DEAR EDITOR
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by disrupted epidermal barrier functions.1 Stratum corneum (SC) consists of corneocytes and a lipid-rich extracellular matrix, which plays a key role in epidermal permeability barrier (EPB) functions.2,3 Major lipid constituents of the SC are ceramides (CERs), free fatty acids (FFAs), cholesterol and triglycerides (TGs).2,3 Staphylococcus aureus (S.aureus) colonization is an important trigger of AD.4 Comprehensive profiling of SC lipids using S.aureus colonization status, and association between S.aureus colonization and skin lipid composition, has never been documented.
In our study cohort, 27 AD and 15 healthy individuals with no history of skin disorders (≥ 18 years) were enrolled under an IRB-approved protocol. All AD participants had elevated trans-epidermal water loss (TEWL), TARC/CCL17, total serum IgE levels and eosinophil counts (clinical markers of AD severity5) compared to non-atopic (NA) participants before and after (FIG E1A–D and FIG 1) age and gender adjustment. We extracted SC lipids using a high-yield, one-step, modified Bligh and Dryer method6, and all major SC lipids were analyzed by LC-MS/MS. CERs are the most abundant lipid class in human SC (50%) and are divided into 12 subclasses.3 Consequently, the profile of SC lipids was compared between AD S. aureus colonized (AD S. aureus+, n=15), AD S. aureus non-colonized (AD S. aureus−, n=12) and NA S. aureus non-colonized participants(n=15). Certain short-chain CERs, such as CER[AH]C34 and CER[AP]C34, were significantly higher in AD than in NA participants as reported earlier (TABLE E1).3 Specific CERs, belonging to 4/12 subclasses, differed between AD S. aureus+ and AD S. aureus− participants. The levels of CER[AH](44, 48, 50 and 52 carbons length), CER[AP](40 carbons length), as well as very-long-chain CERs, e.g. CER[EOH](66, 68 and 70 carbons length) and CER[EOS](70 carbons length), were significantly lower in AD S. aureus+ than AD S. aureus− participants (TABLE E1 and FIG 1). After age and gender adjustment, CER[AH]C38, CER[AH]C48, CER[AP]C40, CER[EOH]C66, CER[EOH]C68 and CER[EOS]C70 were significantly lower in AD S. aureus+ participants (TABLES 1 and E1). Basal TEWL negatively correlated with levels of certain CERs, including CER[AH]C48, CER[AH]C50, CER[AP]C36, CER[EOH]C66, CER[EOH]C68, CER[EOS]C70, CER[NDS]C52 and CER[NDS]C54. Interestingly, CER[NDS]C52 and CER[NDS]C54 were significantly lower in AD S. aureus− compared to NA participants but comparable between AD S. aureus+ and AD S. aureus− participants, suggesting that this subgroup of lipids might be only involved in EPB homeostasis. However, CER[AH]C38 and CER[AP]C40 were only significantly lower in AD S. aureus+ compared to AD S. aureus− participants, and no correlation with TEWL values was observed. This suggests that those CERs might only exhibit antimicrobial activities or provide a survival advantage for S. aureus. The level of very-long-chain CERs (e.g., CER[EOH]C66, CER[EOH]C68 and CER[EOS]C70) was significantly decreased in AD S. aureus+ compared to AD S. aureus− participants and negatively correlated with TEWL (TABLES 1 and E1).
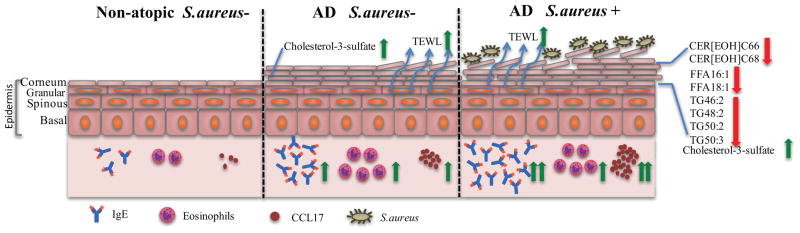
FIG 1. Hypothetical model of altered lipid distribution in skin of AD S. aureus− and AD S. aureus+ participants.
Basal trans-epidermal water loss (TEWL), serum IgE, serum thymus and activation-regulated chemokine (TARC/CCL17) and eosinophils are significantly elevated in AD S. aureus− and AD S. aureus+ participants compared to non-atopic (NA) participants (green arrows). The level of CER[AH]C48, CER[EOH]C66, CER[EOH]C68, CER[EOS]C70, FFA16:1, FFA18:1, TG46:2, TG48:2, TG50:2 and TG50:3 is significantly down-regulated in AD S. aureus+ participants compared to AD S. aureus− (red arrows). The level of cholesterol-3-sulfate is significantly increased in AD S. aureus− and AD S. aureus+ participants compared to NA (green arrows). AD, Atopic dermatitis; S. aureus, Staphylococcus aureus.
