Abstract
Introduction
Sensitive detection of cognitive decline over the course of preclinical Alzheimer’s disease is critical as the field moves toward secondary prevention trials.
Methods
We examined Aβ-related change in several variations of the preclinical Alzheimer cognitive composite (PACC) and each individual PACC component in clinically normal (CN) older participants in the Harvard Aging Brain Study. We then examined the PACC variations in the Alzheimer’s Disease Cooperative Study Prevention Instrument Study as a replication cohort.
Results
Aβ+ CN individuals demonstrated longitudinal decline on all individual PACC components and all PACC variations. Aβ group differences emerged earlier when Free and Cued Selective Reminding Test Free Recall was included in the PACC. PACC decline was associated with Clinical Dementia Rating progression.
Discussion
This independent data set and a replication cohort confirm the ability of the PACC to capture both early and late cognitive decline during the preclinical stages of Alzheimer’s disease, which may prove advantageous in the prevention trial design.
Keywords: Preclinical Alzheimer’s disease, Secondary prevention, Cognitive composite, Amyloid PET
1. Background
The pathophysiological processes of Alzheimer’s disease (AD) begin at least a decade before clinical symptoms emerge [1,2], providing a window to intervene before widespread neuronal damage has ensued. Abnormal accumulation of Aβ is common among older clinically normal (CN) individuals and consistently associated with cognitive decline over time [3–8], supporting the framework that Aβ+ CN individuals are indicative of a preclinical stage of AD [2].
There has been a recent shift in the implementation of clinical trials in the AD field, such that several clinical trials aimed at preventing cognitive decline in CN individuals at risk for AD dementia are ongoing [9–12]. Work from observational studies consistently shows that Aβ+ CN individuals show greater decline than Aβ− CN individuals on cognitive composites spanning multiple domains [5,7,13,14]. Data-driven approaches have similarly suggested that multidomain cognitive composites are optimal for capturing gradual decline for more than the decade before dementia [15,16]. To establish a cognitive end point for use in secondary prevention trials, Donohue et al. developed a composite based on cognitive domains demonstrating gradual decline in the decade before AD dementia, resulting in the preclinical Alzheimer cognitive composite (PACC) [17]. The PACC was conceived as a multicognitive domain composite heavily weighted toward episodic memory, including both a list learning memory task and paragraph recall, plus a timed executive function task, and a global cognition measure. The PACC was initially tested in three separate CN cohorts: Aβ-related decline was examined in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Australian Imaging, Biomarker and Lifestyle Study of Ageing (AIBL), whereas group differences based on APOE4 and Clinical Dementia Rating (CDR) progression were examined in the Alzheimer’s Disease Cooperative Study Prevention Instrument (ADCS-PI) [17]. Given limited overlap in neuropsychological tests across cohorts, cohort-specific PACC versions were examined: the ADNI PACC used ADAS-cog Word Recall, Logical Memory Delayed Recall, Mini-Mental State Examination (MMSE), and Digit Symbol; AIBL used CVLT Delayed Recall, Logical Memory Delayed Recall, MMSE, and Digit Symbol; ADCS-PI used the Free and Cued Selective Reminding Test (FCSRT), NYU Paragraph Recall score [18], modified MMSE [19], and Digit Symbol. These analyses showed significant Aβ-related decline at Year 3 in AIBL and in the ADCS-PI APOE4+ group at Year 3. ADNI results were less clear, with a significant Aβ group difference at Year 2 that did not remain significant at Year 3.
Although the initial work investigating cohort-specific versions of the PACC was promising [17], until now, longitudinal PACC data with all the specific test components used in the current prevention trials in a cohort characterized by Aβ status has been unavailable. Sufficient neuropsychological follow-up has recently become available in the Harvard Aging Brain Study (HABS) (mean = 3.6 ± 1.3 years, similar to the length of the A4 Study), providing an opportunity to investigate the PACC in a CN cohort that has all four neuropsychological PACC tests and baseline Aβ status measured with PET imaging. The overarching goal of the present study was to contribute to validation of the PACC by determining whether Aβ+ CN individuals show significantly different change over time compared with Aβ− CN individuals over the time frame of a current prevention trial. Secondary aims were to examine Aβ-related change in each of the individual PACC components and the impact of adding or eliminating individual PACC components. Additional analyses, which focused on Aβ+ CN individuals who progressed or remained stable on the CDR, were performed to establish whether the PACC could detect cognitive decline throughout the entire continuum of preclinical AD (because Aβ+ CN individuals who progress to CDR 0.5 within a few years are likely at a later preclinical stage). Finally, we tested the PACC variations in the ADCS-PI study, which also contains the FCSRT [17] by examining differences across APOE4 and CDR progressor groups to determine whether there was consistency across the HABS and the ADCS-PI.
