Abstract
Mast cell leukemia (MCL) is a very rare subtype of systemic mastocytosis (SM). We have identified 13 such patients (5.9%) among 218 patients with SM seen at our institution between 1994 and 2016. Patients with MCL had poor survival (median 31.6 months); response to various therapies was rare and not durable. Clinical course may be affected by concurrent associated hematologic neoplasm and different genetic profiles. More research is required to decipher this rare and enigmatic SM subtype.
Keywords: Systemic mastocytosis, mast cell leukemia
Introduction
Systemic mastocytosis (SM) is rare myeloproliferative neoplasm of clonal mast cells. Mast cell leukemia (MCL)(1, 2) is a rare subtype of SM and seen in <1% of SM patients.(3) Other subtypes of SM include indolent (ISM), smoldering (SSM), aggressive (ASM) and SM with associated hematologic neoplasm (SM-AHN).(4) The diagnosis of MCL is based on the presence of ≥20% mast cells in the bone marrow smears, but most MCL cases are aleukemic type (aMCL), characterized by <10% peripheral blood mast cells. Till date, only two reports from European colleagues have systematically characterized MCL. One report reviewed 51 cases of MCL collected from various centers in France(1) and another report(5) reviewed 28 cases of MCL from Germany. The outcome of MCL was significantly inferior as compared to other subtypes of SM, with median survival of <2 years. As reported by other colleagues, none of the currently available therapies have shown durable long term response in MCL. Infrequently, durable partial remissions were observed with single agent cladribine and more recently with midostaurin (a multi tyrosine kinase inhibitor, including abnormal KITD816V kinase, present in SM), now approved new therapy in the US for patients with ASM, SM-AHD, and MCL.(5, 6) Survival outcomes do not appear to improve after allogeneic stem cell transplantation.(7) Recently, the mutation profiles of ASM and MCL patients were reported.(8–10) The majority of patients exhibited KIT D816V mutation but frequently showed concurrent mutations in additional genes. Commonly observed concurrent mutations genes were SRSF2, TET2, ASXL2, and K/N-RAS. It is possible that the clinical course of ASM and the mutation profile is dependent upon the molecular characteristics of the AHN. In addition, presence of SRSF2/ASXL2/RUNX1 mutations (S/A/R) in patients with MCL was proposed to be an independent predictor for poor survival. (5)
Patients and Methods
In this study, we present our single center experience with MCL. We reviewed 218 patients with SM who presented to our institution between 1994 and 2016. This study was approved by the institutional review board. The survival of patients was calculated from the date of initial presentation to the date of last follow up/death. Kaplan-Meier survival analysis was conducted with GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA). In a subset of 8 patients with available bone marrow samples, we performed an amplicon-based targeted next generation sequencing (NGS) using a panel of 49 cancer-related genes (Thunderbold Myeloid Panel, Rain Dance Technologies) using MiSeq system (Illumina, Inc., San Diego, CA). The median coverage for most genes was between 3000X–4000X, facilitating confident variant calling at 1% sensitivity. A minimum of 250X coverage was required for variant calling.
Results
We identified 13 patients with MCL (5.9%). All 13 patients presented with aMCL; none had circulating mast cells. In 4 of 13 patients, associated hematologic neoplasm (AHN) was present: 2 with myelodysplastic syndrome, 1 with chronic myelomonocytic leukemia, and 1 with multiple myeloma. All patients had de novo aMCL except for one patient who had aMCL progressed from ASM. The median age at diagnosis was 62 years (range 24–75 years). Seven patients were males and 6 were females. Twelve (92%) were Caucasians and one patient was African- American. During the initial clinical presentation, 85% had constitutional symptoms (progressive fatigue, weight loss, night sweats), 54% had urticarial skin rash (macular, maculopapular and erythematous), 31% gastrointestinal symptoms (diarrhea) and 23% presented with bone pains. Imaging studies were performed in 6 patients: 5/6 had enlarged liver and/or spleen, 3 patients had lymphadenopathy (retroperitoneal, peripheral or mediastinal), 3 patients presented with bony lesions (2 sclerotic and one lytic) and 1 patient had adrenal gland involvement. Overall, the median hemoglobin level was 10 g/dL (range 7.7–12.9), median white blood cell count (WBC) 8 ×109/L (3.4–44.2), median platelet count 149 ×109/L (13–625) and absolute neutrophil count (ANC) 4 ×109/L (2–31). Serum tryptase was >200 ng/ml in all the patients. Median mast cell percentage in the bone marrow aspirate was 36% (range 20–64). Two patients also had liver biopsy demonstrating SM. Twelve patients had bone marrow karyotyping and 11/12 (92%) were diploid, one patient had a del5q abnormality (this patient had an AHN with myelodysplastic syndrome). Eleven of 13 patients were evaluated for CD2 and CD25 that showed aberrant CD25 expression in 8/11; CD2 in 5/11; negative for both in 3 patients. Two patients had prior malignancy. Details of the baseline characteristics are in Table 1.