TABLE 1.
Changes in Stratum Corneum Lipids Profile Related to S.aureus Status in Atopic Dermatitis Participants
| AD S. aureus+/AD S. aureus− | AD S. aureus−/NA S. aureus− | Atopic/Non-Atopic | BasalTEWL (g/(m2h)) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Geometric Mean Ratio[1] (95% CI) | Geometric Mean Ratio[1] (95% CI) | Geometric Mean Ratio[1] (95% CI) | Geometric Mean Ratio[2] (95% CI) | |||||
| Carbon Chain Length (Intensity) | ||||||||
| p-value | p-value | p-value | p-value | |||||
| CER[EOH] C66 | 0.52 (0.34, 0.79) | 0.003** | 1.05 (0.70, 1.59) | 0.797 | 0.76 (0.54, 1.06) | 0.103 | 0.40 (0.25, 0.63) | <0.001*** |
| CER[EOH] C68 | 0.57 (0.38, 0.86) | 0.008** | 1.13 (0.77, 1.67) | 0.521 | 0.86 (0.62, 1.18) | 0.332 | 0.41 (0.25, 0.68) | <0.001*** |
| Cholesterol-3-sulfate | 0.86 (0.67, 1.10) | 0.210 | 1.50 (1.18, 1.90) | 0.001** | 1.38 (1.14, 1.68) | 0.002** | 2.52 (1.02, 6.27) | 0.046* |
| FFA 16:1 | 0.18 (0.06, 0.52) | 0.003** | 1.75 (0.61, 4.99) | 0.288 | 0.73 (0.31, 1.73) | 0.466 | 0.97 (0.78, 1.21) | 0.782 |
| FFA 18:1 | 0.43 (0.28, 0.66) | <0.001*** | 1.49 (0.98, 2.25) | 0.061 | 0.97 (0.69, 1.37) | 0.872 | 1.18 (0.69, 2.05) | 0.534 |
| TG 46:2 | 0.26 (0.07, 0.96) | 0.043* | 1.48 (0.42, 5.23) | 0.532 | 0.75 (0.27, 2.12) | 0.583 | 0.84 (0.71, 1.00) | 0.049* |
| TG 48:2 | 0.45 (0.25, 0.80) | 0.008** | 1.34 (0.76, 2.34) | 0.300 | 0.90 (0.57, 1.42) | 0.635 | 0.74 (0.50, 1.10) | 0.134 |
| TG 50:2 | 0.48 (0.28, 0.83) | 0.009** | 1.63 (0.97, 2.73) | 0.063 | 1.13 (0.74, 1.73) | 0.551 | 0.84 (0.54, 1.31) | 0.430 |
| TG 50:3 | 0.44 (0.26, 0.74) | 0.003** | 1.62 (0.97, 2.72) | 0.064 | 1.07 (0.70, 1.64) | 0.739 | 0.83 (0.53, 1.28) | 0.381 |
Most significantly altered lipids are represented. For details see Table E1 and E2. NS: Not Significant;
P<0.05;
P<0.01,
P<0.001.
Red and green colors indicate significant down-regulation and up-regulation, respectively. Orange and blue colors indicate significant negative-correlation and positive-correlation with basal TEWL, respectively.
Geometric mean ratio of SC lipid between any two of the three diagnostic groups adjusted for age and gender.
Geometric mean ratio of basal TEWL for every 10-fold increase in a SC lipid, adjusted for diagnostic group, age and gender.
Cholesterol and cholesterol-3-sulfate are abundant in human epidermis. Here, the level of cholesterol was comparable among all groups as reported earlier (TABLE E1).7 Interestingly, compared to NA participants, cholesterol-3-sulfate was significantly increased in AD participants, irrespective of S. aureus colonization, age and gender (TABLE 1, E1 and FIG 1). Increased cholesterol-3-sulfate was also associated with increased basal TEWL (TABLES 1 and E1), suggesting that increased cholesterol-3-sulfate level may be a risk factor for AD in general and a possible contributor to barrier disruptions. A recent study indicated that cholesterol-3-sulfate cycle disruption accounts for EPB abnormality in X-linked ichthyosis.8
FFAs are crucial for barrier functions2 and FFA chain length has been reported to be altered in AD skin.3 We found that levels of two unsaturated FFAs, FFA16:1 and FFA18:1, were significantly lower in AD S. aureus+ than in AD S. aureus− participants but comparable between AD S. aureus− and NA participants, regardless of age and gender adjustment (TABLE 1, E2 and FIG 1). There was no evidence of an association between basal TEWL and FFAs (TABLES 1 and E2), suggesting that dysregulation of FFAs are more likely responsible for skin antimicrobial defense and not barrier function. Consistently, exogenous FFA16:1 has been reported as a potent bacterial growth inhibitor.9
TGs that are broken down to FFAs, play a critical role in EPB maintenance.2,3 Notably, the levels of a group of TGs (e.g., TG46:1, TG48:1, TG48:2, TG50:1, TG50:2, TG50:3) were significantly lower in AD S. aureus+ participants and independent of age and gender adjustment (TABLE 1, E2 and FIG 1). Whereas TG46:2 and TG56:2 were significantly reduced in AD S. aureus+ participants after adjusting for age and gender (TABLE 1, E2). Increased basal TEWL was only associated with decreased TG46:2 (TABLE 1, E2). These results suggest a potential role for TGs in cutaneous anti-microbial defense.