2. Methods
2.1. Participants
Two hundred seventy-seven CN participants from the HABS were included (Table 1). Participants were recruited from the community through media and outreach events. At baseline, all participants were with CDR = 0, within education-adjusted norms on the Logical Memory Delayed Recall and MMSE ≥25.
Table 1.
HABS demographics. APOE4 status was missing on 10 Aβ− and 4 Aβ+ participants in the HABS
| All HABS | Aβ− | Aβ+ | |
|---|---|---|---|
| N (%) | 277 | 206 (74.4%) | 71 (25.6%) |
| Age (y)* | 73.5 ± 6.0 | 72.9 ± 6.0 | 75.2 ± 5.7 |
| Female (%) | 59% | 59% | 61% |
| Education | 15.8 ± 3.1 | 15.6 ± 3.1 | 16.4 ± 2.8 |
| APOE4+ (%)* | 29.3% | 18.4% | 61.2% |
| Follow-up (y) | 3.7 ± 1.3 | 3.7 ± 1.3 | 4.0 ± 1.1 |
| Logical Memory | 13.7 ± 3.3 | 13.6 ± 3.4 | 14.1 ± 3.0 |
| Digit Symbol | 47.3 ± 10.7 | 47.3 ± 11.1 | 47.1 ± 9.5 |
| MMSE | 29.0 ± 1.1 | 29.1 ± 1.1 | 28.8 ± 1.0 |
| FCSRT-Total | 47.6 ± 0.9 | 47.6 ± 0.9 | 47.7 ± 0.8 |
| FCSRT-Free | 33.3 ± 5.4 | 33.3 ± 5.3 | 33.2 ± 5.8 |
| FCSRT-96 | 80.54 ± 6.07 | 80.11 ± 6.42 | 80.68 ± 5.95 |
Abbreviations: FCSRT, Free and Cued Selective Reminding Test; HABS, Harvard Aging Brain Study; MMSE, Mini-Mental State Examination.
NOTE. Means and standard deviations are listed for continuous variables.
Variables with significant differences across Aβ groups (P < .05).
Study protocols were approved by the Partners Institutional Review Board, and all participants provided informed consent.
2.2. PIB-PET imaging
For HABS, C11-PIB was synthesized and administered at MGH (Siemens ECAT EXACT HR+ scanner) [20]. Distribution volume ratio images were created with Logan plotting (40–60 min, cerebellar reference), and a global cortical aggregate was used to dichotomize participants into Aβ− and Aβ+ groups using a cutoff of 1.20 [20,21].
2.3. Neuropsychological testing
The PACC comprises (1) Logical Memory Delayed Recall, (2) MMSE Total score, (3) WAIS-R Digit Symbol coding, and (4) the FCSRT. Measures were z-transformed based on the baseline mean and standard deviation and averaged. We elected to average z-scores rather than sum across z-scores as done by Donohue et al. [17] to facilitate comparison across PACC variations with different number of components. The same version was administered each year for Logical Memory, Digit Symbol, and MMSE, whereas the FCSRT had alternate versions (A-B-C-A-B-C). The PACC is administered in the HABS by six certified neuropsychological testing raters. These are research assistants who are trained and certified in administration of all the neuropsychological tests by a licensed clinical neuropsychologist and recertified every year.
The Logical Memory score reported is Delayed Recall of an orally presented short story (Logical Memory IIa) [22]. The WAIS-R Digit Symbol score includes the number of items correctly completed in 90 seconds [23] and the MMSE is a global cognitive measure [24]. Logical Memory and Digit Symbol were scored according to the standard methods.
The FCSRT [25] is a multimodal associative memory measure, in which learning is enhanced by providing a visual and a semantic category cue. During the testing phase, the semantic cue is provided for items that were not freely recalled. Thus, two primary scores are generated: (1) Free Recall is the sum of items freely recalled (up to a total of 48) and (2) Total Recall is the sum of free and cued recall (up to a total of 48). Given that Free and Total scores may capture different aspects of associative memory failure in preclinical AD [26–30], we examined the contributions of these measures as separate scores into the PACC and a measure that combined these scores into a single component (yielding a maximum score of 96 for that component, and referred to herein as FCSRT-96) (eMethods 1; eFig. 1–3).
To examine the contribution of different individual components on the PACC, we iteratively eliminated individual components and examined the ratio between the β estimate and standard error describing the difference between Aβ+ and Aβ− groups. On the basis of the results of the HABS, a subset of PACC variations was additionally explored using data from the ADCS-PI study, as this cohort also includes the FCSRT [17].
2.4. CDR progression
Functional progression on the CDR [31] was used to investigate the relationship of PACC decline with clinically relevant change in daily life function. Aβ+ participants were categorized based on whether they progressed to CDR 0.5 at any time during follow-up. In addition, PACC slopes were calculated across participants and used as a predictor in a survival analysis examining time to CDR 0.5.
