Abstract
Objective
Higher IQ correlates with lower systemic inflammation, consistent with an association between lower IQ and disease risk. The present study examined the role of repetitive thought (RT) in the relationship between IQ and interleukin (IL)-6. RT is thinking attentively, repeatedly, and frequently about oneself and one’s world and is characterized by Valence (positive-negative), Purpose (searching-solving), and Total quantity (much-little).
Methods
Estimated IQ and RT dimension scores were assessed at baseline in a sample of older adults (N=120, Mage=74 years), who thereafter had blood drawn up to 10 times semiannually (n=799). Models adjusted for BMI, chronological age, and statin medication.
Results
Higher IQ was associated with lower IL-6 (γ=−0.225, SE=0.111, p=.045). Of the RT dimensions, only more Total RT predicted lower IL-6 (γ= −0.037, SE=0.011, p=.001), an effect that was not moderated by Valence or Purpose. More Total RT accounted for part of the effect of IQ on IL-6 (indirect effect = −0.06 [CI = −0.14, −0.002]). There was also a significant interaction between IQ and Total RT (F(1,119)=6.97, p=.009), in which more Total RT was more strongly associated with lower IL-6 for people with lower IQ.
Conclusions
Although some forms of RT such as worry may have negative health correlates for older adults, engaging in RT per se can be healthy insofar as it also encompasses planning, processing, and coping. Older adults with higher IQ were more likely to engage in RT, but those with average IQ benefitted the most with regard to a marker of systemic inflammation.
Keywords: cognitive function, IQ, repetitive thought, inflammation, interleukin-6, older adults
People with better premorbid cognitive function enjoy a reduced risk of premature mortality, whether cognitive function is operationalized as childhood intelligence (see (1) for a meta-analytic review) or as results of brief cognitive testing in older adulthood (e.g., 2–4). Cognitive function predicts all-cause mortality and cause-specific deaths from cardiovascular disease, stroke, and infection as well as from dementia (2). The relationship between cognitive function and all-cause mortality may be explained in part by its relationship to systemic inflammation. Inflammatory markers such as interleukin-6 (IL-6) and C-reactive protein (CRP) predict mortality from multiple causes (5). Among young and midlife adults, better cognitive function associated with lower inflammatory markers including CRP, fibrinogen, and erythrocyte sedimentation rate (1, 6, 7). Among older adults, better executive cognitive function and processing speed correlated with lower inflammatory markers including CRP and IL-6 (8). The relationship between cognitive function and inflammation held true for childhood IQ (1), suggesting that although inflammation may compromise brain health and cognitive function, cognitive function may also protect against development of systemic inflammation. Consistent with this causal direction, better presurgical cognitive function predicted less increase in IL-6 after surgery among coronary artery bypass patients (9).
A number of potential mechanisms for cognitive function’s effects on inflammation and health have been proposed (see (10) for a review): Better cognitive function is associated with better health behavior and higher socioeconomic position and may also index better system integrity (i.e., general physiological makeup). However, statistical adjustment for these factors does not completely explain the relationship (7, 11). Another potential pathway and the focus of the present study is repetitive thought (RT). RT can be defined as thinking attentively, repeatedly, and frequently about oneself and one’s world (12). The construct encompasses discrete forms of RT (e.g., worry, planning, processing, reflection, rumination) and characterizes them along three orthogonal dimensions: Valence (positive or negative content), Purpose (searching, questioning, and uncertainty or solving, planning, and certainty), and Total (high or low propensity for RT of all kinds).
In older adults, cognitive function correlated with RT dimension scores: executive functions were specifically related to Valence, and IQ was specifically related to more solving Purpose and higher Total RT. The finding for Total indicates that people with higher IQ reported engaging in more RT regardless of Valence or Purpose (13). Although some forms of RT have been linked to poorer health and higher risk biomarkers (e.g., worry; 14–16), other forms may be beneficial (e.g., emotional processing; 17). In addition, engaging in RT per se may have some benefits. Total RT was more highly correlated cross-sectionally with higher perceived stress (r = .52) than with higher depressive symptoms (r = .30) or lower psychological well-being (r = .16; 13). Thinking about stressors and feelings, even negative feelings, may have positive consequences: the Total dimension of RT was also prospectively associated with higher perception of personal growth (18). Among HIV seropositive men, cognitive processing (“deliberate, effortful, or long-lasting thinking”) after bereavement was associated with a greater likelihood of finding meaning and lower mortality risk (19, p. 980). In general, thinking more about both the positive and negative aspects of oneself and one’s world may promote a number of adaptive responses, including better self-understanding and more effective planning and coping with stress (20), and provide another pathway from cognitive function to health.
