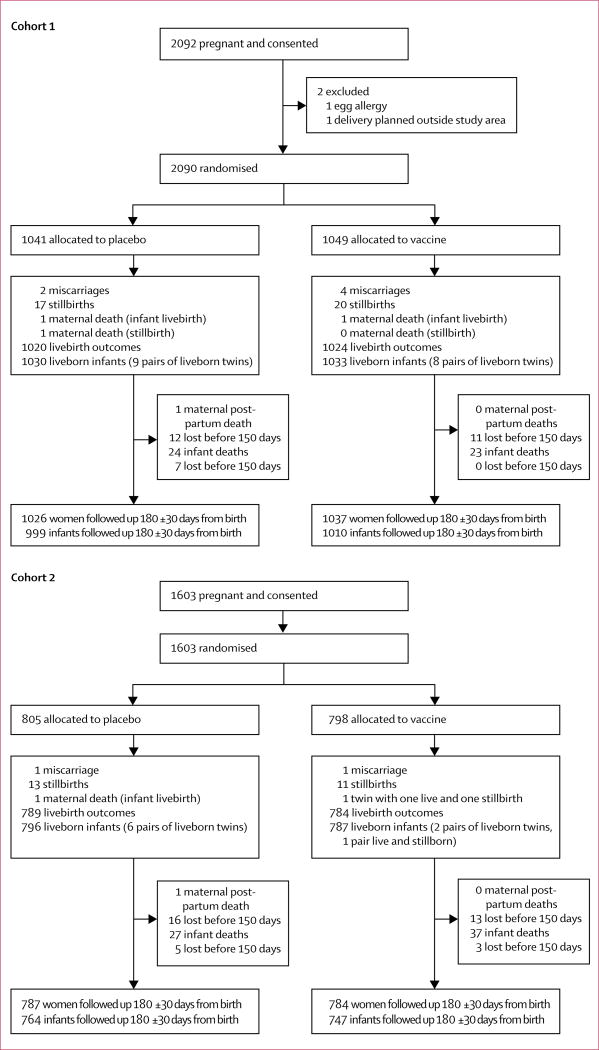
Between April 25, 2011, and April 24, 2012, in the first cohort study, we enrolled and followed up 2090 pregnant women (1041 allocated to placebo and 1049 allocated to vaccine), and their 2063 liveborn infants (1030 with placebo and 1033 with vaccine). Seven of these infants were excluded from influenza and birth outcomes analyses because their births occurred within 2 weeks of maternal immunisation. Between April 25, 2012, and April 24, 2013, in the second study, we enrolled 1603 women who delivered 1583 livebirths (ten of whom were excluded because birth occurred within 2 weeks of vaccination; figure 1). Vaccination occurred until Sept 9, 2013.
Figure 1.
Trial profile
At the time of immunisation, placebo and vaccine recipients in each cohort were similar (table 1). In both cohorts, the mean age at enrolment was 23 years and mean gestational age at immunisation was 23·5 weeks. 3513 (96%) of 3646 infants had at least 1 day of morbidity and infants were followed up for morbidity for a median of 174 days (IQR 166–179). 3666 (99%) of 3693 mothers had at least 1 day of morbidity during pregnancy with a median of 100 days (IQR 67–131). Post partum follow-up for women and infants was a median of 180 days (IQR 171–180).
Table 1.
