Abstract
Background and Objectives
Addictions to heroin or to cocaine are associated with substantial psychiatric comorbidity, including depression. Poly-drug self-exposure (e.g., to heroin, cocaine, cannabis or alcohol) is also common, and may further affect depression comorbidity.
Methods
This case-control study examined the relationship of exposure to the above drugs and depression comorbidity. Participants were recruited from methadone maintenance clinics, and from the community. Adult male and female participants (n=1,201) were ascertained consecutively by experienced licensed clinicians. The instruments used were the SCID-I, and Kreek-McHugh-Schluger-Kellogg (KMSK) scales, which provide a rapid dimensional measure of maximal lifetime self-exposure to each of the above drugs. This measure ranges from no exposure to high unit dose, high frequency, and long duration of exposure.
Results
A multiple logistic regression with stepwise variable selection revealed that increasing exposure to heroin or to cocaine was associated greater odds of depression, with all cases and controls combined. In cases with an opioid dependence diagnosis, increasing cocaine exposure was associated with a further increase in odds of depression. However, in cases with a cocaine dependence diagnosis, increasing exposure to either cannabis or alcohol, as well as heroin, was associated with a further increase in odds of depression.
Discussion and Conclusions
This dimensional analysis of exposure to specific drugs provides insights on depression comorbidity with addictive diseases, and the impact of poly-drug exposure.
Scientific Significance
A rapid analysis of exposure to drugs of abuse reveals how specific patterns of drug and poly-drug exposure are associated with increasing odds of depression.
Introduction
Addictions to heroin or cocaine are associated with psychiatric comorbidity, especially depression, based primarily on the study of categorical diagnoses (1, 2). This comorbidity affects the course and severity of individual patients’ conditions (1). Preclinical literature shows that the pattern, duration and amount of exposure to specific drugs of abuse can affect behavioral and depressant-like effects, and underlying neurobiological changes (3–7). The underlying variables which affect comorbidity of addictive diseases and depression are not well understood, and may include genetic, epigenetic and environmental factors, as well as neurobiological changes that occur due to exposure to specific types of drugs of abuse (8, 9).
Poly-drug exposure in persons with heroin or cocaine addiction diagnoses is common, and can include exposure to other substances, principally cannabis and alcohol. The impact of specific types of poly-drug exposure on depression-like effects has been studied in a limited number of preclinical studies (10, 11), therefore our understanding of the neurobiology of this phenomenon is limited.
Recent research in psychiatry and addiction has placed emphasis on “dimensional” analysis of bio-behavioral phenomena, in addition to categorical diagnoses (12–14). A validated set of scales, the Kreek-McHugh-Schluger-Kellogg (KMSK) scales, provide a rapid dimensional measure of maximal lifetime self-exposure to specific drugs in humans, ranging from no exposure to heavy exposure (15, 16). Some studies have examined dimensionally how drug exposure and poly-drug exposure are associated with depression, in diagnosed participants (17–19). Some of the instruments used in these studies could not be easily applied in the field, due primarily to their length. The KMSK scales thus potentially provide the opportunity to rapidly examine the relationship of drug exposure and depression comorbidity, in a dimensional manner. The main hypothesis of this study was that maximal exposure to specific drugs of abuse, as well as specific patterns of poly-drug exposure, will have characteristic association with major depression comorbidity.
Methods
This was a case-control study, with two consecutive cohorts of sequentially ascertained participants, examined as outpatients in a research hospital. “Cases” herein are participants with an addictive disease diagnosis (DSM IV criteria), and “controls” are participants without an addictive disease diagnosis. We further stratified all participants (cases or controls) by the presence of depression diagnosis. This is a secondary analysis of participants systematically ascertained for genetic association studies (20).
In this secondary base study, cases and controls were ascertained sequentially (cumulatively sampled) from several methadone maintenance clinics, and also from the community, within a large urban area (21) (Table 1).
Table 1.