The present study was based on a sample of older adults whose trait RT was characterized using the dimensional model and among whom IQ was correlated with more solving Purpose and higher Total RT (13). A subset of this sample also had blood drawn every six months for up to 5 years following their psychological assessment. This subsample was used to test the hypothesis that IQ would be prospectively associated with lower IL-6. IL-6 levels increase with “normal” aging, but higher levels increase the risk for many diseases of older age, including osteoporosis, lymphoma, and Alzheimer’s disease (21, 22) Elevated IL-6 also increases risk of premature mortality among healthy older adults (23, 24). IL-6 stimulates production of C-reactive protein, which is also considered a risk factor for morbidity (particularly cardiovascular) and mortality (23, 24) but has been described as “a surrogate for interleukin-6 action” (22, p. 132).
The relationship between IQ and IL-6 was then probed further by testing whether RT mediated this effect: People with higher IQ engaged in more solving Purpose and more Total RT, which accounted for part of the relationship between IQ and IL-6. Finally, a moderation model was tested to explore whether any relationship between RT and IL-6 was equal across levels of IQ. This model was motivated in particular by the question of whether engaging in more Total RT would mainly benefit individuals with higher IQ or benefit people across a range of IQs.
Methods
Participants
Participants were 120 community-dwelling older adults. The sample mean age was 74 years at enrollment (range, 60–93 years). All participants were married at enrollment, although only one member of the couple was enrolled to avoid dyadic dependency in the data. The sample was 42% male and 58% female, consistent with the sex ratio in older age. A majority of the sample was white (96%) and the rest, African-American (4%). Median education was 16 years (range= 7–22), and median annual income was $60,000 (range= $12,000-$400,000). Exclusion criteria included diseases or disorders affecting the immune system, chemotherapy or radiation treatment within the past 5 years, unwillingness to undergo vaccination or venipuncture, immunomodulatory medications including opioids and steroids, and more than two of the following classes of medications: psychotropics, antihypertensives, hormone replacement, or thyroid supplements. No participants were cognitively impaired in baseline testing for verbal memory and executive cognitive functioning.
Procedure
Participants were recruited from a volunteer subject pool maintained by the Sanders-Brown Center on Aging at the University of Kentucky. All study materials and procedures were approved by the University of Kentucky Institutional Review Board. Prospective participants were contacted and screened by phone. Those who were interested and eligible gave informed consent, were enrolled, and completed questionnaire measures verbally with the assistance of a research assistant and response cards. Interviews occurred at 6-month intervals over 5 years (up to 10 waves) between November 2006 and April 2011. At each completed wave, participants received a $20-dollar gift card. Following each interview visit, study nurses drew blood. Blood draws were scheduled for the morning to reduce extraneous variance from circadian rhythms in cytokines (25). On average, participants had 6.7 available IL-6 data points (SD= 2.4, median =7, range = 1–10). Of the 120 participants, 114 had valid IL-6 data at Wave 1, 91 at Wave 2, 107 at Wave 3, 105 at Wave 4, 86 at Wave 5, 92 at Wave 6, 70 at Wave 7, 76 at Wave 8, 30 at Wave 9, and 28 at Wave 10. Of the 1,200 possible IL-6 data points, missing data were present because 61 people missed one or more blood draw appointments or had problems with blood withdrawal (98 person-waves missing), 30 people dropped out (152 person-waves missing), 12 people had extreme values that were dropped from analysis (see below; 12 person-waves) and 5 people died (24 person-waves missing). In addition, 54 people did not complete all 10 waves before the end of the study (115 person-waves missing). Overall, 799 waves of data were available for analysis.
Measures
IQ
An estimate of full-scale IQ was derived from the North American Adult Reading Test (26), administered by an interviewer at baseline. Participants were asked to read a list of 61 irregularly spelled words. Each incorrectly pronounced word counted as one error. The estimated score has the same population distribution as other measures of IQ (mean = 100; SD = 15). Correlations between estimated IQ and results of IQ testing using a full battery (Wechsler Adult Intelligence Scale) were highest for verbal (r = .83) and full-scale (r = .75) IQ and lower for performance IQ (r = .40) (26). In the absence of dementia, it is considered to estimate premorbid, crystallized intelligence rather than fluid intelligence. Therefore, this measure largely reflects cognitive capacity prior to any potential cognitive changes due to age or health in this sample.