Baseline maternal characteristics at the time of vaccination
| Cohort 1 | Cohort 2 | Combined | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Placebo (N=1041) |
Vaccine (N=1049) |
Placebo (N=805) |
Vaccine (N=798) |
Placebo (N=1846) |
Vaccine (N=1847) |
|
| Age (years) | 23·0 (4·6) | 23·2 (4·5) | 23·2 (4·7) | 23·4 (5·0) | 23·1 (4·7) | 23·3 (4·7) |
|
| ||||||
| Gestational age | ||||||
| 17–25 weeks | 768 (74%) | 774 (74%) | 385 (48%) | 379 (48%) | 1153 (63%) | 1153 (62%) |
| 26–34 weeks | 273 (26%) | 275 (26%) | 420 (52%) | 419 (53%) | 693 (38%) | 694 (38%) |
|
| ||||||
| Gestational age (weeks) | 22·2 (5·2) | 22·0 (5·3) | 25·2 (4·2) | 25·3 (4·3) | 23·5 (5·1) | 23·4 (5·2) |
|
| ||||||
| Systolic/diastolic blood pressure (mm Hg) | 99·9 (9·8)/66·3 (8·6) | 100·4 (9·8)/66·7 (9·4) | 100·7 (9·8)/67·1 (8·8) | 100·7 (9·4)/66·9 (8·6) | 100·3 (9·8)/66·6 (8·7) | 100·5 (9·6)/66·7 (9·1) |
|
| ||||||
| Height (cm) | 151·5 (5·6) | 151·7 (5·6) | 151·7 (5·5) | 151·6 (5·3) | 151·6 (5·6) | 151·6 (5·5) |
|
| ||||||
| Weight (kg) | 48·0 (7·2) | 48·8 (7·9) | 48·3 (7·1) | 48·4 (7·5) | 48·1 (7·1) | 48·6 (7·8) |
|
| ||||||
| BMI (kg/m2) | 20·9 (2·8) | 21·2 (3·0) | 20·9 (2·7) | 21·1 (2·9) | 20·9 (2·7) | 21·1 (3·0) |
| <16 | 14 (1%) | 14 (1%) | 9 (1%) | 8 (1%) | 23 (1%) | 22 (1%) |
| 16–18·5 | 177 (17%) | 159 (15%) | 139 (17%) | 128 (16%) | 316 (17%) | 287 (16%) |
| 18·5–25 | 765 (74%) | 773 (74%) | 597 (74%) | 589 (74%) | 1362 (74%) | 1362 (74%) |
| >25 | 81 (8%) | 98 (9%) | 60 (8%) | 71 (9%) | 141 (8%) | 169 (9%) |
|
| ||||||
| No education | 427 (43%) | 446 (44%) | 300 (39·8%) | 298 (40·5%) | 727 (42%) | 744 (43%) |
|
| ||||||
| Nulliparous | 417 (40%) | 413 (39%) | 372 (46%) | 346 (43%) | 789 (43%) | 759 (41%) |
|
| ||||||
| Gravidity | 1·3 (1·5) | 1·3 (1·5) | 1·1 (1·4) | 1·1 (1·3) | 1·2 (1·5) | 1·2 (1·4) |
|
| ||||||
| Parity | 1·2 (1·6) | 1·2 (1·5) | 1·0 (1·4) | 1·0 (1·6) | 1·1 (1·5) | 1·1 (1·5) |
|
| ||||||
| History of child deaths* | 103 (18%) | 86 (15%) | 51 (12%) | 44 (10%) | 154 (15%) | 130 (13%) |
|
| ||||||
| History of stillbirths* | 45 (7%) | 35 (6%) | 14 (3%) | 20 (4%) | 59 (6%) | 55 (5%) |
Data are mean (SD) or n (%). BMI=body-mass index.
Among women with at least one previous live birth; includes 11 pregnancies in cohort 2, six in the placebo group and five in the vaccine group (vaccine group: three infants, one twin pair), who were vaccinated within 14 days of delivery. Includes nine pregnancies in cohort 1, four in placebo and five in vaccine group, who were vaccinated within 14 days of delivery.
In 216 975 home visits, 5874 nasal swabs were collected from mothers and infants. Influenza viruses were documented in 24 of the 36 study months (appendix), including 1–17 isolates each month during the 10-month period of continuous circulation of influenza virus at the beginning of the study (appendix). We detected 254 cases of laboratory-confirmed influenza that met study criteria (tables 2, 3); 182 episodes were due to influenza type A, including 61 H1N1 strains, 89 H3N2 strains, and 32 for which the subtype could not be determined, and 129 influenza B viruses, including 28 Victoria lineage, 88 Yamagata lineage, and 13 non-subtypeable strains (appendix). Influenza A H3N2 and B/Victoria virusespredominated in 2011, both A H1N1 and B/Yamagata-like viruses predominated in 2012, and A H3N2 predominated in 2013. Two women and six infants each had two distinct influenza episodes (infected with different subtypes including A H1N1 and B at each episode). One infant had two distinct illness episodes 21 days apart with a nasal swab that tested positive for A H1N1 at each episode.