Study Demographics
| Cohort 1. Ascertained 4/4/02–5/27/05 | |||
|---|---|---|---|
| Controls (n=140): | Cases (n=477): | t or χ2 [df]; p value | |
| Mean Age (SEM) | 33.7 (SD= 11.1) | 40.6 (SD=10.6) | t [465]=6; p<0.001 |
| Gender: N (% of total) | |||
| Male | 61 (43.6%) | 324 (67.9%) | χ2=27; p<0.001 |
| Female | 79 (56.4%) | 152 (32.1%) | |
| Depression Diagnosis: N (% of total within gender) | |||
| Male | 4 (1.0%) | 60 (15.6%) | N/A (one cell had low “N”)a |
| Female | 11 (4.8%) | 48 (20.8%) | |
| Ethnic/Cultural Group: N (% of total) | |||
| African-American | 69 (49.3%) | 209 (43.8%) | χ2=6.7; n.s. |
| Caucasian | 41 (29.3%) | 126 (26.4%) | |
| Hispanic | 17 (12.1%) | 105 (22.0%) | |
| Remaining/Mixed | 13 (9.3%) | 37 (7.8%) | |
| Cohort 2. Ascertained 6/9/05–8/1/13 | |||
| Controls (n=163) | Cases (n=421) | t or χ2 [df]; p value | |
| Mean Age (SEM) | 33.0 (SD=11.2) | 44.6 (SD=8.6) | t=[581]11.8; p<0.001 |
| Gender: N (% of total) | |||
| Male | 76 (46.6%) | 257 (61.0%) | χ2=9.97; p<0.002 |
| Female | 87 (53.4%) | 164 (39.0%); n=1 M to F transgender | |
| Depression Diagnosis: N (% of total within gender) | |||
| Male | 3 (1.0%) | 74 (22.2%) | N/A (one cell had low “N”)a |
| Female | 8 (3.1%) | 65 (24.9%) | |
| Ethnic/Cultural Group: N (% of total) | |||
| African-American | 60 (36.8%) | 241 (57.2%) | χ2=24.2; p<0.0001 |
| Caucasian | 41 (25.2%) | 70 (16.6%) | |
| Hispanic | 31 (19.0%) | 72 (17.1%) | |
| Remaining/Mixed | 31 (19.0%) | 38 (9.1%) | |
χ2 analysis on the data from both cohorts combined was significant (χ2=7.04; p=0.008).
Recruitment, inclusion and exclusion criteria
Participants were recruited through Institutional Review Board (IRB)-approved posted notices and newspaper advertisements in the area. Study Inclusion criteria: Participants were required to be ≥18 years of age, competent to understand study procedures, and to understand and sign the informed consent in English. Study Exclusion criteria: Participants with uncontrolled schizophrenia or other psychotic mental illnesses which prevented them from understanding study procedures or informed consent, were excluded (22).
Participants were excluded from the control category if they had any of the following: a) current or continuing abuse of alcohol, or at least one instance of drinking to intoxication during the previous 30 days, b) any use of illicit drugs including opiates, cocaine, and amphetamines (but excluding cannabis) during the 30 days prior to ascertainment, c) if they had used illicit drugs (with the exception of cannabis) for at least three times a week for a period of at least 1 month, in their lifetime, and d) if they had used cannabis on more than 12 days in the 30 days prior to ascertainment. The latter criterion (i.e., d) was originally adopted based on the approximate frequency of cannabis use in controls in this urban area (22). Cohorts and ascertainment: Cohort 1 was composed of 617 consecutive participants (4/4/02–5/27/05). Cohort 2 was composed of 579 consecutive participants (6/9/05–8/1/13).
As mentioned above, participants were also recruited through postings at several methadone maintenance clinics in New York City. Current or prior abuse of other drugs in addition to heroin or opioids was not an exclusion criterion for these participants. Participants with addictive diseases were also ascertained as part of community recruitment in the area, therefore the present cases are not only representative of participants in current or previous methadone maintenance treatment. All ascertainments were completed during a standardized face-to-face interview with a trained licensed clinician (e.g., Ph.D. Psychologist, M.D., D.O., Nurse Practitioner or Registered Nurse).
Questionnaires and diagnostic categories
Participants were ascertained with the SCID I/P (Version 2.0) (23), and with KMSK questionnaires for lifetime exposure to each of heroin, cocaine, cannabis or alcohol, during a standardized face-to-face interview (15). KMSK scores provide a rapid dimensional measure of maximal lifetime self-exposure to each substance of interest, and can be obtained within an interview in ≈15 minutes. The scale for each drug ranges in integers from “0” (no exposure/never used), to a maximal score (i.e., 13 for heroin and alcohol, 14 for cannabis and 16 for cocaine). The exposure score is a composite of estimated unit doses, frequency (e.g., times/day) and duration (e.g., in years), at the time in a participant’s life when use was the heaviest (15). Prior studies show that KMSK scores at or above a specific “cutpoint” for each drug have high concurrent validity with the respective DSM IV dependence diagnosis (15, 16).
