Abstract
The surgical treatment of lung malignancies often results in persistent symptoms, psychosocial distress, and decrements in quality of life (QOL) for cancer patients and their family caregivers (FCGs). The potential benefits of providing patients and FCGs with preparatory education that begins in the preoperative setting has been explored in multiple medical conditions, with positive impact observed on postoperative recovery, psychological distress, and QOL. However, few studies have explored the benefits of preparatory educational interventions to promote self-management in cancer surgery, including lung surgery. This paper describes the systematic approach used in the development of a multimedia self-management intervention to prepare cancer patients and their FCGs for lung surgery. Intervention development was informed by 1) contemporary published evidence on the impact of lung surgery on patients and FCG, 2) our previous research that explored QOL, symptoms, and caregiver burden after lung surgery, 3) the use of the chronic care self-management model (CCM) to guide intervention design, and 4) written comments and feedback from patients and FCGs that informed intervention development and refinement. Pilot-testing of the intervention is in process, and a future randomized trial will determine the efficacy of the intervention to improve patient, FCG, and system outcomes.
Keywords: Surgery, lung malignancies, family caregivers, quality of life
Introduction
Surgery is one of the most common and effective treatments for primary and metastatic malignancies of the lung. Like all cancer treatments, surgery has a negative impact on a range of physical and psychosocial health outcomes. Studies have shown that lung surgery substantially reduces all dimensions of quality of life (QOL) in the immediate postoperative period [1]. Unfortunately, a large proportion of patients continue to experience functional limitations and persistent symptoms such as pain and dyspnea [2,3]. Patients often report anxiety related to hospitalization, recovery, potential complications, and outcomes of surgery, but the majority were not directed to resources that would help to alleviate their anxiety [4]. Heightened pre-op anxiety may adversely impact physical preparation and contribute to a stress response that can impede recovery [5]. Persistent unmet needs related to self-care and monitoring symptoms following discharge may result in suboptimal recovery and unexpected hospital readmissions [6]. Patient and family caregiver (FCG) preparatory education has long been seen as critical to surgical recovery, and is an essential tool for promoting better understanding of treatment and recovery. The potential benefits of pre-operative preparatory education on physical and psychological outcomes have been tested in patients undergoing non-cancer surgery, with observed reductions in hospital length of stay, anxiety, and depressive scores [7]. This paper reviews the impact of lung surgery on patient and FCG QOL and describes the development process of a multimedia intervention (video, print) to prepare cancer patients and FCGs for lung surgery.
The Impact of Lung Surgery on Quality of Life
Figure 1a and 1b presents a summary of the physical, psychological, social, and spiritual well-being experiences of lung surgery patients and FCGs using the City of Hope QOL model [8]. Many studies have described the QOL changes in patients following lung surgery. Most suggest that surgery has a negative impact on short-term QOL, specifically in the physical and functional well-being domains [9]. Transient declines in physical and functional well-being are common, with gradual recovery in most patients at 6–9 months following surgery [2,3,9–12]. However, long-term physical well-being issues can persist for some patients 2–3 years following surgery [2,9,10,13]. The most prevalent symptoms following lung surgery are pain, fatigue, dyspnea and cough [9,10]. Most studies report a one-month transient increase in pain severity, with recovery observed between 3–9 months postoperatively [2,14–17]. Dyspnea may persist 2–3 years after surgery, with approximately 53% of patients experiencing persistent dyspnea and 40% with worsening fatigue [2]. The significance of persistent symptoms and functional impairments may vary depending on the type of surgery and factors such as age and co-morbidities [18,19,20].
Figure 1.