Table 2.
Primary outcomes
| Cohort 1 | Cohort 2 | Combined | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||||
| Placebo | Vaccine | Risk ratio (95% CI) |
p value | Placebo | Vaccine | Risk ratio (95% CI) |
p value | Placebo | Vaccine | Risk ratio (95% CI) |
p value | |||||||
|
|
|
|
|
|
|
|||||||||||||
| Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | |||||||
| Maternal | ||||||||||||||||||
|
| ||||||||||||||||||
| All influenza-like illness | 166 (642·4) | 258·4 | 157 (665·0) | 236·1 | 0·91 (0·71–1·16) | 0·431 | 98 (455·6) | 215·1 | 62 (451·1) | 137·4 | 0·64 (0·45–0·91) | 0·014 | 264 (1098·0) | 240·4 | 219 (1116·1) | 196·2 | 0·81 (0·66–0·99) | 0·041 |
| Influenza-like illness (pregnancy) | 78 (261·7) | 298·0 | 66 (272·2) | 242·5 | 0·81 (0·58–1·14) | 0·226 | 50 (160·3) | 311·9 | 30 (161·2) | 186·1 | 0·60 (0·38–0·94) | 0·026 | 128 (422·0) | 303·3 | 96 (433·4) | 221·5 | 0·73 (0·56–0·96) | 0·022 |
| Influenza-like illness (postpartum) | 88 (380·7) | 231·1 | 91 (392·8) | 231·7 | 1·01 (0·73–1·41) | 0·950 | 48 (295·2) | 162·6 | 32 (289·9) | 110·4 | 0·68 (0·41–1·11) | 0·123 | 136 (676·0) | 201·2 | 123 (682·7) | 180·2 | 0·90 (0·68–1·18) | 0·446 |
|
| ||||||||||||||||||
| Infant | ||||||||||||||||||
|
| ||||||||||||||||||
| All laboratory-confirmed influenza | 69 (322·9) | 213·7 | 60 (335·2) | 179·0 | 0·84 (0·59–1·19) | 0·324 | 36 (256·2) | 140·5 | 14 (247·1) | 56·7 | 0·40 (0·22–0·74) | 0·003 | 105 (579·0) | 181·3 | 74 (582·3) | 127·1 | 0·70 (0·52–0·95) | 0·020 |
| Low birthweight (<2500 g) | 220 (28%) of 780 | NA | 193 of (23%) 807 | NA | 0·85 (0·72–1·00) | 0·052 | 145 (25%) of 581 | NA | 122 (21%) of 573 | NA | 0·85 (0·69–1·05) | 0·141 | 365 (27%) of 1361 | NA | 315 (23%) of 1380 | NA | 0·85 (0·75–0·97) | 0·016 |
Data are n (person-years) or n (%) of N for low birthweight.
Data are incidence per 1000 person-years.
NA=not applicable.
Table 3.