The KMSK questionnaires also collect data on basic age trajectory of exposure, as age of first use and of heaviest use for each drug (in whole years). If a participant entered a range of ages for heaviest use, the first year in the range is taken as the onset of heaviest use, and analyzed herein. The KMSK scales are freely available at http://lab.rockefeller.edu/kreek/kmsk (13, 24, 25).
Statistical analyses
Univariate analyses
Univariate analyses, including Mann-Whitney t-tests and contingency analyses were completed with the GraphPad Prism program. The p<0.05 α level was set for rejection of null hypotheses.
Multiple logistic regressions
Three multiple logistic regressions were performed using Akaike Information Criterion (AIC) stepwise selection, and were performed in: a) all participants combined (i.e., all cases and controls), b) all cases with an opioid dependence diagnosis, c) all cases with a cocaine dependence diagnosis. The dependent variable for all regressions was presence or absence of depression diagnosis (binary). The “glm” function and “MASS” and “LogisticDx” packages in “R” software were used for parameter estimation, selection of variables based on the AIC, and goodness of fit evaluation. Additionally, a Monte Carlo experiment with 1,000 balanced re-samples (with equal numbers of depressed and “non-depressed” participants) was performed, with two goals: 1) to evaluate the fitted models’ performance in hold-out samples; 2) to obtain more robust confidence interval estimates for the Odds Ratios of the stepwise-selected variables.
Independent variables for demographics were: gender [females as reference group], ethnicity [Caucasian as reference group], cohort [cohort 1 as reference group]) and age of ascertainment [in whole years]. In the regression which included all participants, a further binary variable was added for controls, versus cases as the reference group. Independent variables for dimensional analysis of drug exposure were: KMSK lifetime exposure scores for heroin, cocaine, alcohol and cannabis. As mentioned above, KMSK scores have high concurrent validity with the respective dependence diagnosis (e.g., heroin KMSK scores ≥“cutpoint” are highly predictive of the opioid dependence diagnosis) (15, 16). Thus, heroin KMSK scores were not entered as an independent variable for the regression in cases with opioid dependence diagnosis, and cocaine KMSK scores were not entered as an independent variable for the regression in cases with cocaine dependence diagnosis. This design therefore allowed a dimensional examination of the phenomenon of poly-drug exposure in participants with a specific dependence diagnosis.
Results
Sample Demographics
Age: Sample demographics are in Table 1. For both cohorts, mean age at the time of ascertainment was greater for participants with a substance abuse or dependence diagnosis (cases), versus controls (Mann-Whitney t-tests). Gender: For both cohorts, males with a substance abuse or dependence diagnosis (cases) were more frequent than females. Ethnicity: A χ2 analysis of ethnicity across cases and controls was not significant in cohort 1, but was significant in cohort 2. These demographic variables are examined further in multiple logistic regression analyses (below).
Drug dependence diagnoses
The main DSM IV dependence diagnoses in the cases were opioid, cocaine, cannabis and alcohol dependence, and cases with multiple dependence diagnoses to these drugs were common. Other dependence diagnoses were relatively uncommon.
Depression and other comorbid diagnoses
Other than drug abuse or dependence diagnoses, the most common DSM IV Axis I diagnosis was depression (Major Depressive Disorder), followed by anxiety. Other Axis I diagnoses were relatively uncommon. Our analysis therefore focuses on depression, the most common comorbid diagnosis. Total numbers of participants with depression diagnoses are shown in Table 1, stratified by gender (26). Due to the low numbers of controls with depression diagnoses, a basic analysis of depression diagnoses was carried out with cohorts combined. A contingency analysis for the presence of depression diagnosis with cohorts combined was significant (χ2 =7.04; p=0.008).
Depression comorbidity and age trajectory of exposure (age of first use and age of heaviest use)
The presence or absence of depression diagnosis did not affect age of heroin first use or of heaviest use, in cases with opioid dependence diagnosis (Mann-Whitney tests, not shown). Also, the presence or absence of depression diagnosis did not affect age of cocaine first use or of heaviest use, in cases with cocaine dependence diagnosis (Mann-Whitney tests, not shown).
Multiple logistic regressions for depression diagnosis as dependent variable
Three multiple logistic regressions with stepwise selection were performed: a) in all cases and controls combined, b) in cases with opioid dependence diagnosis, and c) in cases with cocaine dependence diagnosis.
Independent variables for demographics
Cases versus controls: As expected based on Table 1, controls had lower odds of depression, compared to cases (participants with an abuse or dependence diagnosis), in the overall regression. Gender: Females had greater odds of depression than males in all regressions. Ethnic/Cultural Groups: African-Americans (AA) had lower odds of depression than Caucasians (Cau) in the overall regression, and in cases with opioid dependence diagnosis, but not in cases with cocaine dependence diagnosis. Cohort effect: Participants in cohort 2 had greater odds of depression than those in cohort 1, in all regressions. Age of ascertainment: Older age of ascertainment was associated with greater odds of depression only in the regression for cases with cocaine dependence.