2.5. Statistical models
Analyses were performed using R v3·3. Linear mixed models (LMMs) were used to examine longitudinal cognitive change [21] and mixed model of repeated measures (MMRMs) analyses explored group differences at each annual assessment without assuming a linear trajectory [17]. We investigated both LMM and MMRM approaches, as LMMs are commonly used in observational studies investigating Aβ-related decline [5,7,13,21], whereas MMRMs are often used in clinical trials [17]. LMMs included main effects of age and Aβ, their interactions with time (from baseline), and a random intercept for each participant. MMRM analyses controlled for baseline composite and age, with a compound symmetric correlation structure and heterogeneous variance [17]. Cox proportional hazards models assessed CDR progression, controlling for age. All P values were two-sided and no correction for multiple comparisons was performed.
3. Results
3.1. Aβ+ decline on the PACC
There were no baseline differences in any individual PACC component across Aβ groups (Table 1). Aβ+ showed a significant decline across all PACC iterations compared with Aβ− using LMMs (P values <.0001; eTable 1). The difference between Aβ groups ranged between −0.075 and −0.151 average z-score units per year. The Aβ+ group consistently showed worse performance over time across each individual PACC measure (eTable 1, Fig. 1). A similar pattern of decline was observed when examining PACC change with respect to APOE4 status rather than Aβ status (eTable 1).
Fig. 1.
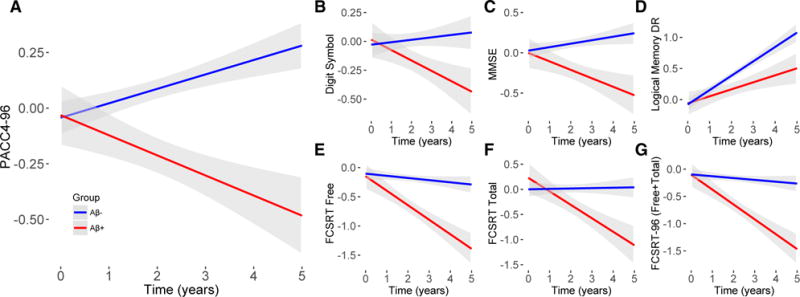
Longitudinal change by Aβ status for the PACC and individual tests in the HABS. Aβ-related decline in present for the PACC4-96 (A) and all individual components (B–F). Z-scores are shown on the y-axis for all tests. Abbreviations: HABS, Harvard Aging Brain Study; PACC, preclinical Alzheimer cognitive composite.
Analyses across PACC iterations were repeated using an MMRM approach. In general, there was significantly worse performance in Aβ+ compared with Aβ− that began after 1 year of follow-up and remained significant after 5 years of follow-up (eTable 2). Examination of effect sizes across PACC iterations revealed that all variations including the FCSRT-Free resulted in qualitatively larger effect sizes at both Years 3 and 5. Removal of Logical Memory Delayed Recall resulted in a smaller effect size at Year 3 but not at Year 5. Removal of the MMSE increased the effect size at Year 3 but not at Year 5 (Fig. 2).
Fig. 2.
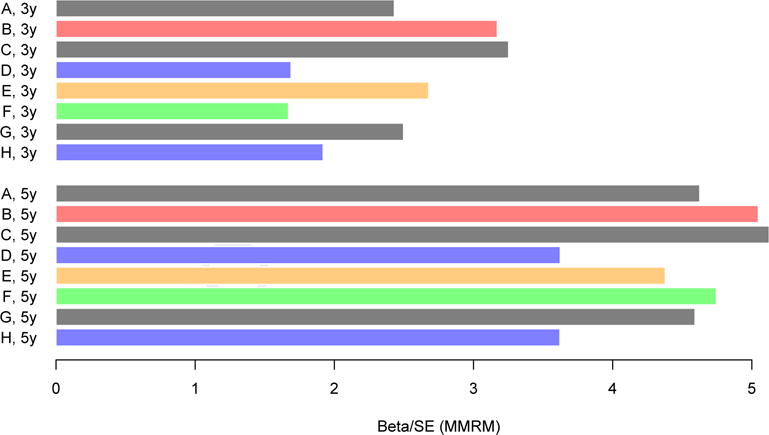
Effect sizes reflecting the group difference between Aβ+ and Aβ− groups after 3 and 5 years of follow-up across PACC iterations from the HABS. Effect sizes reflect the β estimate of the group difference between Aβ+ and Aβ− groups, divided by the standard error of that estimate. Blue is used to highlight PACC iterations that have excluded the FCSRT-Free, green is used to highlight the PACC iteration that excludes LMDR, and orange is used to highlight the PACC iteration that excludes the MMSE. Red is used to highlight the PACC-96. Removal of the FCSRT-Free results in smaller effect sizes at Year 3 and 5 (blue). Removal of LMDR results in a smaller effect size at Year 3 but not at Year 5 (green). Removal of the MMSE results in a larger effect size at Year 3 but not at Year 5 (orange). All other iterations are shown in gray. PACC iterations are as follows: (A) five components (FCSRT-Free, FCSRT-Total, LMDR, DS, and MMSE); (B) FCSRT-Free and FCSRT-Total combined (FCSRT-96, LMDR, DS, and MMSE); (C) no FCSRT-Total (FCSRT-Free, LMDR, DS, and MMSE); (D) no FCSRT-Free (FCSRT-Total, LMDR, DS, and MMSE); (E) no MMSE (FCSRT-Free, FCSRT-Total, LMDR, and DS); (F) no LMDR (FCSRT-Free, FCSRT-Total, DS, and MMSE); (G) no DS (FCSRT-Free, FCSRT-Total, LMDR, and MMSE); and (H) neither FCSRT measures (LMDR, DS, and MMSE). Abbreviations: FCSRT, Free and Cued Selective Reminding Test; HABS, Harvard Aging Brain Study; MMRM, mixed model of repeated measure; MMSE, Mini-Mental State Examination; PACC, preclinical Alzheimer cognitive composite.