Selected secondary outcomes
| Cohort 1 | Cohort 2 | Combined | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||||||
| Placebo | Vaccine | Effect size (95% CI)‡ |
p value | Placebo | Vaccine | Effect size (95% CI)‡ |
p value | Placebo | Vaccine | Effect size (95% CI)‡ |
p value | |||||||
|
|
|
|
|
|
|
|||||||||||||
| Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | Cases* | Incidence† | |||||||
| Maternal | ||||||||||||||||||
|
| ||||||||||||||||||
| All laboratory-confirmed influenza | 30 (639·4) | 46·9 | 17 (662·4) | 25·7 | 0·55 (0·30 to 0·99) | 0·047 | 14 (454·0) | 30·8 | 14 (449·4) | 31·2 | 1·02 (0·48 to 2·18) | 0·956 | 44 (1093·4) | 40·3 | 31 (111·8) | 27·9 | 0·69 (0·44 to 1·10) | 0·122 |
| Laboratory-confirmed influenza (pregnancy) | 15 (260·6) | 57·6 | 7 (271·3) | 25·8 | 0·45 (0·18 to 1·10) | 0·078 | 9 (159·8) | 56·3 | 12 (160·7) | 74·7 | 1·38 (0·56 to 3·37) | 0·484 | 24 (420·5) | 57·1 | 19 (431·9) | 44·0 | 0·77 (0·42 to 1·43) | 0·414 |
| Laboratory-confirmed influenza (postpartum) | 15 (378·9) | 39·6 | 10 (391·1) | 25·6 | 0·65 (0·28 to 1·47) | 0·299 | 5 (294·1) | 17·0 | 2 (288·7) | 6·9 | 0·41 (0·08 to 2·09) | 0·282 | 20 (672·9) | 29·7 | 12 (679·9) | 17·6 | 0·59 (0·28 to 1·23) | 0·162 |
|
| ||||||||||||||||||
| Infant | ||||||||||||||||||
|
| ||||||||||||||||||
| All influenza-like illness | 409 (349·9) | 1168·8 | 439 (363·6) | 1207·4 | 1·04 (0·88 to 1·22) | 0·674 | 328 (270·0) | 1214·9 | 272 (258·7) | 1047·5 | 0·87 (0·71 to 1·05) | 0·145 | 737 (619·9) | 1188·9 | 710 (622·3) | 1140·9 | 0·96 (0·85 to 1·09) | 0·556 |
| Preterm (<37 weeks’ gestation) | 145 of (14%) 1023 | NA | 137 (13%) of 1027 | NA | 0·94 (0·76 to 1·17) | 0·584 | 102 (13%) of 790 | NA | 88 (11%) of 782 | NA | 0·87 (0·67 to 1·14) | 0·314 | 247 (14%)of 1813 | NA | 225 (12%) of 1809 | NA | 0·91 (0·77 to 1·08) | 0·289 |
| SGA‡ (intergrowth) | 334 (45%) of 741 | NA | 327 (42%) of 764 | NA | 0·95 (0·85 to 1·06) | 0·374 | 229 (41%) of 559 | NA | 211 of (39%) 543 | NA | 0·95 (0·82 to 1·10) | 0·475 | 563 (43%) of 1300 | NA | 538 (41%) of 1307 | NA | 0·95 (0·87 to 1·04) | 0·121 |
| SGA (Alexander) | 400 (51%) of 775 | NA | 387 (48%) of 802 | NA | 0·93 (0·85 to 1·03) | 0·182 | 275 (47%) of 580 | NA | 250 (44%) of 573 | NA | 0·92 (0·81 to 1·04) | 0·198 | 675 (50%) of 1355 | NA | 637 (46%) of 1375 | NA | 0·93 (0·86 to 1·01) | 0·068 |
| Birthweight, g | 2741·0 (465·3) | NA | 2770·0 (440·6) | NA | 28·9 (−15·6 to 73·5) | 0·203 | 2789·8 (441·8) | NA | 2851·8 (458·6) | NA | 62·0 (10·0 to 113·9) | 0·019 | 2761·9 (455·9) | NA | 2803·9 (449·9) | NA | 42·1 (8·2 to 76·0) | 0·015 |
| Gestational age, weeks | 39·4 (2·7) | NA | 39·4 (2·6) | NA | 0·002 (−0·23 to 0·23) | 0·986 | 39·3 (2·4) | NA | 39·5 (2·4) | NA | 0·16 (−0·07 to 0·40) | 0·168 | 39·4 (2·6) | NA | 39·4 (2·5) | NA | 0·07 (−0·09 to 0·24) | 0·386 |
Gestational age <23 weeks and ≥50 weeks dropped because not feasible. Only weights measured within 72 h of birth are presented; weight missing or measured ≥72 h after birth were excluded. Gestational age calculated as date of birth minus date of last menstrual period (estimated recall period of 3–4 weeks). Seven livebirths in cohort 1, three placebo and four in vaccine group, excluded due to vaccination within 14 days of delivery. Nine livebirths in cohort 2, five placebo and five in vaccine group, excluded due to vaccination within 14 days of delivery. Eight dropped from SGA (Alexander) for improbable SGA and weight combination (cohort 1, four placebo, four vaccine). SGA=small for gestational age.