Repetitive thought
Prior to the present analysis, the structure of trait RT was established and calculation of dimension scores was performed on a larger sample (n = 179), of which the present sample (n = 120) is a majority subset (13). At their first interview, participants were administered multiple repetitive thought measures assessing worry, intrusive thoughts, rumination, depressive rumination (symptom focus, self-analysis, and self-reproach), reflection, emotional processing, and savoring (reminiscing, savoring, and anticipation). Median item-total correlation for scale scores was .29, indicating individual differences in RT Total. Scale scores were also subjected to multidimensional scaling, which replicated the Valence and Purpose dimensions previously identified in younger adults (12). Dimension scores for each individual were then created by multiplying standardized scale scores by dimension weights. Dimension scores were stable over a 3-year follow-up (r = .52–.66; 18).
Covariates
Three covariates were selected that could account for extraneous variance in IL-6 without overcontrolling or compromising statistical power (27, 28): BMI, age at study entry, and statin use. Height and weight (for BMI) were self-reported at the first interview; age was calculated as the difference in years between date of birth and the first interview date; and statin use was coded by a study nurse from prescription medication lists.
Including BMI in the models adjusted for differences in IL-6 due to adiposity. Although BMI is potentially related to IQ, preliminary evidence suggested that the relationship was modest (r = −.13) and therefore overcontrol was unlikely. One female participant was missing BMI, which was replaced with the sex-specific mean. Including age at study entry adjusted for increases in systemic inflammatory markers with age. Including statin use adjusted for its anti-inflammatory effects (29).
An exploratory model added depressive symptoms from the Geriatric Depression Scale (GDS; 30) due to known relationships between RT and depressive symptoms (13) and between depressive symptoms and inflammation (31, 32, 33). The GDS assesses depressive symptoms excluding somatic complaints commonly associated with aging. The GDS was administered at each wave and demonstrated good internal consistency at all waves (α=.69–.82). Because the other explanatory variables in the model were at Level 2 (people), the Level 2 mean across all waves was used.
IL-6
IL-6 was assayed from serum using high-sensitivity enzyme-linked immunosorbent assays (ELISA; R&D Systems). Median inter-assay CV across the study period was 6.44 (range = 3.1–13.3) and median intra-assay CV was 2.17 (range = 1.4–3.7). IL-6 was log10-transformed to improve normality. Raw IL-6 values for included data ranged from 0.53 – 92.65 pg/mL (median = 2.05 pg/mL across all waves). Twelve extreme values (> 400 pg/mL) were dropped from analysis; study records suggested that these were due to acute infections. Taking the median quartiles (across waves) as the best characterization of the distribution, they were comparable to quartiles from a large sample of older adults selected as “high functioning” (23, p. 507): 25th percentile, 1.46 pg/mL in the comparison sample vs. 1.33 pg/mL in the present sample; median, 2.08 pg/mL in the comparison sample vs. 2.01 pg/mL in the present sample; 75th percentile, 3.19 pg/mL in the comparison sample vs. 3.07 pg/mL in the present sample.
Data analysis
To account for the repeated assessment of IL-6 with different people having different numbers of assessments, data were analyzed using a multilevel model (SAS PROC MIXED) that utilized all available data. People were at Level 2 and IL-6 assessments at Level 1. Models included a random intercept to account for individual differences in IL-6 at the first assessment as well both a fixed and random effect of time (as semiannual wave number), centered around the first wave. The fixed effect of time accounts for higher likelihood of dropout among people with higher IL-6, that is, for data that were not MCAR. The random effect of time accounts for autoregressive relationships between IL-6 assessments. All predictors were at Level 2 and were centered around the sample mean for analysis. Fixed effects were estimated using maximum likelihood, with Kenward-Roger correction for estimation bias and degrees of freedom. Alpha was set at .05 for all inferential tests. Fixed effects are reported as unstandardized γ weights, which can be interpreted in the same manner as unstandardized beta weights in regression. IL-6 was standardized for analysis, so the fixed effects should be interpreted as the SD difference in IL-6 associated with a 1-unit difference in the predictor. Aikake’s information criterion (AIC) is reported for each model; the AIC provides an index of fit that takes model parsimony into account. Note that AIC provided by SAS accounts for the number of random but not fixed effects in the model. Therefore, twice the number of fixed effects was added to the reported AIC. Mediation was tested using the Rmediation program (34; https://amplab.shinyapps.io/MEDCI/), which provided an estimate of the indirect effect along with its 95% confidence interval. Simple slopes were estimated by recentering predictors at high and low values.