3.2. PACC decline is associated with CDR progression
Sixty-two CN indivduals progressed to CDR 0.5 at follow-up (25 Aβ+ and 37 Aβ−). A survival analysis revealed greater risk of progression in Aβ+ compared with Aβ− (hazards ratio = 1.84, P = .021). Slopes reflecting change across all eight PACC iterations were significantly associated with risk of CDR progression, with each −0.10 z-score units per year being associated with Hazards ratios ranging between 1.40 and 1.65 (eTable 3).
To determine whether Aβ-related PACC decline differed by CDR progressor status, the Aβ+ group was divided into those that progressed to CDR 0.5 versus those that remained stable (eTable 4). The only individual components to show differences across groups at baseline were FCSRT-Free and FCSRT-96, which was significantly lower in the Aβ+ progressor group compared with the Aβ+ stable group (eTable 4). LMMs revealed that Aβ+ progressors showed greater decline across all PACC iterations and individual PACC components compared with Aβ+ stable and Aβ− groups (Fig. 3, eTable 5). The Aβ+ stable group did not differ from the Aβ− group across any PACC iteration or individual PACC component except FCSRT-Free (P = .0024; Fig. 3E) and FCSRT-96 (P = .0071; Fig. 3G).
Fig. 3.
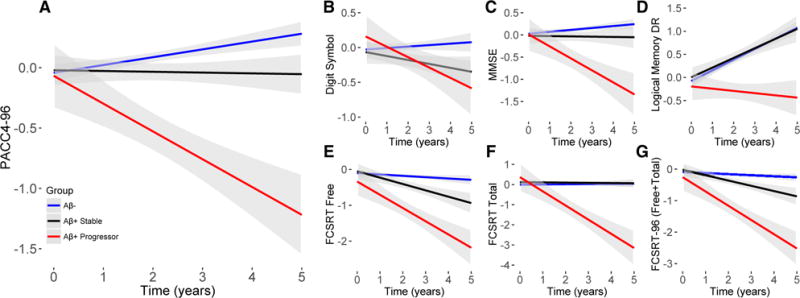
PACC decline by CDR progressor status in the HABS. A consistent pattern is present for the PACC4-96 (A) and individual components (B–F), such that decline is strongest in Aβ+ participants that also progress on the CDR 0.5. FCSRT-Free Recall is the only measure to show significant decline in the Aβ+ stable group (D). Z-scores are shown on the y-axis for all tests. Abbreviations: CDR, Clinical Dementia Rating; FCSRT, Free and Cued Selective Reminding Test; HABS, Harvard Aging Brain Study; PACC, preclinical Alzheimer cognitive composite.
Analyses examining the PACC iterations were repeated using an MMRM approach, revealing a significant decline in the Aβ+ progressor group that emerged after 2 years of follow-up compared with the Aβ− group for most PACC iterations. Significant differences between the Aβ+ progressor and Aβ+ stable groups emerged at 4 years of follow-up (eTable 6). Effect sizes comparing Aβ+ progressors and the Aβ− group at Year 3 were similar, with the greatest reduction observed after removing Logical Memory. Likewise, effect sizes across PACC iterations at Year 5 were similar, with the greatest reduction observed after removing both FCSRT measures (Fig. 4).
Fig. 4.