Data are n (person-years); n (%) of N for preterm (<37 weeks gestation), SGA (intergrowth), and SGA (Alexander); or mean (SD) for birthweight and gestational age.
Data are incidence per 1000 person-years.
Effect size is risk ratio for all outcomes except gestational age and birthweight, which are difference between mean values (95% CI).
We detected no significant difference between groups for maternal influenza-like illness in cohort 1 (9% efficacy, 95% CI −16 to 29) but there was a significant reduction in cohort 2 for vaccine compared with placebo (36%, 9 to 55; table 2). There was a significant difference in the two cohorts combined (19%, 1 to 34).
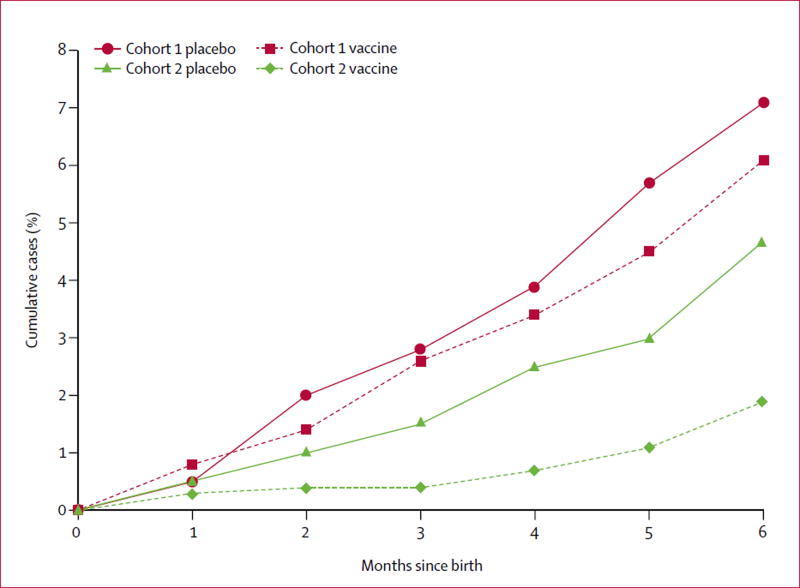
The incidence of laboratory-confirmed influenza in infants was not significantly different in cohort 1 (16% efficacy, 95% CI −19 to 41), but was in cohort 2 (60%, 26 to 88; table 2; appendix). The incidence of laboratory-confirmed influenza in infants for both cohorts combined was lower in the vaccine group (30%, 5 to 48; table 2). We report cumulative rates of laboratory-proven influenza cases in infants aged 0–6 months in figure 2 and the cumulative risk ratio by age of infants is in the appendix. Low birthweight (<2500 g) did not differ significantly between groups in each cohort, but there was a significant difference when both cohorts were combined (15% efficacy, 95% CI 3–25; table 2).
Figure 2.
Laboratory-confirmed influenza in infants in cohort 1 and cohort 2
Secondary outcomes are described in table 3. The incidence of maternal laboratory-confirmed influenza was lower in the vaccine group than in the placebo group in cohort 1, but not in cohort 2 or the two cohorts combined (table 3, appendix). There was no significant difference between groups for influenza-like illness in infants, preterm birth, or small for gestational age births, and the gestational ages were similar in each cohort and the combined groups (table 3). A post-hoc analysis showed the mean birthweight for both cohorts combined was significantly greater in the vaccine group than in the placebo group (table 3).
Among infants, the attack rate of proven influenza was 6·7% in cohort 1 and 4·7% in cohort 2 in the two placebo cohorts (appendix). We compared haemagglutination inhibition antibody titres for each of the two vaccine formulations used during the 3-year study (appendix). Before immunisation, haemagglutination inhibition antibody titres to influenza vaccine antigens were relatively low and similar in vaccine and placebo recipients for all vaccine antigens, with the exception of the antibody titre for the A H3N2/Victoria antigen (appendix). Vaccinated women had a broad range of significant increases in antibody titres to all antigens, with geometric mean increases ranging from 2·4 to 9·0 times. Cord antibody titres reflected maternal antibody concentrations, with significantly greater antibody concentrations to all vaccine antigens in children of immunised mothers compared with mothers who received placebo (p<0·0001; appendix).