Altogether, our work suggests that some lipids associate with bacterial colonization and others with physiologic evidence of barrier dysfunction. S.aureus colonization might be a confounder for disease severity in relation to lipid composition. A recent report identified 2430 differentially expressed genes in AD skin, with those involved in lipid metabolism and biosynthesis among the top Gene Ontology-terms that were enriched, indicating the importance of lipid metabolism in atopic skin.10 Present study characterizes the unique lipid profile observed in AD and well-recognized AD sub-phenotypes. The conclusions that can be made about cause and effect are limited from our cross-sectional study design but the ability to evaluate lipid composition in a fast, reliable and non-invasive way makes it feasible to perform a longitudinal study. Present results may be important for therapeutic applications that could reverse S. aureus colonization and improve skin barrier in AD patients.
Supplementary Material
Acknowledgments
Funding: This project has been funded in whole or in part by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) Atopic Dermatitis Research Network contracts HHSN272201000020C and HHSN272201000017C, grants U19 AI117673-01 and UM2AI117870, and ONAMI pre-GAP funding. Clinical Trial Research Centers was supported in part by the Colorado Clinical and Translational Science Award/Colorado Clinical & Translational Sciences Institute grant UL1 RR025780 from National Center for Research Resources/NIH and from NIH/National Center for Advancing Translational Sciences (grant UL1 TR000154).
We gratefully acknowledge Dr. Taifo Mahmud and Khaled Almabruk for help with performing the MIC assay. We thank Dr. Fred Stevens from OSU Mass Spectrometry Facility for advice on mass spectrometry analyses. We also thank Drs. Mark Zabriskie and Theresa Filtz of the OSU College of Pharmacy for continuous support and encouragement.
Abbreviations
- AD
atopic dermatitis
- TARC/CCL17
Thymus- and activation-regulated chemokine/Chemokine (C-C motif) ligand 17
- CER
ceramides
- CER[AH]
consisting of α-hydroxy fatty acid and 6-hydroxysphingosines
- CER[AP]
consisting of α-hydroxy fatty acid and phytosphingosines
- CER[EOH]
consisting of ester-linked ω-hydroxy fatty acid and 6-hydroxysphingosines
- CER[EOS]
consisting of ester-linked κ-hydroxy fatty acid and sphingosines
- CER[NDS]
consisting of non-hydroxy fatty acids and dihydrosphingosines
- EPB
epidermal permeability barrier
- FFAs
free fatty acids
- FLG
filaggrin gene
- LC-MS/MS
liquid chromatography-mass spectrometry
- MIC
minimum inhibitory concentration
- NA
non-atopic
- S. aureus
Staphylococcus aureus
- SC
stratum corneum
- TEWL
trans-epidermal water loss
- TGs
Triglycerides
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- 1.Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151–61. doi: 10.2332/allergolint.13-RAI-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feingold KR, Elias PM. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim Biophys Acta. 2014;1841:280–94. doi: 10.1016/j.bbalip.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.van Smeden J, Janssens M, Gooris GS, et al. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta. 2014;1841:295–313. doi: 10.1016/j.bbalip.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Glatz M, Horiuchi K, et al. Dysbiosis and Staphylococcus aureus Colonization Drives Inflammation in Atopic Dermatitis. Immunity. 2015;42:756–66. doi: 10.1016/j.immuni.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CH, Yu HS. Biomarkers for itch and disease severity in atopic dermatitis. Curr Probl Dermatol. 2011:136–48. doi: 10.1159/000323307. [DOI] [PubMed] [Google Scholar]
- 6.Eg B, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Joo KM, Hwang JH, Bae S, et al. Relationship of ceramide-, and free fatty acid-cholesterol ratios in the stratum corneum with skin barrier function of normal, atopic dermatitis lesional and non-lesional skins. J Dermatol Sci. 2015;77:71–4. doi: 10.1016/j.jdermsci.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Elias PM, Williams ML, Choi EH, et al. Role of cholesterol sulfate in epidermal structure and function: lessons from X-linked ichthyosis. Biochim Biophys Acta. 2014;1841:353–61. doi: 10.1016/j.bbalip.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons JB, Yao J, Frank MW, et al. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bacteriol. 2012;194:5294–304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole C, Kroboth K, Schurch NJ, et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J Allergy Clin Immunol. 2014;134:82–91. doi: 10.1016/j.jaci.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.