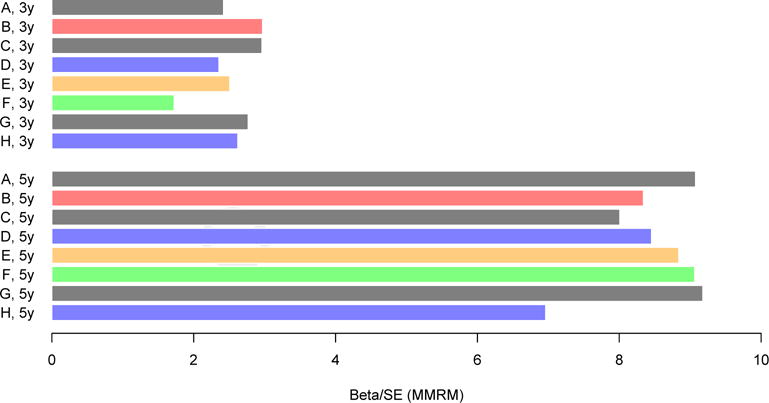
Effect sizes reflecting the group difference between the subset of Aβ+ CDR progressors and the Aβ− group after 3 and 5 years of follow-up across PACC iterations from the HABS. Effect sizes reflect the β estimate of the group difference between Aβ+ and Aβ− groups, divided by the standard error of that estimate. Blue is used to highlight PACC iterations that have excluded the FCSRT-Free, green is used to highlight the PACC iteration that excludes LMDR. Orange is used to highlight the PACC iteration that excludes the MMSE. Red is used to highlight the PACC-96. Removal of LMDR results in a smaller effect size at Year 3 but not at Year 5 (green). Removal of both FCSRT measures results in a smaller effect sizes at Year 5 (H). All other iterations are shown in gray. PACC iterations are as follows: (A) five components (FCSRT-Free, FCSRT-Total, LMDR, DS, and MMSE); (B) FCSRT-Free and FCSRT-Total combined (FCSRT-96, LMDR, DS, and MMSE); (C) no FCSRT-Total (FCSRT-Free, LMDR, DS, and MMSE); (D) no FCSRT-Free (FCSRT-Total, LMDR, DS, and MMSE); (E) no MMSE (FCSRT-Free, FCSRT-Total, LMDR, and DS); (F) no LMDR (FCSRT-Free, FCSRT-Total, DS, and MMSE); (G) no DS (FCSRT-Free, FCSRT-Total, LMDR, and MMSE); (H) neither FCSRT measures (LMDR, DS, and MMSE). Abbreviations: FCSRT, Free and Cued Selective Reminding Test; HABS, Harvard Aging Brain Study; MMRM, mixed model of repeated measure; MMSE, Mini-Mental State Examination; PACC, preclinical Alzheimer cognitive composite.
3.3. Similar pattern of PACC decline in ADCS-PI
Given that analyses within the HABS suggest an early involvement of FCSRT-Free in preclinical AD, we additionally examined group differences across a subset of PACC iterations based on APOE4 status in CN from the ADCS-PI, as this cohort included the FCSRT [17]. Consistent with the HABS, results with PACC iterations that incorporated Free Recall showed an earlier group difference, revealing significantly worse performance in the APOE4+ group at Year 2 and 3 (P values ≤.037). The PACC variation without FCSRT-Free was significantly worse in APOE4+ compared with APOE4− only at Year 3 (P = .002; Table 2).
Table 2.
MMRM analysis of PACC iterations. Results are shown for ADCS-PI differences by (A) APOE4 group and (B) CDR progressor group
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| (A) ADCS-PI APOE4 analysis | |||
| N (APOE4−/APOE4+) | 281/95 | 256/86 | 225/75 |
| A. Five components (FCSRT-Free, FCSRT-Total, LMDR, DS, MMSE) | −0.125 ± 0.068 P = .069 | −0.266 ± 0.127 P = .037 | −0.594 ± 0.189 P = .002 |
| B. FCSRT-Free and FCSRT-Total combined (FCSRT-96, LMDR, DS, MMSE) | −0.126 ± 0.066 P = .056 | −0.198 ± 0.077 P = .011 | −0.412 ± 0.123 P < .001 |
| C. No FCSRT-Total (FCSRT-Free, LMDR, DS, MMSE) | −0.122 ± 0.066 P = .065 | −0.184 ± 0.074 P = .013 | −0.377 ± 0.118 P = .001 |
| D. No FCSRT-Free (FCSRT-Total, LMDR, DS, MMSE) | −0.108 ± 0.079 P = .17 | −0.251 ± 0.149 P = .093 | −0.682 ± 0.221 P = .002 |
| B) ADCS-PI CDR progressor analysis | |||
| N (stable/progressor) | 421/27 | 380/24 | 333/21 |
| A. Five components (FCSRT-Free, FCSRT-Total, LMDR, DS, MMSE) | −0.553 ± 0.115 P< .001 | −0.806 ± 0.178 P < .001 | −1.555 ± 0.246 P < .001 |
| B. FCSRT-Free and FCSRT-Total combined (FCSRT-96, LMDR, DS, MMSE) | −0.606 ± 0.110 P < .001 | −0.732 ± 0.117 P < .001 | −1.205 ± 0.181 P < .001 |
| C. No FCSRT-Total (FCSRT-Free, LMDR, DS, MMSE) | −0.614 ± 0.109 P < .001 | −0.724 ± 0.114 P < .001 | −1.156 ± 0.178 P < .001 |
| D. No FCSRT-Free (FCSRT-Total, LMDR, DS, MMSE) | −0.672 ± 0.129 P < .001 | −0.889 ± 0.205 P < .001 | −1.651 ± 0.286 P < .001 |
Abbreviations: ADCS-PI, Alzheimer’s Disease Cooperative Study Prevention Instrument; CDR, Clinical Dementia Rating; FCSRT, Free and Cued Selective Reminding Test; MMRM, mixed model of repeated measure; MMSE, Mini-Mental State Examination; PACC, preclinical Alzheimer cognitive composite.