Incidences of adverse events were statistically similar between placebo and vaccine groups. Miscarriages occurred in three (0·2%) of 1846 participants in the placebo group versus five (0·3%) of 1847 in the vaccine group, stillbirths occurred in 31 (1·7%) of 1846 versus 33 (1·8%) of 1847, and congenital defects occurred in 18 (1·0%) of 1846 versus 20 (1·1%) of 1847 (appendix). Five women died in the placebo group due to tuberculosis, haemorrhage, suicide, or unknown causes, and two women died in the vaccine group due to haemorrhage and an unknown cause. 111 infants died (51 [3%] with mothers who received placebo and 60 [3%] with mothers who received vaccine; appendix).
Discussion
In this report, we summarise the results of a randomised placebo-controlled trial of 3693 mothers immunised throughout the year with influenza vaccine in a subtropical region, with results broadly generalisable to the south Asian region. Our data describe the substantial burden of influenza infection during pregnancy in south Asia. We showed that maternal vaccination with a trivalent inactivated influenza vaccine significantly reduced laboratory-confirmed influenza infection in infants, influenza-like illness in women, and the proportion of babies born with low birthweight over 2 years, although the effect was not present in each annual cohort. Our findings suggest that vaccination throughout the year might have an advantage over limited seasonal use of influenza vaccine, with its reduced probability of influenza strain-vaccine match, in subtropical and tropical regions where influenza is present for many months.28 Circulating viruses varied substantially, leading to limited periods of antigenic match between the trivalent inactivated vaccine used and the variety of circulating influenza viruses.10,14 The detailed results of strain-specific vaccine effect will be presented in a subsequent paper.
In this study, the rate of low birthweight infants was reduced but preterm births and small for gestational age births were not affected. This discrepancy from studies done in Africa6,7 could be explained by the increase of birthweight in children of immunised mothers occurring mainly among term infants who were low birthweight, a group that represents 15% of births in south Asia. Although the increase in mean birthweight of 42 g with vaccination was not as great as that reported in a study done in Bangladesh (90 g overall and 200 g in the subset of mothers with reduction of influenza disease),8 the effect of maternal immunisation on birthweight was significant in cohort 2 and when the cohorts were combined. These increases in birthweight contrast with the lack of effect on birthweight of vaccination reported in maternal influenza immunisation studies done in Africa6,7 in regions with lower rates of maternal malnutrition.
Improved influenza vaccines with broader antigenic range are needed to increase effectiveness, especially in tropical and subtropical regions, as well as worldwide. Longer shelf-life would also improve vaccination in tropical regions where influenza viruses circulate almost throughout the year and the shift from northern hemisphere to southern hemisphere vaccines can be problematic. In Nepal, both type B strains circulated in 2011 and 2012, yet the trivalent vaccine includes only one B strain. A quadrivalent vaccine with both type B antigens, which is available in some countries, would probably have resulted in a greater effect.12,14
The strengths of this study included the placebo-controlled randomised design, with active weekly prospective household observation of all participants to determine accurate gestational ages, illness episodes, and birth outcomes. A limitation of our study was the criterion of fever as part of the diagnosis of maternal influenza-like illness, which might account for the substantially lower proportion of nasal specimens collected and fewer cases of proven influenza illness in the mothers compared with infants, for whom respiratory symptoms alone were required. Routine viral culture was difficult to do, so only a subset of samples had culture done to verify genetic characteristics. An additional possible limitation was the lack of first trimester ultrasound for accurate dating of gestational ages. However, maternal recall was on average within 2–3 weeks of the date of the last menstrual period because of the frequent home surveillance to identify and enrol eligible participants.