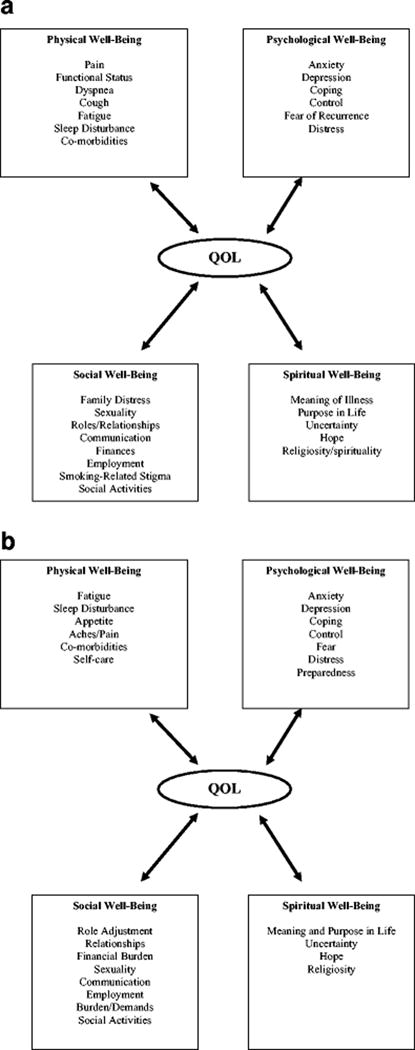
a. Quality of Life in Lung Surgery Patients
b. Quality of Life in Lung Surgery Family Caregivers
Studies have shown that the majority of patients experience improved psychological well-being after surgery compared with baseline but levels were lower in reference to the general population [9]. However, a small subset of patients experienced persistent psychological distress over time (25–33%) [9,21]. Factors that provoke anxiety in resectable patients are often not surgical risks of perioperative morbidity or mortality, but the physical and mental handicaps that hinder recovery postoperatively [22,23]. The association with smoking has resulted in substantial stigma with lung malignancies, and patients report higher self-blame, poorer self-esteem, and more psychological maladjustment [24,25]. The physical and psychological effects of surgery can also negatively impact social well-being, and lead to increased family distress, reduced participation in social activities, and relationship problems [26]. Conversely, spirituality has a protective effect on overall QOL and symptom distress in lung cancer patients, with improved psychological well-being in patients who reported more meaning in life [27,28].
Our research program included two sub-analysis of QOL outcomes from an NCI-funded Program Project that tested the efficacy of an interdisciplinary palliative care intervention in stage I-IV lung cancer patients. The first analysis described the longitudinal changes in physical function, symptoms, and QOL in early stage lung cancer patients (stage I–III) [29]. We found that overall symptom distress was highest at baseline and 2 months post-enrollment, but improved at 12 months. The most prevalent symptoms at baseline included fatigue, anxiety, dyspnea, sleep disturbances, and cough, all with prevalence of greater than 69%. In a separate analysis, we described the 12-month trajectory of five symptoms (fatigue, sleep, dyspnea, cough, pain) for patients who were surgically treated and had pre-operative baseline data [30]. All symptom scores were highest at baseline but improved across the 12-month trajectory, with the least amount of changes observed for dyspnea and pain.
Equally important, lung surgery can profoundly affect the well-being of FCGs. There is scant evidence in the current literature that focused exclusively on the QOL needs of FCGs in lung surgery. In studies with lung cancer FCGs that included patients who were surgically treated, FCG physical and mental health were lower than population norms [31]. Greater than 50% of FCGs reported negative emotional consequences of caregiving, and 33% reported negative consequences to their physical health [31]. Common FCG symptoms resulting from providing care include sleep disruption and fatigue [31]. Perceived caregiver burden was a significant correlate of depressive symptoms in FCGs, and female caregivers were more likely to report negative life changes with the caregiving role [32,33]. In a qualitative study, challenges in coping with a family member’s lung cancer included a profound sense of uncertainty regarding the future (38%), managing the patient’s emotional reaction (33%), and performing practical tasks such as patient care coordination (14%) [34]. A large proportion of FCGs experienced one or more adverse social and financial changes. These included disengagement from social and leisure activities (56%), reduced work hours (45%), having to quit work or make major lifestyle changes due to caregiving (18%), and loss of most or all of their family savings (18%) [35]. Reductions in social activities and loss of main income were associated with more caregiver psychological distress [35]. Research related to spirituality suggests that similar to patients, FCGs also derive meaning in their cancer experience. Finding meaning in a loved one’s cancer resulted in more positive adjustments to the caregiving role [27].