Independent variables for dimensional analysis of drug exposure (KMSK exposure scores)
We report below data for drugs that were retained in each multiple regression model. Odds ratios for exposure were calculated per 1-unit score in KMSK scales for each drug.
Overall regression
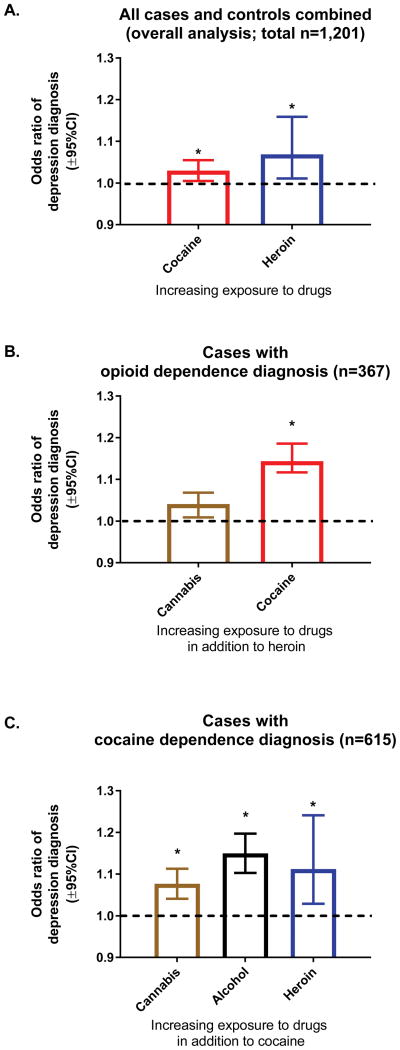
In the overall regression with all cases and controls combined, exposure to either heroin or cocaine were each associated with greater odds of depression (see Fig. 1.
Figure 1.
Impact of drug exposure on depression, in all participants combined (all cases and controls), in cases with opioid dependence diagnosis, and in cases with cocaine dependence diagnosis (panels A–C, respectively). Bars represent odds ratios (±95%CL) of depression diagnosis with increasing exposure for drugs that were retained in the multiple regression models. Odds ratios are presented for a 1-score increment in each KMSK exposure scale. *Indicates significant independent variables.
Cases with opioid dependence diagnoses
In cases with opioid dependence diagnosis, greater cocaine exposure was associated with increased odds of depression comorbidity (Fig. 1).
Cases with cocaine dependence diagnoses
In cases with cocaine dependence diagnoses, greater exposure to cannabis or alcohol, as well as heroin, were each associated with increased odds of depression (Fig. 1).
Discussion
This study provided a dimensional analysis of the relationship of exposure to specific drugs of abuse with depression comorbidity, in participants diagnosed with specific addictive diseases. Of practical relevance, KMSK scales used herein provided a rapid measure of drug exposure to several major drugs of abuse, obtained within clinical interviews.
Gender differences
Females had higher odds of depression compared to males, in the overall multiple logistic regression analysis (with all cases and controls combined). This gender difference was also observed in regressions limited to cases with opioid or cocaine dependence. The increased odds of depression in females with specific addictive disease diagnoses is consistent with previous studies (1, 27). The specific bio-behavioral factors which underlie this difference are unclear. However, gender differences have been reported in both MOPr and dopaminergic receptor populations in human PET studies (28, 29). It is also known that major neurobiological effects of drugs of abuse, including MOPr agonists, cocaine, alcohol and cannabis can be sexually dimorphic (30–33). Some preclinical studies have also reported that exposure to specific drugs of abuse has sexually dimorphic depressant-like effects (34–36). Overall, this analysis confirms that women with either opioid or cocaine dependence diagnoses have greater odds of depression comorbidity than men.
Ethnic/Cultural group differences
Participants of African-American background had lower odds of depression in the overall regression analysis, compared to Caucasians. These reduced odds of depression were also observed in African-American cases with opioid dependence diagnosis, but not those with cocaine dependence diagnosis. A lower prevalence of depression diagnoses in African-Americans has been previously reported in persons with opioid addiction (1, 37), but not necessarily in other clinical conditions (38). Such variations may be due to genetic (19), environmental, or cross-cultural differences in both reporting of symptoms and in their diagnosis (39), as well as other social and cultural factors (40, 41). Since African-Americans with cocaine dependence diagnoses did not have decreased odds of depression in this study, it may be postulated that factors in addition to environment and cross-cultural presentation or diagnosing, are involved in the differential risk of depression comorbidity.