NOTE. Group differences at each annual follow-up visit are listed (β ± standard error). Significant effects are displayed in italics (P < .05).
We also tested the PACC variations comparing the ADCS-PI CDR progressors versus CDR stable groups. Consistent with the HABS analyses, ADCS-PI CDR progressors showed significant decline compared with the CDR stable group across all PACC iterations (Table 2).
4. Discussion
We found that Aβ+ CN participants in the HABS demonstrated a significant longitudinal decline on the PACC. Aβ group differences on the PACC remained significant across all iterations that systematically excluded individual components and when components were examined individually (Logical Memory, MMSE, Digit Symbol, FCSRT-Free Recall, and FCSRT-Total Recall). These results demonstrate that an Aβ-related effect on cognition is observable over a relatively short follow-up period and is captured by a prespecified multidomain cognitive composite. These findings further support the PACC as a valid approach to gauge efficacy of secondary prevention trials in Aβ+ CN participants.
The main finding that Aβ+ CN participants show significant PACC decline is consistent with work across multiple clinical research groups showing Aβ-related decline across different cognitive domains [3,5,7,8,14]. Data-driven approaches have also supported the use of a multidomain composite. Specifically, work by Langbaum et al. has shown that a combination of six to seven tests spanning episodic memory, executive function, language, visuospatial ability, and global function optimally captured cognitive decline 5 years before clinical symptoms [15]. The ability of multidomain cognitive composites to detect decline in at risk CN and in the years before AD diagnosis emphasizes that a composite spanning multiple domains can be used to measure cognitive decline many years before the onset of AD clinically evident symptoms.
Consistent with other studies [32], Aβ+ participants in the HABS were more likely to progress to CDR 0.5 compared with Aβ−. Separation of the Aβ+ group based on CDR progression revealed that Aβ-related PACC decline is predominantly driven by the subset of Aβ+ that progress on the CDR. This is not surprising, given that the selection of the PACC components was heavily influenced by studies examining decline associated with progression to MCI and dementia [33–35]. Although this finding provides confidence that PACC decline is associated with clinically relevant functional decline, it also suggests that PACC decline is honed for change that occurs relatively late in preclinical AD. However, iteratively eliminating components from the PACC suggest that individual components may differentially impact the ability to detect early and late change in preclinical AD. Specifically, we found that removal of the MMSE [16] resulted in greater Aβ group differences in the PACC at Year 3, but not after 5 years of follow-up. Thus, the MMSE may have limited signal early during the course of preclinical AD but starts to show decline among Aβ+ at later follow-up. Although inclusion of the FCSRT-Free Recall measure into the PACC consistently improved effect sizes related to differences between Aβ+ and Aβ− groups, the Total Recall score performed well when examining the 5-year change in the subset of Aβ+ progressors. Thus, the FCSRT-Total score and MMSE may capture change closer to clinical symptoms and may be particularly relevant in the long-term extension studies of current secondary prevention trials to demonstrate clinical meaningfulness [12]. Conversely, FCSRT-Free Recall and Logical Memory Delayed Recall consistently improved the effect sizes at shorter follow-up. Interestingly, the FCSRT-Free score was the only test to show a significant decline among Aβ+ who remained stable on the CDR, highlighting that this measure may change very early in the continuum of preclinical AD. Likewise, PACC iterations that incorporated Free Recall revealed earlier significant differences across APOE4+ groups in the ADCS-PI Study. Logical Memory Delayed Recall improved the difference between Aβ+ and Aβ− after a short follow-up of 3 years but not after 5 years of follow-up. This early Aβ-related effect of Logical Memory may be diminished at longer follow-up because of practice effects (the same version is given every year in the HABS in contrast to alternate versions that are used in clinical trials). Thus, sensitive tests of memory may be particularly relevant for capturing very early decline in preclinical AD.