We performed several secondary analyses using data from our previous studies to describe caregiver burden, skills preparedness, and QOL in FCGs of lung cancer patients, including those who were surgically treated [36,37]. Overall, FCGs experienced high levels of caregiver burden, particularly in subjective demand burden (perceived demands of caregiving re-sponsibilities) and subjective stress burden (emotional response to caregiving). FCG’s reported moderate levels of psychological distress that worsened over time, and psychological well-being domain had the worst QOL score [36]. Problem areas that are associated with increased FCG psychological distress included the self-care component (problems related to FCG self care and maintenance of QOL), the FCG role component (perceptions of the caregiving role in terms of demands and preparedness), and the FCG stress component (emotional response to the caregiving role) [37].
Intervention Overview
Conceptual Framework
The framework driving the design of this multimedia intervention is the chronic care self-management model (CCM). The CCM transforms a reactive health care system into one that improves patient outcomes through planning, proven strategies, management, and patient activation [38,39]. The model results in healthier patients, more satisfied providers, and lower health care utilizations, and can be applied to cancer surgical populations [40,41]. The CCM recognizes six essential elements of high-quality care. These include: 1) systems that promote safe, high quality care; 2) effective, efficient clinical care that includes patient and FCG self-management support; 3) care that is evidence-based and family-centered; 4) efficient and effective care through organized data; 5) care that empowers and prepares patients and FCGs to manage healthcare; and 6) inclusion of community resources [41]. The six essential elements and responsive intervention content are described in Table 1.
Table 1.
Essential Elements of the Chronic Care Model (CCM) Applied to Preparing Patients and Family Caregivers in Lung Surgery
| CCM Essential Elements | Intervention Content |
|---|---|
| 1. Systems that promote safe, high quality surgical care | Supporting patients and family units by providing quality information before and after lung surgery that is preparatory, patient-centered, and cost-effective. |
| 2. Effective, efficient clinical care that includes patient and family self-management support | Provide self-management information to enhance patient and family caregiver self-efficacy in preparing for lung surgery and recovery. |
| 3. Evidence-based, patient- and family-centered care | Incorporate teaching and counseling content that is culturally appropriate, evidence-based, and personalized to patient and family’s individual needs. |
| 4. Efficient and effective care through organized data | Design and develop efficient and effective methods of intervention implementation by integrating technology with traditional print/written materials (video-assisted, wed-based, etc). |
| 5. Care that empowers and prepares patients and families to manage their health | Integrate evidence-based self-management strategies to empower and prepare patients and families before and after surgery. |
| 6. Inclusion of community resources | Identify and provide patients and families with community resources that are culturally appropriate, and include these resources as an essential element of the intervention content. |
Cancer patients and their FCGs make day-to-day decisions (self-manage) about their illnesses. Self-management education complements traditional patient education in supporting patients to live the best possible quality of life with their cancer. Our intervention incorporates patient activation, a construct that focuses on taking action to maintain and improve one’s health [42]. A central concept in self-management is self-efficacy, which is defined as confidence to carry out a behavior necessary to reach a desired goal [43]. Self-efficacy is enhanced when patients and FCGs succeed in building confidence in their ability to manage their health. Whereas traditional patient education offers information and technical skills, self-management education enhances activation and self-efficacy for patients and FCGs.
There is an emerging body of literature that assesses the efficacy of multimedia interventions for cancer patient education. A recent systematic review and meta-analysis of video interventions found that this educational technology was as effective, and in some RCTs, superior in knowledge transfer compare to traditional methods such as print materials [44]. Furthermore, with the presentation focusing on basic, thorough, and uniform information, videos may be especially suitable for patients and families who are non-native English speakers and those with low health literacy levels [44].
Intervention Content
Table 2 provides a list of content for the 15-minute multimedia intervention. The intervention is dyadic because it contains content to support both patients and FCGs, and the intervention is administered to both at the same time using the same medium (video program and accompanying handbook). The utilization of audio/visual and print materials will provide patients and FCGs with different methods of learning, in accordance with adult principles of learning.
Table 2.