Age trajectory of drug exposure
Ages of first use and of heaviest use of each drug are collected in KMSK questionnaires. In cases with opioid dependence diagnosis, ages of first use or heaviest use of heroin did not differ, when stratified by presence or absence of depression. A similar finding was obtained for cocaine, in cases with cocaine dependence diagnosis. This suggests that the age trajectory of exposure to heroin or cocaine was not significantly affected by the presence of depression herein, in cases with the respective dependence diagnosis. As a limitation, we were unable to collate systematic data on the age of onset of depression, based on the instruments used herein. Therefore we cannot examine further whether the age trajectory of exposure was associated with different onset of depression. Other studies have examined the relative onset of specific addictive diseases and comorbid depression (42, 43). These prior studies showed different temporal patterns in subsets of patients (e.g., onset of addictive disease preceding onset of depression or vice versa). The underlying mechanisms in these different groups are not understood. It is possible that further dimensional analyses of drug exposure may enhance these efforts in the future.
Dimensional examination of drug exposure and depression comorbidity
In all cases and controls combined, greater exposure to either heroin or cocaine exposure was associated with increased odds of depression diagnoses. Some prior studies have reported that heroin, cocaine, alcohol, cannabis exposure were a risk factor for depression comorbidity, using primarily categorical diagnoses (19, 43, 44). In the analysis with all cases and controls combined, exposure to either alcohol or cannabis were not detected as significant. However alcohol or cannabis exposure were detected as significant when the analysis was limited to cases with cocaine dependence diagnoses (see below). Practical dimensional measures such as KMSK scales may be used further to determine how exposure to specific drugs, even at “sub-diagnostic” levels, can affect odds of depression (45). Future studies with larger numbers of participants without addictive disease diagnoses may therefore utilize such an approach.
Patterns of poly-drug exposure and depression comorbidity
Poly-drug exposure is relatively common in clinical populations with addictive diseases, and may also differ depending on the stage of exposure (e.g., early vs late stages) (46). Poly-drug abuse is a challenge, both for clinical prognosis and disease nosology (47, 48). The present study provides new insight into how specific patterns of poly-drug exposure affect depression comorbidity, in persons with specific dependence diagnoses.
Thus, we found here that in cases with opioid dependence, cocaine exposure increased the odds of depression comorbidity. Dual use of heroin and cocaine is commonly reported, and is associated with greater depression comorbidity than single-drug use, based on studies using primarily categorical diagnoses (48–50). In preclinical studies, cocaine and MOPr agonists such as heroin share qualitatively similar effects with each other, for example on striatal dopamine dialysate levels, acutely or chronically (51–53). Dopaminergic systems are known to affect hedonic states, therefore it could be postulated that a shared downstream mechanism can underlie the increase in depression comorbidity in cocaine/heroin poly drug-users users.
By contrast, the regression in cases with cocaine dependence diagnosis revealed that increasing exposure to either cannabis or alcohol, as well as heroin, resulted in greater odds of depression comorbidity. Prior clinical studies, using more complex clinical instruments, found that poly-drug exposure to cocaine or cannabis was associated with increased risk of depression, in persons with alcohol dependence (54). One intriguing conclusion from this analysis is that even a relatively small increase in exposure to cannabis or alcohol (as detected dimensionally with KMSK scales) results in further increased odds of depression, in cases with cocaine dependence. It is known that CB1 receptor systems interact with the effects of cocaine, from preclinical and clinical studies (55, 56). Alcohol and cocaine also share some downstream neurobiological effects (51).
Taken together, these findings suggest that specific types of poly-drug exposure are associated with differential depression comorbidity, likely based on the neurobiological mechanisms which are affected by specific drug combinations. Therefore, the mechanisms underlying depression comorbidity in cocaine/heroin poly-drug users are not necessarily the same as those in cocaine/cannabis poly-drug users (as an example). Recent fMRI neuroimaging studies also show that persons with a categorical depression diagnosis could be further sub-divided into several “biotypes”, based on specific neurophysiological characteristics (57).
Few preclinical studies have focused on how poly-drug exposure affects the emergence of depression-like behaviors or neurobiological changes (10, 11). Therefore knowledge on the neurobiological mechanisms of poly-drug exposure and depression is limited. Of translational value, this study indicates specific patterns of poly-drug exposure associated with increased odds in depression comorbidity, and these could be potentially modeled in future preclinical studies.