Results
Table 1 shows descriptive statistics and correlations among model predictors. There were statistically significant relationships among the covariates; for example, higher BMI was associated with younger chronological age and higher likelihood of statin use. However, although IQ tended to be related to lower BMI and older age, these relationships were not statistically significant. As previously reported in the full sample (13), IQ was unrelated to RT Valence (p = .50) but related to more solving Purpose (p = .005) and more Total (p = .038). The sample as a whole had higher than average IQ, in the context of a relatively large range (84–127) and interquartile range (108–119). Therefore, the findings should be interpreted as effects of IQ in the range from average to above average.
Table 1.
Descriptive statistics for and correlations among model predictors and covariates (N = 120).
| Variable | Mean | SD | Correlations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | |||
| 1. BMI | 27.5 | 4.9 | - | ||||||
| 2. Age at entry | 74.1 | 5.6 | −.26* | - | |||||
| 3. Statin use | 58% | .19* | .01 | - | |||||
| 4. RT Valence | 0.12 | 6.2 | .02 | −.02 | .00 | - | |||
| 5. RT Purpose | −0.14 | 4.2 | −.12 | .06 | .18* | .04 | - | ||
| 6. RT Total | 0.17 | 5.3 | .08 | −.21* | .02 | −.14 | −.02 | - | |
| 7. IQ | 113.1 | 8.0 | −.13 | .14 | −.02 | .06 | .25* | .19* | - |
p < .05.
Note: BMI = body mass index; RT = repetitive thought; IQ = intelligence quotient. Higher Valence is more positive and less negative; higher Purpose is more solving and less searching.
The first hypothesis, that higher IQ would predict lower IL-6, was supported. Table 2 shows the results of the models. In Model 1, which included only IQ, each 15-point difference in IQ was associated with 0.21 SDs lower IL-6. However, this difference was not statistically significant (p = .092). In Model 2, however, there was evidence that covariates accounted for “error” variance in the model, as the γ for IQ was slightly larger (−0.23) and statistically significant (p = .045).
Table 2.
Results of multi-level models predicting log-transformed IL-6 (N = 120, n = 799).
| Null model | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||
|---|---|---|---|---|---|---|---|
| Fixed effect | 1-unit scaling | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) | γ (SE) |
| Intercept | 1 SD (Z) | 0.027 (0.066) | 0.034 (0.065) | 0.209 (0.100) | 0.186 (0.101) | 0.201(0.100) | 0.195 |
| IQ | 15 points | −0.205† (0.121) | −0.225*(0.111) | −0.143(0.114) | −0.146(0.113) | ||
| Time | 1 wave | −0.130**(0.024) | −0.130**(0.024) | −0.131**(0.024) | −0.129**(0.024) | ||
| BMI | 1 point | 0.056**(0.013) | 0.058** (0.012) | 0.057**(0.012) | 0.056**(0.012) | ||
| Age at entry | 1 year | 0.040**(0.011) | 0.031** (0.011) | 0.033**(0.011) | 0.031*(0.011) | ||
| Statin use | 1 = yes, 0 = no | 0.104(0.121) | 0. 138 (0.120) | 0.124(0.119) | 0.137(0.119) | ||
| RT Valence | 1 point | −0.010(0.010) | −0.009(0.010) | 0.003(0.012) | |||
| RT Purpose | 1 point | −0.004(0.014) | 0.001(0.014) | −0.002(0.014) | |||
| RT Total | 1 point | −0.037*(0.011) | −0.033*(0.014) | −0.038*(0.012) | |||
| GDS | 1 point | 1.079(0.678) | |||||
| Random effect | |||||||
| Intercept variance | 0.417 | 0.402 | 0.349 | 0.366 | 0.347 | 0.331 | |
| Time slope variance | - | - | 0.009 | 0.009 | 0.009 | 0.009 | |
| Residual variance | 0.564 | 0.565 | 0.520 | 0.521 | 0.522 | 0.521 | |
| Model fit | |||||||
| −2LL | 2036.2 | 2033.3 | 1949.8 | 1943.5 | 1941.9 | 1939.4 | |
| AIC | 2042.2 | 2043.3 | 1979.8 | 1981.5 | 1983.9 | 1985.4 |
p < .10;
p < .05;
p < .001.
Note: BMI = body mass index; RT = repetitive thought; IQ = intelligence quotient. Higher Valence is more positive and less negative; higher Purpose is more solving and less searching.