Intervention Content
|
COMPONENT 1 (PRE-OP): Part 1 What to Expect Before SurgeryWhat to Expect on the Day of Surgery |
|
|
|
|
| Part 2 What to Expect After Surgery –Recovery in the Hospital |
|
|
| |
|
COMPONENT 2 (BEFORE DISCHARGE): Part 3 What to Expect When Healing at Home |
|
|
| |
|
COMPONENT 3: (AFTER DISCHARGE) Telephone Support |
|
There are three main components within the intervention, and these are administered at different times in the perioperative setting. This design was selected to diminish information overload and burden for patients and FCGs. The first component is delivered pre-operatively, and consists of content provided in Parts 1 and 2 of the intervention, which prepares patients and FCGs for what to expect before and up to discharge after surgery. The second component is delivered post-operatively prior to discharge, and consists of content provided in Part 3 of the intervention, which prepares patients and FCGs on what to expect after going home where recovery is expected to continue. Finally, the third component consists of telephone support and assessment during the first two weeks post-discharge and prior to the first post-operative visit.
As part of our intervention development and refinement process, we presented the first version of the multimedia intervention to seven patients and FCGs for review. Reviewers were participants on a previous study that tested an interdisciplinary palliative care intervention in lung cancer, which included surgically treated patients and their FCGs. Patients and FCGs reviewed a copy of the intervention content, and were asked to provide written comments and feedback on specific and overall content. Overall, patients and FCGs endorsed the need and potential benefits of the intervention content, dose, and timing. Patient/FCG reviewers confirmed the importance of including information on additional community resources, the practical tips for FCGs on caring for patients, and the importance of addressing anxiety as part of the intervention (see Table 3). Based on feedback, areas that needed more emphasis and/or content included breathing exercises, the expected sleep disturbance during hospitalization, coughing, and the need for more information on the different types of lung surgery.
Table 3.
Intervention Comments and Recommendations
| Stress the coughing and use of the “bear.” (Patient) |
| Expect not to get much sleep in the hospital. (Patient) |
| Bringing up lots of gunk while coughing is important. (Patient) |
| My whole family could have used this! (Patient) |
| A great book! Helpful for patients and families (FCG) |
| The additional resources section was very helpful. (FCG) |
| The parts for family members are very helpful, like giving tips on how we can help the patient. (FCG) |
| I especially liked the part about worry and fear. Very important. (Patient) |
| The right amount of information if it’s not read all at once. (Patient) |
| Re-word “quit smoking.” It’s a turn off. (Patient) |
| Like how it’s broken down into sections. Better that way since there can be lots of information! (FCG) |
| More information on the kinds of lung surgery. It’s hard to remember what the doctor told you. (Patient) |
Conclusions
In this paper, we aimed to present the systematic development of a multimedia intervention to better prepare patients and FCGs for lung surgery. Cancer preparatory interventions that begins in the preoperative setting have potential for improving important patient-reported, surgical, and system outcomes, and more evidence from well-designed interventional studies are needed to confirm these benefits. We designed a multimedia intervention based on a review of the contemporary literature as well as our own studies. We incorporated several key components that are lacking in current cancer preparatory educational interventions [5]. These include the addition of postoperative follow-up sessions to facilitate self-management and assess for QOL needs, inclusion of practical information on engaging in self-management activities that enhance recovery, integration of a multimedia approach to offer alternative methods of learning, and the inclusion of content to support FCG needs. A more holistic approach to “prehabilitation” that addresses physical and psychological needs has the potential to improve QOL as well as important metrics such as length of hospital stay [45]. Interventions that integrate self-management strategies to improve patient and FCG preparation for lung surgery are cost-effective approaches to impact these important metrics, particularly for high-risk patients [46–48]. Our interdisciplinary research team is currently pilot-testing the multimedia intervention in lung surgery patients and FCGs, using a sequential enrollment design to assess its feasibility, usability, and acceptability. This will lead to a larger randomized trial to confirm the efficacy of the intervention.