Given that individual PACC components vary in their ability to measure cognitive decline throughout the continuum of preclinical AD, the choice of a particular PACC variation might be motivated by the preclinical population and the duration in a given trial. For instance, the A4 Study chose to restrict inclusion based on Logical Memory performance, such that very high performing CN participants are not eligible, to maximize ability to detect decline for more than 3 years. Thus, the A4 Study is likely enrolling a more advanced preclinical stage compared with the HABS cohort and may benefit from the inclusion of the FCSRT-Total score. For other ongoing secondary prevention trials, such as the EARLY (“A5”) Study (https://clinicaltrials.gov/ct2/show/NCT02569398) that do not restrict eligibility on cognitive criteria and will enroll participants down to age 60, the inclusion of the Free Recall component may be particularly relevant to detect very early Aβ-related decline. Other secondary prevention trials in younger participants with autosomal dominant AD mutations, including the Dominantly Inherited Alzheimer Network and the Alzheimer Prevention Initiative trials in the Colombian PS-1 kindred and in APOE e4/4 homozygotes, are using similar multicognitive domain composites [36,37]. To further optimize sensitivity across the spectrum of preclinical AD, future iterations of the PACC may consider inclusion of additional cognitive domains, such as semantic fluency, which has demonstrated robust Aβ-related decline [38]. In addition, computerized testing with challenging memory tests and reaction time measures may improve the sensitivity to the earliest changes in preclinical AD [39,40].
Our study has several limitations. The HABS cohort is a highly educated convenience sample from the Boston area and may not be representative of the general population. Furthermore, the HABS only incorporates annual testing, whereas most prevention trials will have more frequent administrations using alternate versions. It is also important to note that the PACC was created to track Aβ-related cognitive decline over time and is unlikely to be sensitive as a general screening test. Indeed, we did not observe any significant differences in any PACC component across Aβ groups at baseline, although baseline differences have been reported in other cohorts [41,42]. Our analyses were focused on investigating an a priori cognitive composite used in current anti-Aβ prevention trials to the HABS, to provide validation that this composite is able to detect Aβ-related decline during the preclinical stage of AD. Future trials may incorporate additional measures, such as semantic fluency and/or computerized tests. It will also be important to explore data-driven approaches [15] and differential weighting of specific components to optimize sensitivity to Aβ-related decline [16]. Finally, it is important to note that the PACC was honed to detect Aβ-related cognitive decline, specifically because current prevention trials are testing anti-Aβ therapies in preclinical AD. The contribution of other age-related neuropathophysiological processes to cognitive decline in the elderly, such as Lewy Body, TDP-43 pathology, and cerebrovascular disease, remains to be elucidated. The Longitudinal Evaluation of Amyloid Risk and Neurodegeneration Study, a companion observational study to the A4 trial funded by the Alzheimer’s Association, will investigate PACC longitudinal change in a cohort who do not show evidence of elevated Aβ accumulation [4]. Future trials may target other pathophysiological processes and may require different composites to detect non–Aβ-related decline.
In summary, the finding that Aβ-related cognitive decline is captured with the PACC in an independent cohort of CN increases our confidence that the ongoing secondary prevention trials will be able to detect a significant drug effect if antiamyloid therapies initiated during the preclinical stages of AD are able to slow disease progression. Furthermore, decline on the PACC is associated with progression to functional impairment as assessed by the CDR. The consistent findings with the PACC in previous cohorts using different neuropsychological tests suggest that it is the combination of cognitive domains [15], rather than the specific tests, that is particularly powerful in detecting decline during the preclinical stages of AD. A combination of measures that capture free versus cued recall aspects of episodic memory may prove robust to cognitive ceiling and floor effects and advantageous to track decline throughout the continuum of preclinical AD and into the early symptomatic stages of AD. Although this study represents an important step in validating cognitive composite outcomes, the ultimate validation will require evidence of a significant therapeutic effect in secondary prevention trials.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: We searched Pubmed to identify studies examining cognitive decline in amyloid positive clinically normal older adults and work using cognitive composites in prevention trials targeting clinically normal individuals at risk for cognitive decline caused by Alzheimer’s disease (AD).
Interpretation: Our analyses confirm that the preclinical Alzheimer cognitive composite, an a priori composite currently used in two large prevention trials, is sensitive to amyloid-related decline. The combination of measures of Free Recall that show early amyloid-related decline, in addition to cued memory measures that decline in the later stages of preclinical AD, may enhance the ability to track decline throughout the continuum of preclinical AD. Furthermore, we found that decline on this cognitive composite predicts functional decline on the Clinical Dementia Rating scale.
Future directions: Continued efforts to develop more sensitive composites as future prevention trials move into even earlier stages of AD are ongoing.
Acknowledgments
The study was supported primarily by P01 AG036694 and R01 AG046396, with contributions from K01 AG051718 and K24AG035007 from AG/NIA/NIH.