In subtropical and tropical regions, the timing of the circulation of influenza virus varies,29 suggesting that seasonal immunisation might not be the best strategy. Variations in the timing and duration of the tropical influenza season might justify the provision of influenza vaccine throughout the year. But this strategy would not counter the antigenic changes that constantly occur with influenza viruses. Most of the vaccine effect in this study was present in the second cohort, which received two formulations of influenza vaccine with a broader range of antigens than in the first. The substantial antigenic variation will require improved vaccines with broader antigenic coverage.30 A vaccination strategy that targets pregnant women and builds on the tetanus toxoid vaccination programmes that operate in many low-resource settings would could make vaccination throughout the year cost-effective and logistically feasible.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for English language studies published until Dec 31, 2016, with no start date restriction, with the terms “influenza”, “influenza vaccine”, “human”, “pregnancy”, “randomised placebo trial”, and “tropical”. We found that relevant publications were published after 2008. Previous studies have shown that standard influenza vaccine is immunogenic, safe in pregnancy, and provides passive antibodies to the infant, as well as clinical protection for both mother and infant. The strategy of maternal immunisation with inactivated influenza vaccine is recommended in many countries and by WHO. Three prospective randomised trials in Asia and Africa have assessed the efficacy of maternal influenza immunisation for infants, ranging from 33% to 63%. To our knowledge, no large prospective placebo-controlled studies have assessed year-round influenza immunisation in subtropical regions where influenza circulates for many months of the year.
Added value of this study
Our study provides new evidence regarding antenatal influenza vaccine when given year-round to pregnant women in a subtropical region. Influenza virus circulated for 24 months during 36 months of observation. The study showed the safety, immunogenicity, and the feasibility of protection of mothers, showing a reduction of influenza in infants, influenza-like illness in mothers, and the proportion of low birthweight infants overall, although the results differed by cohort.
Implications of all the available evidence
In regions where influenza virus circulates for many months of the year, a universal strategy of immunising women during pregnancy might provide better protection than attempting to predict when influenza will be present. The development of influenza vaccines with broader antigenic coverage will increase the efficacy of the seasonal year-round immunisation strategies.
Acknowledgments
MCS, JKa, LCM, HYC, and JMT have received grants from the Bill & Melinda Gates Foundation. JAE has received grants and personal fees from GlaxoSmith Kline, Pfizer, and Gilead; research support from Roche; and personal fees from Abbvie. HYC has received grants from PATH and the Thrasher Foundation. MM has received funding from Merck, Sanofi, and PATH.
This study was funded by the Bill & Melinda Gates Foundation (grant 50274). We thank Jean Sheffield, Maureen Maguire, Kusam Thapa, and Ramesh Adhikari for their service on the data and safety monitoring board, and Niteen Wairagkar for his support throughout the project. We thank Min Levine and Xiyan Xu of the Centers for Disease Control and Prevention for haemagglutination inhibition antibody assays and viral cultures. GVK Biosciences provided independent monitoring and auditing support. We thank Michelle Hughes for maintaining good clinical practice for the trial, and for appropriate storage and shipment of specimens. We thank Gretchen Langdon for administrative support, shipping of research items to and from the field site, and cataloguing of specimens.
Footnotes
See Online for appendix
Contributors
MCS, JMT, JKa, JAE, and JKu designed the study. MCS conceived the study and secured funding for the project. SKK, SCL, LSh, JMT, LCM, and JKa supervised the conduct of the study in the field. LSt set up the vaccine cold chain and specimen management in the field. JKa was the study statistician, and JKa and LCM analysed the data. JKu did the PCR assays. HYC assisted in study implementation, data analysis, and cord blood collection. NK analysed data. MM did viral cell cultures and serum haemagglutination inhibition antibody assays. AMR provided data analysis support and managed specimen cataloguing and shipments. All authors read and approved the final manuscript.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Ortiz JR, Englund JA, Neuzil KM. Influenza vaccine for pregnant women in resource-constrained countries: a review of the evidence to inform policy decisions. Vaccine. 2011;29:4439–52. doi: 10.1016/j.vaccine.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 2.Burney LE. Influenza immunization: statement. Public Health Rep. 1960;75:944. [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Vaccines against influenza, WHO position paper—November 2012. Wkly Epidemiol Rec. 2012;87:461–76. [PubMed] [Google Scholar]