Design considerations and limitations
Only a relatively small number of controls (i.e., participants without an addictive disease diagnosis) were diagnosed with depression (1). The relatively small number of controls with depression in these cohorts may have limited our capacity to detect how “sub-diagnostic” exposure to specific drugs would affect odds of depression. This limitation may be remedied by future studies of drug exposure and depression, with a larger number of participants without addictive disease diagnoses.
Age of ascertainment was older in cases versus controls. One possible reason for this is the recruitment in long-standing methadone treatment programs, where participants may be older than volunteers from the general community. However, older age was only associated with greater odds of depression in cases with cocaine dependence diagnoses, and therefore was not likely to be a general confounder herein.
As is the case with many case-control studies, recall bias cannot be excluded herein (58). Other study designs and sampling strategies could be used to confirm and extend these studies, to address this possible concern. Likely due to the chronological aspects of these cohorts (with participants of at least 18 years of age in 2002–2013), illicit prescription opioid exposure was low, with heroin being the principally reported MOPr agonist. KMSK questionnaires also include separate forms for illicit use of prescription opioids, which may also be associated with substantial depression comorbidity (59). Future studies may examine dimensionally how exposure to illicit prescription opioids impacts depression comorbidity.
Further dimensional studies of cannabis exposure and depression comorbidity would also be of value, for cohorts reaching adulthood at later times, given evolving trends in the legal availability of cannabis. Of interest, increasing cannabis exposure was associated with greater odds of depression in cases with cocaine dependence diagnosis. Exposure to cannabinoid CB1-receptor agonists such as Δ9-tetrahydrocannabinol (especially in adolescence) is known to cause depressant-like effects in preclinical models (30, 34). Some, but not all, studies report an increase in depression comorbidity in cannabis users (18, 60), and it is possible that differential cannabis exposure across studies is a major reason for these apparent discrepancies.
Summary and conclusions
This study provided a rapid dimensional analyses of how exposure to major drugs of abuse is associated with depression comorbidity, in clinically diagnosed participants. The study also provided valuable dimensional data on the association of specific patterns of poly-drug exposure and depression comorbidity.
Acknowledgments
The authors would like to gratefully acknowledge funding by NIH grants DA05130, DA09444, DA12848 and also NIH-GCRC and NIH-CTSA funding to the Rockefeller University Hospital (M01-RR00102 and UL1RR024143). We also gratefully acknowledge support by the Dr. Miriam and Sheldon Adelson Medical Research Foundation and New York State Office of Alcoholism and Substance Abuse Services.
We are grateful for outstanding ascertainment by several clinicians, including Scott Kellogg Ph.D., James Schluger M.D., Lisa Borg M.D., Charles Lilly M.D., Mark Green M.D., Heather Hofflich D.O., Gavin Bart M.D., Kathy Bell R.N., Dorothy Melia R.N., Brenda Ray N.P. (in memoriam) and Elizabeth Ducat N.P..
Abbreviations
- 95%CL
95% confidence limits
- AA
African-American
- AIC
Akaike information criterion
- Cau
Caucasian
- CB1
Cannabinoid receptor-1
- KMSK scale
Kreek-McHugh-Schluger-Kellogg scale for exposure to drugs of abuse
- N.S
non-significant
Footnotes
Declaration of Interest: The authors have no conflicts of interest related to this manuscript.