Table-1.
Description of patient characteristics and outcomes in patients with aleukemic mast cell leukemia (aMCL)
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis | 73 | 47 | 62 | 59 | 24 | 42 | 74 | 59 | 47 | 69 | 73 | 75 | 72 |
| Ethnicity | Caucasian | Caucasian | African-American | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Sex | Male | Male | Female | Female | Female | Male | Female | Male | Male | Female | Male | Female | Male |
| Associated clonal hematologic non-mast cell lineage disease (AHNMD) | MDS | None | Myeloma | None | None | None | CMML | None | None | None | None | MDS | None |
| B findings (If yes then which type) | MC>30%, Dysmyelopoiesis | >30% MC in bone marrow, Dysmyelopoiesis | MC>20%, Tryptase>200 | MC>20%, Tryptase>200 | Splenomegaly, MC>20% | MC>30%, Hepatosplenomegaly | Dysmyelopoiesis, Tryptase>200 | Hepatosplenomegaly; MC>30%, Tryptase>200 | MC>30%; Hepatosplenomegaly | MC>30%, Tryptase> 200 | NA | >30% | |
| C Findings (Y/N) | N | Y | N | N | Y | Y | Y | Y | N | NA | Y | Y | Y |
| C Findings (If yes then which type) | NA | Anemia (Hb < 9.0); Hepatomegaly with ascites | NA | NA | Anemia (Hb<10gm/d L) | Skeletal lesions | Anemia/Thrombocytopenia | Anemia/Thrombocytopenia | NA | NA | Hepatomegaly with ascites | Anemia/thrombocytopenia | Skeletal lesions |
| Initial Clinical Presentation (0 = Bone Marrow Alone, 1= Extramedullary, 2= Both) | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 0 | 2 |
| Cutaneous signs and symptoms (Y/N) | N | N | Y-Erythematous rash | Y-Mast cells | Y-Erythematous lesions | Y-Maculopapular rash | Y-Macular rash | Y-Pruritic rash | Y-Macular rash | NA | N | N | N |
| Constitutional symptoms-Type | Y - Fatigue and weight loss | Y-Weight loss | Y-Weight loss | N | Y-Fatigue | Y-Fatigue, weight loss | Y-Fatigue | Y-Fatigue and Night sweats | Y-Weight loss | NA | Y-Fatigue | Y-Fatigue | Y-Irritability and Fatigue |
| Allergic/Anaphylactic symptoms (Y/N) | N | N | Y | N | Y | y | Y | N | N | NA | N | N | N |
| GI Symptoms (Y/N) | N | N | N | N | Y | Y | Y | Y | N | NA | N | N | N |
| Skeletal symptoms (Y/N) | N | N | N | N | N | Y | Y | N | N | NA | N | N | Y |
| Neurological (Y/N) | Y-Neurogenic bladder | Y-Flashes of light | Y | N | N | N | N | Y | Y | NA | Y | N | Y |
| Other systemic symptoms | None | None | None | None | None | None | None | None | None | None | None | None | None |
| Site of Relapse (BM or Extramedullary) | NA | NA | NA | Liver, adrenals | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| History of SM (Y/N) | Y | Y | N | Y | Y | Y | Y | Y | Y | NA | Y | Y | Y |
| Survival status 0=Alive, 1 = dead | Dead | Dead | Dead | Alive | Dead | Alive | Dead | Alive | Dead | NA | Dead | Dead | Dead |
| Overall Survival (Months) | 2.3 | 7.4 | 35.4 | 131.3 | 5.6 | 111.3 | 18.1 | 1.4 | 95.6 | 31.6 | 31.1 | 26.9 | |
| CD2 in BM (Y/N)-IHC | NA | Y | N | NA | NA | Y | N | Y | Y | N | N | N | N |
| CD25 in BM (Y/N)-IHC | NA | Y | NA | NA | NA | Y | N | Y | Y | N | Y | N | Y |
| CD2 in BM (Y/N)-FC | NA | Y | NA | Y | NA | Y | Y | N | Y | NA | Y | N | N |
| CD25 in BM (Y/N)-FC | NA | Y | NA | Y | NA | Y | NA | Y | Y | NA | Y | N | Y |
| CD117 in BM (Y/N)-FC | NA | Y | NA | Y | NA | Y | Y | Y | Y | NA | Y | Y | Y |