Models 3 and 4 show the intervening or mediating role of RT. In Model 3, which included RT dimensions and covariates, the only RT dimension that was significantly associated with IL-6 was RT Total (p = .001). Each 1 SD increase in Total RT was associated with −0.20 SDs lower IL-61. When IQ and RT dimensions were entered together in Model 4, the effect of IQ decreased from −.23 to −.14 and was no longer statistically significant (p = .21). In addition, the indirect path from IQ to Total RT to IL-6 was statistically significant (−0.059, SE = 0.037, 95% CI = −0.143, −0.002). Therefore, the hypothesis that Total RT would mediate between IQ and IL-6 was supported.
The mediational model indicated that people with higher IQ engaged in more repetitive thought, which accounted for part of their lower IL-6. However, it is possible that some RT process (not captured by Valence and Purpose) differs between people with different IQs, and therefore more Total RT might not have equal effects across the range of IQs. Therefore, a final model was fit that tested the interactions between RT dimensions and IQ. There was a statistically significant interaction between IQ and Total RT (F (1,119) = 6.97, p = .009); the other RT dimensions did not interact with IQ (Valence, p = .79; Purpose, p = .69). Figure 1 illustrates the interaction between Total RT and IQ. At 10 IQ points below the sample mean (IQ = 103), more RT Total was significantly related to lower IL-6 (slope γ = −0.081, SE = 0.021, p < .001); at 10 IQ points above the sample mean (IQ = 123), this effect was not present (slope γ = 0.00, SE = 0.018, p > .99). Therefore, the hypothesis that IQ would interact with RT was supported, but in an unexpected direction. Among people with above average IQ, IL-6 was lower regardless of their Total RT. Among people with average IQ, IL-6 was lower for those who engaged in more Total RT.
Figure 1.
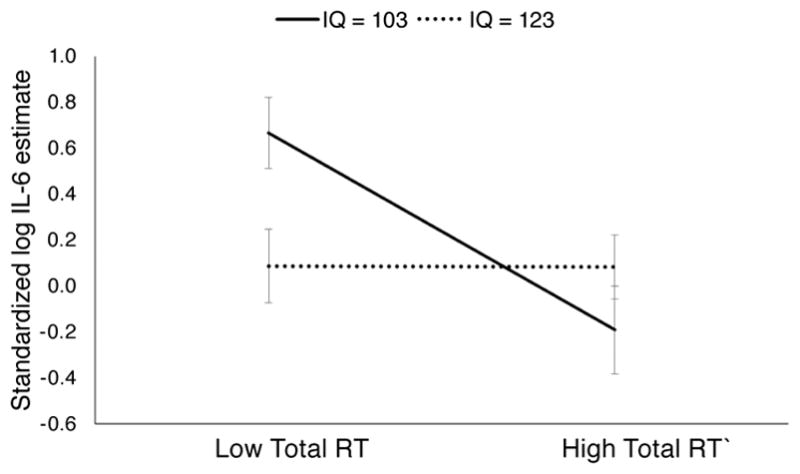
Standardized log IL-6 for people 10 IQ points below the sample mean (103) and 10 points above (123) as predicted by low (−1SD) Total repetitive thought (RT) and high (+1SD) Total RT. Model estimates are shown with their standard errors.
Exploratory Analyses
Additional analyses explored quadratic relationships between the RT dimensions and IL-6 by adding the squared, centered dimension scores to the models. There was a statistically significant quadratic effect of Total RT (F(1,123) = 10.32, p = .002), where the negative slope for Total RT was steeper at lower levels (γ = 0.0050, SE = 0.0015). However, adding quadratic effects to the mediational model did not further reduce the IQ γ, indicating no additional mediation above and beyond the linear effect; furthermore, the quadratic effect was not moderated by IQ (F(1,134 = 0.44, p = .51).
The effect of adding average depressive symptoms across the study period (i.e., the Level 2 effect of depressive symptoms) is shown in Model 5 of Table 2. More depressive symptoms tended to associate with higher IL-6 (p = 0.114), but other effects in the model were substantively unchanged and the statistically significant effect of Total RT increased slightly.
Finally, interactions between IQ, RT, and aging (i.e., change over the study period) or baseline age were tested to explore whether the effects of IQ and RT were dynamic over the study period and/or stronger at young-old or old-old age. Interactions with aging or baseline age were added to the models shown in Table 2, Models 2 and 3. There were no statistically significant interactions with aging (ps = .068 – .65). A nearly statistically significant negative interaction term between aging and RT total (γ = −0.009, SE = 0.005, p = .068) indicated that higher RT Total tended to be associated with even lower IL-6 at later study waves. There were also no statistically significant interactions with baseline age (ps = .21 – .82). Therefore, there was no evidence to reject the null hypothesis that the effects of IQ and RT hold equally across participant ages and across the study period.