Footnotes
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jalz.2017.01.018.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD, et al. Amyloid-beta assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79:1636–44. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–86. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, et al. Effect of amyloid on memory and non-memory decline from pre-clinical to clinical Alzheimer’s disease. Brain. 2013;137:221–31. doi: 10.1093/brain/awt286. [DOI] [PubMed] [Google Scholar]
- 6.Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–7. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen RC, Wiste HJ, Weigand SD, Rocca WA, Roberts RO, Mielke MM, et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. doi: 10.1001/jamaneurol.2015.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snitz BE, Weissfeld LA, Lopez OL, Kuller LH, Saxton J, Singhabahu DM, et al. Cognitive trajectories associated with beta-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80:1378–84. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills SM, Mallmann J, Santacruz AM, Fuqua A, Carril M, Aisen PS, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–43. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–9. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368:1169–71. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 13.Clark LR, Racine AM, Koscik RL, Okonkwo OC, Engelman CD, Carlsson CM, et al. Beta-amyloid and cognitive decline in late middle age: findings from the Wisconsin Registry for Alzheimer’s Prevention study. Alzheimers Dement. 2016;12:805–14. doi: 10.1016/j.jalz.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:666–74. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim YY, Snyder PJ, Pietrzak RH, Ukiqi A, Villemagne VL, Ames D, et al. Sensitivity of composite scores to amyloid burden in preclinical Alzheimer’s disease: introducing the Z-scores of attention, verbal fluency, and episodic memory for nondemented older adults composite score. Alzheimers Dement (Amst) 2016;2:19–26. doi: 10.1016/j.dadm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–70. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–79. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 20.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau PET imaging in aging and early Alzheimer’s disease. Ann Neurol. 2016;79:110–9. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, Betensky RA, Hedden T, Schultz AP, Amariglio RE, Rentz DM, et al. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014;71:1379–85. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Memory Scale-Revised. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- 23.Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Grober E, Merling A, Heimlich T, Lipton RB. Free and cued selective reminding and selective reminding in the elderly. J Clin Exp Neuropsychol. 1997;19:643–54. doi: 10.1080/01688639708403750. [DOI] [PubMed] [Google Scholar]
- 26.Di Stefano F, Epelbaum S, Coley N, Cantet C, Ousset PJ, Hampel H, et al. Prediction of Alzheimer’s disease dementia: data from the GuidAge Prevention Trial. J Alzheimers Dis. 2015;48:793–804. doi: 10.3233/JAD-150013. [DOI] [PubMed] [Google Scholar]
- 27.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- 28.Grober E, Sanders AE, Hall C, Lipton RB. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24:284–90. doi: 10.1097/WAD.0b013e3181cfc78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–67. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 30.Wagner M, Wolf S, Reischies FM, Daerr M, Wolfsgruber S, Jessen F, et al. Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology. 2012;78:379–86. doi: 10.1212/WNL.0b013e318245f447. [DOI] [PubMed] [Google Scholar]
- 31.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 32.Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D, et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology. 2013;80:1784–91. doi: 10.1212/WNL.0b013e3182918ca6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derby CA, Burns LC, Wang C, Katz MJ, Zimmerman ME, L’Italien G, et al. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307–14. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–13. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 35.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–78. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reiman EM, Langbaum JB, Tariot PN, Lopera F, Bateman RJ, Morris JC, et al. CAP—advancing the evaluation of preclinical Alzheimer disease treatments. Nat Rev Neurol. 2016;12:56–61. doi: 10.1038/nrneurol.2015.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papp KV, Mormino EC, Amariglio RE, Munro C, Dagley A, Schultz AP, et al. Biomarker validation of a decline in semantic processing in preclinical Alzheimer’s disease. Neuropsychology. 2015;30:624–30. doi: 10.1037/neu0000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim YY, Villemagne VL, Laws SM, Pietrzak RH, Ames D, Fowler C, et al. Performance on the Cogstate Brief battery is related to amyloid levels and hippocampal volume in very mild dementia. J Mol Neurosci. 2016;60:362–70. doi: 10.1007/s12031-016-0822-8. [DOI] [PubMed] [Google Scholar]
- 40.Rentz DM, Dekhtyar M, Sherman J, Burnham S, Blacker D, Aghjayan SL, et al. The feasibility of At-Home iPad Cognitive Testing for use in clinical trials. J Prev Alzheimers Dis. 2016;3:8–12. doi: 10.14283/jpad.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burnham SC, Bourgeat P, Dore V, Savage G, Brown B, Laws S, et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 2016;15:1044–53. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 42.Soldan A, Pettigrew C, Cai Q, Wang MC, Moghekar AR, O’Brien RJ, et al. Hypothetical preclinical Alzheimer disease groups and longitudinal cognitive change. JAMA Neurol. 2016;73:698–705. doi: 10.1001/jamaneurol.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