| Imaging Findings | NA | MRI: Retroperitoneal and thoracic lymphadenopathy, Hepatosplenomegaly | NA | CT Scan - Large liver mass, adrenalmets, osseousmets. | CT Scan - Inguinal adenopathy and Splenomegaly. | CT- Spleen, liver involvement, diffuse adenopathy, Sclerotic bone lesions | CT Scan - Negative for organomegaly | CT Scan - Hepatosplenomegaly | NA | NA | NA | NA | NA |
| % Mast cells in Marrow Aspirate | 44 | 64 | 36 | 40 | 23 | 51 | 28 | 23 | 39 | 36 | 20 | 34 | 41 |
| % Mast cells in Marrow Biopsy | 95 | 30 | 40 | 80 | 70 | 81 | NA | 70 | 100 | 100 | 80 | 80 | 30 |
| % Mast cells in Peripheral Blood | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemoglobin (g/dL) | 8.6 | 9 | 11.2 | 12.5 | 8.5 | 12.1 | 7.9 | 7.7 | 11 | NA | 12.9 | 7.8 | 11.7 |
| WBC × 103/µL | 8.5 | 6.5 | 14.6 | 6.9 | 19.1 | 31.7 | 44.2 | 17.2 | 5.3 | NA | 7.5 | 5.1 | 3.4 |
| Platelet Count × 103/µL | 13 | 137 | 137 | 247 | 220 | 625 | 83 | 62 | 161 | NA | 383 | 230 | 67 |
| Tryptase (ng/dL) | NA | >200 | 235 | >200 | 201 | 200 | 201 | 379 | 201 | NA | 201 | NA | 204 |
| Liver Size (cm) by PE | 2 | 0 | 0 | 6 | 0 | 4 | 0 | 6 | 2 | NA | 6 | 0 | 0 |
| Lymphadenopathy (Y/N) by PE | NA | N | N | Y | N | N | N | Y | N | NA | N | N | N |
| Spleen(cm) by PE | 10 | 4 | 0 | 5 | 0 | 0 | 0 | 9 | 7 | NA | 0 | 0 | 0 |
| ECOG Performance Status | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | NA | 1 | 1 | 1 |
| Karyotype | Complex | Diploid | Diploid | Diploid | Diploid | Diploid | Diploid | Diploid | NA | NA | Diploid | Diploid | Diploid |
| Other Prior Malignancy (Y/N) | Y | N | N | N | N | N | Y | N | N | NA | N | N | N |
| Initial Therapy | Unknown | Stem Cell Transplant | Imatinib and prednisolone with Antihistaminic | Cladribine with ara-C | Ara-C with Daunorubicin, Imatinib with prednisone followed by Allogenic SCT | Denileukin Difitox with Antihistaminic | Dasatinib | Midostaurin with antihistaminic | Dasatinib with antihistaminic | NA | None | Imatinib | Cladribine |
| Overall Response | NA | NA | NA | Progression | NA | No Response | Ineval(<3mos) | NA | Clinical Improvement | NA | NA | No response | NA |
| Second Line Treatment | Daclizumab | ||||||||||||
| Second Line Response | No Response | ||||||||||||
| Cause of Death | Unknown | Multiorgan Failure | Unknown | Sepsis | Unknown | Unknown | Unknown | Unknown | Unknown | ||||
NA – Not available, Y – Yes, N- No, BM-Bone marrow, SM, Systemic Mastocytosis, IHC – Immunohistochemistry, FC – Flow Cytometry, PE-physical examination
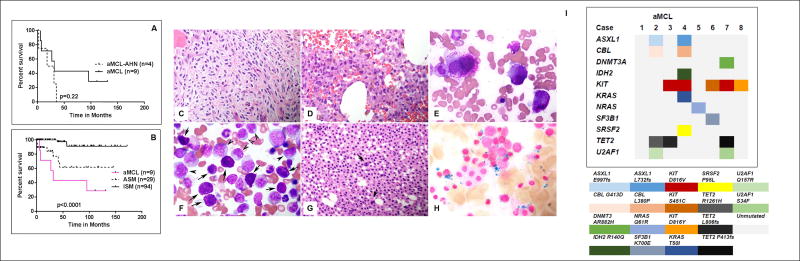
Treatments administered to these patients were heterogeneous. Two patients received imatinib as a part of clinical study (since then approved in the US for adult patients with ASM without KITD816V mutation, or with unknown mutational status) with no response, 2 received dasatinib as a part of a clinical study (one patient, positive for KITD816V mutation, had significant improvement in hepatosplenomegaly and pruritus but progressed after 1 year of dasatinib therapy), 2 received cladribine based therapy with no response, and 2 underwent allogeneic stem cell transplant (SCT) and had disease progression within 6 months after SCT. Overall, the median follow up was 111 months (1.4–131), with 3 patients alive at the time of last follow up (one additional patient was lost to follow up). The median overall survival (OS) for all 13 patients was 31 months; those with AHN (n=4) appeared to have had worse outcome: Figure 1A, shows the median overall survival (OS) of aMCL vs aMCL-AHN (31.6 months vs 24.6 months respectively; p=0.22). We also compared OS of our 9 aMCL patients without AHN, to OS of patients with either ASM or ISM seen at our institution: the median OS was significantly inferior in patients with aMCL (31.6 months) as compared to “not reached” in both ASM and ISM respectively (p<0.0001) (Figure 1B).