Discussion
In this sample of healthy, community-dwelling older adults, individuals with higher IQ had lower levels of IL-6, a proinflammatory cytokine that is associated with health risk in older adults. A large study also found that executive cognitive functioning and processing speed were associated with lower IL-6 in older adults (8). However, in that study, it was not possible to determine whether cognitive function predicted lower IL-6, lower IL-6 preserved cognitive function, or some combination thereof. In contrast, in the present study, a “premorbid” estimate of cognitive function prospectively predicted IL-6 levels, indicating that cognitive function may protect against “inflammaging” (35). Furthermore, this effect was not due to BMI, chronological age, or statin use. It was detected in the average to above average range of IQ and in a demographically homogeneous sample. Although these restrictions of range also restrict generalizability, they also demonstrate that the effect is not due to demographic confounds or specifically to low IQ.
In the present sample, IQ was not correlated with current family income (r = .08, p = .049) and modestly correlated with highest education (r = .29, p = .005). Current income among older adults can be a poor index of socioeconomic status because retirees may be relying more on assets than income; furthermore, education can also be a poor index in older cohorts due to sex differences in education (i.e., women from older cohorts were less likely to pursue higher education than men from those cohorts). Perhaps for these reasons, adding income and education to Model 2 did not substantially affect the size of the effect of IQ (γ = −.21, p = .049). However, the complex relationships between socioeconomic status and IQ (36) suggest that their separate and joint effects on inflammation continue as a focus of investigation.
The relationship between IQ and IL-6 was partially mediated by Total RT, the propensity to engage in RT of all sorts. Higher Total RT has correlated with “negative” psychological states such as perceived stress and with “positive” psychological states such as perceived growth (13, 18). People with higher IQ also engaged in more Total RT, perhaps indicating that people with more cognitive resources are likely to use those resources to understand both positive and negative aspects of themselves and their worlds. Cognitive engagement with both positive and negative aspects, as well as engaging in both searching and solving, may reflect psychological flexibility and a larger “range of regulatory strategies” (37, 38). Such flexibility could improve both psychological and physical health (39).
More positive Valence was associated with lower IL-6, but this relationship was much smaller than the relationship with Total and was not statistically significant. In the exploratory analyses of interactions, there was some suggestion that more positive valence was associated with lower IL-6 in the presence of high solving Purpose and high Total or in the presence of high searching Purpose and low Total, but the simple main effects were not statistically significant (see Supplemental Digital Content 1). Another possibility is that more negative RT does not affect everyone to the same degree or in the same way. For example, in a daily diary study, there were large individual differences in the relationship between negative RT Valence and daily depressive symptoms. For some people, more negative RT on a particular day was associated with an increase in depressive symptoms on that day, but for others, mood was hardly affected (40). Likewise, neutrally valenced RT was associated with higher post-vaccination IL-6 among caregivers but lower IL-6 among controls (41). Identifying moderators of the relationship between RT Valence and inflammation is an important direction for future research.
Although higher IQ predisposed people to higher Total RT, people with average IQ had similar IL-6 to those with above-average IQ if they also had high Total RT. The model indicated that the highest IL-6 was among people with average IQ who engaged in little RT. At least within the range of IQs represented in this study, more RT was beneficial. This finding, if replicated, suggests a potential target for intervention: although IQ is not modifiable, it may be possible to teach people to recruit RT to improve their health, for example, using techniques from cognitive therapy. Expressive writing may serve many of the same functions as high Total RT and has been associated with better physiological functioning and health (42, 43).
There are limitations to the present study, including the homogeneity and limited IQ range of the sample as noted above. These sample characteristics reduce confounding by demographic qualities but also limit generalizability. These findings should be replicated in more diverse samples both with regard to demographics and IQ. In addition, there were strengths and weaknesses related to the assessments. First, the use of a measure of “premorbid” IQ was a strength of the study because it suggested causal direction. However, IQ was estimated from a brief test rather than measured with a more intensive testing battery. Furthermore, the test reflected crystallized and verbal intelligence to a greater degree than fluid and performance intelligence. The latter declines more on average with age and is difficult to estimate retrospectively. Truly prospective studies will be required to test premorbid effects of fluid intelligence on inflammation in older age. Second, the comprehensive assessment of RT dimensions was a strength of the study insofar as the entire RT repertoire of each person was measured rather than one RT construct. Although these dimensions had good temporal stability, future research could use briefer means of measuring RT dimensions (40) to examine whether there is correlated change in RT and IL-6 that is potentially moderated by IQ. Third, inflammation was reflected in only one marker (IL-6). However, IL-6 is associated with health risk in older adults (21, 22, 23, 24), and it was measured multiple times and prospectively, which is a desirable design when testing the effects of psychological traits (44).