References
- 1.Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015 doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mason BJ, Kocsis JH, Melia D, et al. Psychiatric comorbidity in methadone maintained patients. J Addict Dis. 1998;17:75–89. doi: 10.1300/J069v17n03_07. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 4.Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–76. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zernig G, Ahmed SH, Cardinal RN, et al. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]
- 6.Valenza M, Picetti R, Yuferov V, Butelman ER, Kreek MJ. Strain and cocaine-induced differential opioid gene expression may predispose Lewis but not Fischer rats to escalate cocaine self-administration. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- 8.Butelman ER, Yuferov V, Kreek MJ. kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–96. doi: 10.1016/j.tins.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed B, Fang N, Mayer-Blackwell B, et al. Chromatin alterations in response to forced swimming underlie increased prodynorphin transcription. Neuroscience. 2012;220:109–18. doi: 10.1016/j.neuroscience.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemens KJ, McGregor IS, Hunt GE, Cornish JL. MDMA, methamphetamine and their combination: possible lessons for party drug users from recent preclinical research. Drug Alcohol Rev. 2007;26:9–15. doi: 10.1080/09595230601036945. [DOI] [PubMed] [Google Scholar]
- 11.Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003;28:2102–16. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- 12.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 13.Crystal HA, Hamon S, Randesi M, et al. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol. 2012;17:181–91. doi: 10.1111/j.1369-1600.2010.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasin DS, Shmulewitz D, Stohl M, et al. Procedural validity of the AUDADIS-5 depression, anxiety and post-traumatic stress disorder modules: Substance abusers and others in the general population. Drug Alcohol Depend. 2015;152:246–56. doi: 10.1016/j.drugalcdep.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellogg SH, McHugh PF, Bell K, et al. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–50. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- 16.Tang YL, Khoury L, Bradley B, Gillespie CF, Ressler KJ, Cubells JF. Substance use disorders assessed using the Kreek-McHugh-Schluger-Kellogg (KMSK) scale in an urban low-income and predominantly African American sample of primary care patients. Am J Addict. 2011;20:292–9. doi: 10.1111/j.1521-0391.2011.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denis CM, Gelernter J, Hart AB, Kranzler HR. Inter-observer reliability of DSM-5 substance use disorders. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco C, Hasin DS, Wall MM, et al. Cannabis Use and Risk of Psychiatric Disorders: Prospective Evidence From a US National Longitudinal Study. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2015.3229. [DOI] [PubMed] [Google Scholar]
- 19.Sherva R, Wang Q, Kranzler H, et al. Genome-wide Association Study of Cannabis Dependence Severity, Novel Risk Variants, and Shared Genetic Risks. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levran O, Peles E, Randesi M, et al. Susceptibility loci for heroin and cocaine addiction in the serotonergic and adrenergic pathways in populations of different ancestry. Pharmacogenomics. 2015;16:1329–42. doi: 10.2217/pgs.15.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miettinen OS. The “case-control” study: valid selection of subjects. J Chronic Dis. 1985;38:543–48. doi: 10.1016/0021-9681(85)90039-6. [DOI] [PubMed] [Google Scholar]
- 22.Bond C, LaForge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient edition (SCID-I/P; version 2.0) Biometrics Research Department, New York State Psychiatric Institute; 1993. [Google Scholar]
- 24.Jackson CB, Varon J, Ho A, Marks KM, Talal AH, Kreek MJ. Identification of substance use and dependence among patients with viral hepatitis. Dig Liver Dis. 2010;42:650–6. doi: 10.1016/j.dld.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depress Anxiety. 2010;27:1077–86. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 27.Dawson DA, Goldstein RB, Moss HB, Li TK, Grant BF. Gender differences in the relationship of internalizing and externalizing psychopathology to alcohol dependence: likelihood, expression and course. Drug Alcohol Depend. 2010;112:9–17. doi: 10.1016/j.drugalcdep.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Soelch C, Szczepanik J, Nugent A, et al. Lateralization and gender differences in the dopaminergic response to unpredictable reward in the human ventral striatum. Eur J Neurosci. 2011;33:1706–15. doi: 10.1111/j.1460-9568.2011.07642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 30.Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D. Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol Teratol. 2016 doi: 10.1016/j.ntt.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16:376–85. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 32.Calipari ES, Juarez B, Morel C, et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard BA, Glick SD. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev Alcohol. 1995;12:231–41. doi: 10.1007/0-306-47138-8_15. [DOI] [PubMed] [Google Scholar]