Figure 1. Clinical, histopathologic and molecular profile of patients with mast cell leukemia (aMCL).
A) Survival in patients with aMCL was not significantly different according to the presence or the absence of associated hematologic neoplasm (AHN) B) Survival of patients with aMCL was significantly inferior when compared with aggressive (ASM) and indolent systemic mastocytosis (ISM). (C–H) Bone marrow features of aMCL (with/without) AHN. Bone marrow biopsies (Hematoxylin and Eosin, original magnification ×400) show a mast cell infiltrate replacing the BM medullary space, comprising spindle shaped mast cells (C) or anaplastic large mast cells (D). BM aspirate smear (E) illustrates the cellular details of anaplastic mast cells from case D (Wright- Giemsa, original magnification ×1000). F–H are examples of aMCL-AHN cases. BM aspirate smear (F) shows a case of aMCL with chronic myelomonocytic leukemia. Mast cells are heavily granulated as indicated by arrows. There are increased monocytes as indicated by arrow heads. Also seen in picture F are easily identified blasts, dysplastic erythroid precursors and dysgranulopoiesis. G–H shows a case of aMCL with refractory anemia with ring sideroblasts. BM biopsy (G) shows sheets of mast cells, and dysplastic megakaryocytes (indicated by arrows); Iron stain reveals numerous ring sideroblasts (H) (original magnification ×1000). I) Summary of targeted next generation sequencing using a 49 gene panel for 8 patients with aMCL. Each column represents an individual patient and each row represents mutated genes in alphabetical order. KIT and TET2 gene mutations were most frequently observed. The amino acid changes resulting from the mutations are represented in different colors.
Bone marrow morphological examination showed an extensive mast cell infiltrate (median 80%, range 50–90%) in all cases. The mast cells varied from round granulated, spindle, to anaplastic, or mixed round and spindle cells. Immature mast cells were seen in 40% cases. The histopathologic features of the BM aspirate and biopsies of 3 representative cases are illustrated in Figure 1C–H. In 2 patients with AHN, granulated mast cells were seen interspersed with monocytes (F) from a patient with CMML and ringed sideroblasts (H). We also performed targeted NGS using a panel of 49 genes in 8 patients with available samples (included 3 patients with aMCL-AHN). Using this panel following mutations were detected – NRAS p.Q61R in one patient while other 7 patients had varying combination of mutations (shown in Figure 1I). Most commonly mutated gene was KIT in 5 patients (3 with D816V, 1 each with S451C and D816Y). Other mutated genes were TET2 in 3 patients, 2 patients each with U2AF1, ASXL1 and CBL and one patient each with SF3B1, DNMT3A, IDH2, KRAS and SRSF2. None had RUNX1 mutation. We could not detect FLT3 mutations.