Previous research has established a relationship between higher IQ and lower inflammatory markers and mortality risk. The present study adds to the research that suggests a prospective effect of cognitive function on inflammation and health. It further suggests that one of the ways that cognitive function improves health is by increasing the degree to which people think broadly and perhaps flexibly about themselves and their world. Finally, by incorporating RT into the relationship between cognitive function and an inflammatory marker, the study identifies a potential target to intervene in the process of “inflammaging” in older adults.
Supplementary Material
Glossary
- AIC
Aikake’s information criterion
- BMI
body mass index
- CRP
C reactive protein
- CV
coefficient of variability
- HIV
human immunodeficiency virus
- IQ
intelligence quotient
- IL
interleukin
- RT
repetitive thought
Footnotes
Exploratory models also tested the 2-way interactions among RT dimensions, but none were statistically significant, all p > .32. The 3-way interaction approached statistical significance, p = .066, with positive solving in the context of a high Total having the lowest predicted IL-6 and negative searching in the context of a low Total having the highest. See Supplemental Digital Content 1 for details of this interaction.
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to declare. The study was made possible by support from the Dana Foundation, the National Institute on Aging (R01-AG026307, K02-033629, P30-AG028383), and the National Institutes of Health (CTSA-UL1TR000117).
References
- 1.Calvin CM, Batty GD, Lowe G, Deary IJ. Childhood intelligence and midlife inflammatory and hemostatic biomarkers: the National Child Development Study (1958) cohort. Health Psychol. 2011;30:710–18. doi: 10.1037/a0023940. [DOI] [PubMed] [Google Scholar]
- 2.Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Psaty BM, Harris TB, Robbins JA, Burke GL, Kuller LH. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64:1251–61. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachs GA, Carter R, Holtz LR, Smith F, Stump TE, Tu W, Callahan CM. Cognitive impairment: an independent predictor of excess mortality: a cohort study. Ann Internal Med. 2011;155:300–8. doi: 10.7326/0003-4819-155-5-201109060-00007. [DOI] [PubMed] [Google Scholar]
- 4.St John PD, Montgomery PR, Kristjansson B, McDowell I. Cognitive scores, even within the normal range, predict death and institutionalization. Age Ageing. 2002;31:373–8. doi: 10.1093/ageing/31.5.373. [DOI] [PubMed] [Google Scholar]
- 5.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson H, Ahlborg B, Dalman C, Hemmingsson T. Association between erythrocyte sedimentation rate and IQ in Swedish males aged 18–20. Brain Behav Immun. 2010;24:868–73. doi: 10.1016/j.bbi.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Phillips AC, Batty GD, van Zanten JJ, Mortensen LH, Deary IJ, Calvin CM, Carroll D. Cognitive ability in early adulthood is associated with systemic inflammation in middle age: the Vietnam experience study. Brain Behav Immun. 2011;25:298–301. doi: 10.1016/j.bbi.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Tegeler C, O’Sullivan JL, Bucholtz N, Goldeck D, Pawelec G, Steinhagen-Thiessen E, Demuth I. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function - data from the Berlin Aging Study II. Neurobiol Aging. 2016;38:112–7. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Poole L, Ronaldson A, Kidd T, Leigh E, Jahangiri M, Steptoe A. Pre-operative cognitive functioning and inflammatory and neuroendocrine responses to cardiac surgery. Ann Behav Med. 2016;50:545–53. doi: 10.1007/s12160-016-9779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death how researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol Sci Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- 11.Calvin CM, Deary IJ, Fenton C, Roberts BA, Der G, Leckenby N, Batty GD. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. Int J Epidemiol. 2011;40:626–44. doi: 10.1093/ije/dyq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segerstrom SC, Stanton AL, Alden LE, Shortridge BE. A multidimensional structure for repetitive thought: what’s on your mind, and how, and how much? J Pers Soc Psychol. 2003;85:909–21. doi: 10.1037/0022-3514.85.5.909. [DOI] [PubMed] [Google Scholar]
- 13.Segerstrom SC, Roach AR, Evans DR, Schipper LJ, Darville AK. The structure and health correlates of trait repetitive thought in older adults. Psychol Aging. 2010;25:505–15. doi: 10.1037/a0019456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006;60:113–24. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 15.Kubzansky LD, Kawachi I, Spiro A, Weiss ST, Vokonas PS, Sparrow D. Is worrying bad for your heart? A prospective study of worry and coronary heart disease in the Normative Aging Study. Circulation. 1997;95:818–24. doi: 10.1161/01.cir.95.4.818. [DOI] [PubMed] [Google Scholar]