- 34.Rubino T, Vigano D, Realini N, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–71. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro-Carvalho A, Lima CS, Nunes-Freitas AL, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Exposure to nicotine and ethanol in adolescent mice: effects on depressive-like behavior during exposure and withdrawal. Behav Brain Res. 2011;221:282–9. doi: 10.1016/j.bbr.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson SR, Hofford RS, Roberts KW, Eitan D, Wellman PJ, Eitan S. Sex differences in affective response to opioid withdrawal during adolescence. J Psychopharmacol. 2010;24:1411–7. doi: 10.1177/0269881109106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salas J, Scherrer JF, Lustman PJ, Schneider FD. Racial Differences in the Association between Non-Medical Prescription Opioid Use, Abuse/Dependence and Major Depression. Subst Abus. 2015 doi: 10.1080/08897077.2015.1129523. [DOI] [PubMed] [Google Scholar]
- 38.Nelson CJ, Balk EM, Roth AJ. Distress, anxiety, depression, and emotional well-being in African-American men with prostate cancer. Psychooncology. 2010;19:1052–60. doi: 10.1002/pon.1659. [DOI] [PubMed] [Google Scholar]
- 39.Probst JC, Laditka SB, Moore CG, Harun N, Powell MP. Race and ethnicity differences in reporting of depressive symptoms. Adm Policy Ment Health. 2007;34:519–29. doi: 10.1007/s10488-007-0136-9. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery BE, Stewart KE, Bryant KJ, Ounpraseuth ST. Dimensions of religion, depression symptomatology, and substance use among rural African American cocaine users. J Ethn Subst Abuse. 2014;13:72–90. doi: 10.1080/15332640.2014.873605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao Z, Richie WD, Bailey RK. Racial and Ethnic Disparity in Major Depressive Disorder. J Racial Ethn Health Disparities. 2015 doi: 10.1007/s40615-015-0188-6. [DOI] [PubMed] [Google Scholar]
- 42.Maremmani AG, Rovai L, Rugani F, et al. Chronology of illness in dual diagnosis heroin addicts: The role of mood disorders. J Affect Disord. 2015;179:156–60. doi: 10.1016/j.jad.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Dakwar E, Nunes EV, Bisaga A, et al. A comparison of independent depression and substance-induced depression in cannabis-, cocaine-, and opioid-dependent treatment seekers. Am J Addict. 2011;20:441–6. doi: 10.1111/j.1521-0391.2011.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kerridge BT, Saha TD, Chou SP, et al. Gender and nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions - III. Drug Alcohol Depend. 2015;156:47–56. doi: 10.1016/j.drugalcdep.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Florez-Salamanca L, Secades-Villa R, Hasin DS, et al. Probability and predictors of transition from abuse to dependence on alcohol, cannabis, and cocaine: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Drug Alcohol Abuse. 2013;39:168–79. doi: 10.3109/00952990.2013.772618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiatry. 2002;59:375–80. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- 48.Nesvag R, Knudsen GP, Bakken IJ, et al. Substance use disorders in schizophrenia, bipolar disorder, and depressive illness: a registry-based study. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1267–76. doi: 10.1007/s00127-015-1025-2. [DOI] [PubMed] [Google Scholar]
- 49.Maremmani I, Pani PP, Mellini A, et al. Alcohol and cocaine use and abuse among opioid addicts engaged in a methadone maintenance treatment program. J Addict Dis. 2007;26:61–70. doi: 10.1300/J069v26n01_08. [DOI] [PubMed] [Google Scholar]
- 50.Bandettini Di Poggio A, Fornai F, Paparelli A, Pacini M, Perugi G, Maremmani I. Comparison between heroin and heroin-cocaine polyabusers: a psychopathological study. Ann N Y Acad Sci. 2006;1074:438–45. doi: 10.1196/annals.1369.044. [DOI] [PubMed] [Google Scholar]
- 51.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imperato A, Mele A, Scrocco MG, Puglisi-Allegra S. Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. Eur J Pharmacol. 1992;212:299–300. doi: 10.1016/0014-2999(92)90349-9. [DOI] [PubMed] [Google Scholar]
- 53.He S, Grasing K. Chronic opiate treatment enhances both cocaine-reinforced and cocaine-seeking behaviors following opiate withdrawal. Drug Alcohol Depend. 2004;75:215–21. doi: 10.1016/j.drugalcdep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Moss HB, Goldstein RB, Chen CM, Yi HY. Patterns of use of other drugs among those with alcohol dependence: Associations with drinking behavior and psychopathology. Addict Behav. 2015;50:192–8. doi: 10.1016/j.addbeh.2015.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Arias M, Roger-Sanchez C, Vilanova I, et al. Effects of Cannabinoid Exposure during Adolescence on the Conditioned Rewarding Effects of WIN 55212-2 and Cocaine in Mice: Influence of the Novelty-Seeking Trait. Neural Plast. 2016;2016:6481862. doi: 10.1155/2016/6481862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alvaro-Bartolome M, Garcia-Sevilla JA. Dysregulation of cannabinoid CB1 receptor and associated signaling networks in brains of cocaine addicts and cocaine-treated rodents. Neuroscience. 2013;247:294–308. doi: 10.1016/j.neuroscience.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 57.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant KA, Arciniega LT, Tonigan JS, Miller WR, Meyers RJ. Are reconstructed self-reports of drinking reliable? Addiction. 1997;92:601–6. [PubMed] [Google Scholar]
- 59.Fischer B, Murphy Y, Kurdyak P, Goldner EM. Depression - A major but neglected consequence contributing to the health toll from prescription opioids? Psychiatry Res. 2016;243:331–4. doi: 10.1016/j.psychres.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 60.Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–24. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]