Discussion
Our data confirm that MCL is a rare variant of SM with a poor outcome, as compared with other subtypes of SM(1). Presence of AHN may further aggravate survival. Overall, our analysis largely confirms the findings recently reported by the German group (5). Shorter OS in their patients (17 vs. 31 months) could possibly be explained by much higher proportion (70 vs 30%) of patients with AHN in that study, with lower hemoglobin and platelet counts, indicating more aggressive disease. Another important explanation could be a difference in the mutation profile of patients in the two studies: we found SRSF2 and ASXL1 co-mutated genes in only one patient (number 4 in Figure 1I) in contrast to 13 patients who had SRSF2/ASXL1/RUNX1 co-mutated genes in the German study(5). Our patient 4 had concurrent poor prognostic mutations (8) in SRSF2, CBL and ASXL1 genes, and lived only 1.4 months.
In summary, MCL is rare variant of SM with heterogeneous clinical course that may be affected by concurrent AHN and different genetic profiles. Overall, these patients have poor outcome. Our data calls for further studies to characterize the genomic profile of patients with SM and identify potential therapeutic targets and disease resistance pathways.
Highlights.
Mast cell leukemia is a rare type of systemic mastocytosis with a poor prognosis.
There is no standard of care treatment and new therapies are needed.
Associated hematologic neoplasm negatively impacts the survival of these patients.
Acknowledgments
This study was supported in part by the NIH/NCI under award number P30CA016672 and by the NCI under award number P01CA049639.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions
P.J. and S.V. contributed to the study design, data collection (N.S. and P.J.), P.J., S.W., K.P. and S.V. wrote the paper and analyzed results. K.P. analyzed molecular data, S.W. evaluated the pathology and provided images, H.K., and S.V. contributed patient samples.
Conflicts-of-Interest Disclosure: None from any authors.
References
- 1.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–95. doi: 10.1182/blood-2012-07-442400. [DOI] [PubMed] [Google Scholar]
- 2.Valentini CG, Rondoni M, Pogliani EM, Van Lint MT, Cattaneo C, Marbello L, et al. Mast cell leukemia: a report of ten cases. Ann Hematol. 2008;87(6):505–8. doi: 10.1007/s00277-007-0430-3. [DOI] [PubMed] [Google Scholar]
- 3.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113(23):5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 4.Valent P, Akin C, Hartmann K, Nilsson G, Reiter A, Hermine O, et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017;77(6):1261–70. doi: 10.1158/0008-5472.CAN-16-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jawhar M, Schwaab J, Meggendorfer M, Naumann N, Horny HP, Sotlar K, et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica. 2017 doi: 10.3324/haematol.2017.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis. New England Journal of Medicine. 2016;374(26):2530–41. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 7.Ustun C, Reiter A, Scott BL, Nakamura R, Damaj G, Kreil S, et al. Hematopoietic stem-cell transplantation for advanced systemic mastocytosis. J Clin Oncol. 2014;32(29):3264–74. doi: 10.1200/JCO.2014.55.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardanani AD, Lasho TL, Finke C, Zblewski DL, Abdelrahman RA, Wassie EA, et al. ASXL1 and CBL mutations are independently predictive of inferior survival in advanced systemic mastocytosis. Br J Haematol. 2016;175(3):534–6. doi: 10.1111/bjh.13865. [DOI] [PubMed] [Google Scholar]
- 9.Jawhar M, Schwaab J, Hausmann D, Clemens J, Naumann N, Henzler T, et al. Splenomegaly, elevated alkaline phosphatase and mutations in the SRSF2/ASXL1/RUNX1 gene panel are strong adverse prognostic markers in patients with systemic mastocytosis. Leukemia. 2016;30(12):2342–50. doi: 10.1038/leu.2016.190. [DOI] [PubMed] [Google Scholar]
- 10.Schwaab J, Schnittger S, Sotlar K, Walz C, Fabarius A, Pfirrmann M, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122(14):2460–6. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]