- 16.Segerstrom SC, Solomon GF, Kemeny ME, Fahey JL. Relationship of worry to immune sequelae of the Northridge earthquake. J Behav Med. 1998;21:433–50. doi: 10.1023/a:1018732309353. [DOI] [PubMed] [Google Scholar]
- 17.Master SL, Amodio DM, Stanton AL, Yee CM, Hilmert CJ, Taylor SE. Neurobiological correlates of coping through emotional approach. Brain Behav Immun. 2009;23:27–35. doi: 10.1016/j.bbi.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segerstrom SC, Eisenlohr-Moul TA, Evans DR, Ram N. Repetitive thought dimensions, psychological well-being, and perceived growth in older adults: a multilevel, prospective study. Anxiety Stress Coping. 2015;28:287–302. doi: 10.1080/10615806.2014.947285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bower JE, Kemeny ME, Taylor SE, Fahey JL. Cognitive processing, discovery of meaning, CD4 decline, and AIDS-related mortality among bereaved HIV-seropositive men. J Consult Clin Psychol. 1998;66:979–986. doi: 10.1037//0022-006x.66.6.979. [DOI] [PubMed] [Google Scholar]
- 20.Watkins ER. Constructive and unconstructive repetitive thought. Psychol Bull. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Ann Rev Med. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 22.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Internal Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Amer J Med. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 24.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality A population-based, prospective study. Thromb Haemostas. 2006;95:511–8. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 25.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005 May 17;12(3):131–40. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 26.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. The Clin Neuropsychologist. 1989;3:129–36. [Google Scholar]
- 27.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segerstrom SC. Biobehavioral controls: threats to psychoneuroimmunology research? Brain Behav Immun. 2009;23:885–6. doi: 10.1016/j.bbi.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 29.Rezaie-Majd A, Maca T, Bucek RA, Valent P, Müller MR, Husslein P, Kashanipour A, Minar E, Baghestanian M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–9. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Dentino AN, Pieper CF, Rao K, Murali K, Currie MS, Harris T, Blazer DG, Cohen HJ. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Amer Geriatr Soc. 1999;47:6–11. doi: 10.1111/j.1532-5415.1999.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 32.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 33.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatr. 2003;54:566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 34.Tofighi D, Thoemmes F. Single-level and multilevel mediation analysis. J Early Adolesc. 2014;34:93–119. [Google Scholar]
- 35.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 36.Nisbett RE, Aronson J, Blair C, Dickens W, Flynn J, Halpern DF, Turkheimer E. Intelligence: new findings and theoretical developments. Amer Psychol. 2012;67:130–59. doi: 10.1037/a0026699. [DOI] [PubMed] [Google Scholar]
- 37.Bonanno GA, Burton CL. Regulatory flexibility: an individual differences perspective on coping and emotion regulation. Persp Psychol Sci. 2013;8:591–612. doi: 10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- 38.Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clin Psychol Rev. 2010;30:865–78. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy J, Segerstrom SC. Intra-individual variability and psychological flexibility: Affect and health in a National US sample. Journal of Research in Personality. 2016 published before print http://dx.doi.org/10.1016/j.jrp.2016.04.002.
- 40.Segerstrom SC, Hardy JK, Evans DR, Boggero IA, Alden LE, Stanton AL. Briefly assessing repetitive thought dimensions: valence, purpose, and total. Assessment. 23:614–623. doi: 10.1177/1073191115586458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segerstrom SC, Schipper LJ, Greenberg RN. Caregiving, repetitive thought, and immune response to vaccination in older adults. Brain Behav Immun. 2008;22:744–52. doi: 10.1016/j.bbi.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederhoffer KG, Pennebaker JW. Sharing one’s story: On the benefits or writing or talking about emotional experience. In: Snyder CR, Lopez SJ, editors. Handbook of positive psychology. New York: Oxford University Press; pp. 573–583. [Google Scholar]
- 43.Smyth JM. Written emotional expression: effect sizes, outcome types, and moderating variables. J Consult Clin Psychol. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]
- 44.Segerstrom SC, Smith GT. Methods, variance, and error in psychoneuroimmunology research: the good, the bad, and the ugly. In: Segerstrom SC, editor. Oxford handbook of psychoneuroimmunology. New York: Oxford University Press; pp. 421–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