- 4.Henkle E, Steinhoff MC, Omer SB, et al. Incidence of influenza virus infection in early infancy: a prospective study in South Asia. Pediatr Infect Dis J. 2011;30:170–73. doi: 10.1097/INF.0b013e3181f63c39. [DOI] [PubMed] [Google Scholar]
- 5.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 6.Madhi SA, Nunes MC, Cutland CL. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. 2014;371:2340. doi: 10.1056/NEJMc1412050. [DOI] [PubMed] [Google Scholar]
- 7.Tapia MD, Sow SO, Tamboura B, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16:1026–35. doi: 10.1016/S1473-3099(16)30054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184:645–53. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedford T, Riley S, Barr IG, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523:217–20. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saha S, Chadha M, Al Mamun A, et al. Influenza seasonality and vaccination timing in tropical and subtropical areas of southern and south-eastern Asia. Bull World Health Organ. 2014;92:318–30. doi: 10.2471/BLT.13.124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloom-Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8:e54445. doi: 10.1371/journal.pone.0054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirve S, Lambach P, Paget J, Vandemaele K, Fitzner J, Zhang W. Seasonal influenza vaccine policy, use and effectiveness in the tropics and subtropics – a systematic literature review. Influenza Other Resp Viruses. 2016;10:254–67. doi: 10.1111/irv.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadha MS, Potdar VA, Saha S, et al. Dynamics of influenza seasonality at sub-regional levels in India and implications for vaccination timing. PLoS One. 2015;10:e0124122. doi: 10.1371/journal.pone.0124122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha S, Chadha M, Shu Y for the Group of Asian Researchers on Influenza (GARI) Divergent seasonal patterns of influenza types A and B across latitude gradient in Tropical Asia. Influenza Other Resp Viruses. 2016;10:176–84. doi: 10.1111/irv.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan PK, Tam WW, Lee TC, et al. Hospitalization incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed subtropical city. Medicine. 2015;94:e2024. doi: 10.1097/MD.0000000000002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tielsch JM, Steinhoff M, Katz J, et al. Designs of two randomized, community-based trials to assess the impact of influenza immunization during pregnancy on respiratory illness among pregnant women and their infants and reproductive outcomes in rural Nepal. BMC Pregnancy Childbirth. 2015;15:40. doi: 10.1186/s12884-015-0470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox N. Influenza seasonality: timing and formulation of vaccines. Bull World Health Organ. 2014;92:311. doi: 10.2471/BLT.14.139428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaudecker EP, Heck JP, MacIntyre ET, et al. Comparison of a new transport medium with universal transport medium at a tropical field site. Diagn Microbiol Infect Dis. 2014;80:107–10. doi: 10.1016/j.diagmicrobio.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–88. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuypers J, Campbell AP, Guthrie KA, et al. WU and KI polyomaviruses in respiratory samples from allogeneic hematopoietic cell transplant recipients. Emerg Infect Dis. 2012;18:1580–88. doi: 10.3201/eid1810.120477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang YW, Lowery KS, Valsamakis A, et al. Clinical accuracy of a PLEX-ID flu device for simultaneous detection and identification of influenza viruses A and B. J Clin Microbiol. 2013;51:40–45. doi: 10.1128/JCM.01978-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Geneva, Switzerland: World Health Organization; 2011. WHO Global Influenza Surveillance Network, Manual for the laboratory diagnosis and virological surveillance of influenza. [Google Scholar]
- 23.Tielsch JM, Darmstadt GL, Mullany LC, et al. Impact of newborn skin cleansing with chlorhexidine on neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Pediatrics. 2007;119:e330–340. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 25.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:8576–78. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 26.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–06. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 27.Katz J, Lee ACC, Kozuki N, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–25. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambach P, Alvarez AM, Hirve S, et al. Considerations of strategies to provide influenza vaccine year round. Vaccine. 2015;33:6493–8. doi: 10.1016/j.vaccine.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso WJ, Yu C, Viboud C, et al. A global map of hemispheric influenza vaccine recommendations based on local patterns of viral circulation. Sci Rep. 2015;5:17214. doi: 10.1038/srep17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz FM. Infant protection against influenza through maternal immunization: A call for more immunogenic vaccines. JAMA Pediatr. 2016;170:832–33. doi: 10.1001/jamapediatrics.2016.1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.