References
- 1.Vijayvergia N, Shah PC, Denlinger CS. Survivorship in Non-Small Cell Lung Cancer: Challenges Faced and Steps Forward. Journal of the National Comprehensive Cancer Network : JNCCN. 2015 Sep;13(9):1151–1161. doi: 10.6004/jnccn.2015.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Jan 10;26(2):233–241. doi: 10.1200/JCO.2006.07.7230. [DOI] [PubMed] [Google Scholar]
- 3.Balduyck B, Hendriks J, Lauwers P, Van Schil P. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung cancer. 2007 Jun;56(3):423–431. doi: 10.1016/j.lungcan.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Wiljer D, Walton T, Gilbert J, et al. Understanding the needs of lung cancer patients during the pre-diagnosis phase. Journal of cancer education : the official journal of the American Association for Cancer Education. 2012 Jun;27(3):494–500. doi: 10.1007/s13187-012-0345-0. [DOI] [PubMed] [Google Scholar]
- 5.Waller A, Forshaw K, Bryant J, Carey M, Boyes A, Sanson-Fisher R. Preparatory education for cancer patients undergoing surgery: A systematic review of volume and quality of research output over time. Patient education and counseling. 2015 May 23;98(12):1540–1549. doi: 10.1016/j.pec.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 6.King J, Chamberland P, Rawji A, et al. Patient educational needs of patients undergoing surgery for lung cancer. Journal of cancer education : the official journal of the American Association for Cancer Education. 2014 Dec;29(4):802–807. doi: 10.1007/s13187-014-0658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco M, Iona L, Fabbro C, Bulfone G, Palese A. Patient education outcomes in surgery: a systematic review from 2004 to 2010. International journal of evidence-based healthcare. 2012 Dec;10(4):309–323. doi: 10.1111/j.1744-1609.2012.00286.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncology nursing forum. 1995 Jul;22(6):915–922. [PubMed] [Google Scholar]
- 9.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung cancer. 2013 Jul;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Cheville AL, Wampfler JA, et al. Quality of life and symptom burden among long-term lung cancer survivors. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012 Jan;7(1):64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of life of long-term survivors of non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Jul 1;20(13):2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. The Journal of community and supportive oncology. 2016 Jan;14(1):37–44. doi: 10.12788/jcso.0205. [DOI] [PubMed] [Google Scholar]
- 13.Rauma V, Sintonen H, Rasanen JV, Salo JA, Ilonen IK. Long-term lung cancer survivors have permanently decreased quality of life after surgery. Clinical lung cancer. 2015 Jan;16(1):40–45. doi: 10.1016/j.cllc.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Socci L, Refai M, Salati M, Xiume F, Sabbatini A. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. The Annals of thoracic surgery. 2007 Aug;84(2):410–416. doi: 10.1016/j.athoracsur.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli A, Pompili C, Koller M. Changes in quality of life after pulmonary resection. Thoracic surgery clinics. 2012 Nov;22(4):471–485. doi: 10.1016/j.thorsurg.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Paull DE, Thomas ML, Meade GE, et al. Determinants of quality of life in patients following pulmonary resection for lung cancer. American journal of surgery. 2006 Nov;192(5):565–571. doi: 10.1016/j.amjsurg.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Moller A, Sartipy U. Long-term health-related quality of life following surgery for lung cancer. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Feb;41(2):362–367. doi: 10.1016/j.ejcts.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MK, Parma CM, Celauro AD, Vigneswaran WT. Quality of life and mood in older patients after major lung resection. Ann Thorac Surg. 2009 Apr;87(4):1007–1012. doi: 10.1016/j.athoracsur.2008.12.084. discussion 1012-1003. [DOI] [PubMed] [Google Scholar]
- 19.Balduyck B, Hendriks J, Lauwers P, Van Schil P. Quality of life after lung cancer surgery: a prospective pilot study comparing bronchial sleeve lobectomy with pneumonectomy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2008 Jun;3(6):604–608. doi: 10.1097/JTO.0b013e318170fca4. [DOI] [PubMed] [Google Scholar]
- 20.Cavalheri V, Jenkins S, Cecins N, et al. Impairments after curative intent treatment for non-small cell lung cancer: A comparison with age and gender-matched healthy controls. Respir Med. 2015 Oct;109(10):1332–1339. doi: 10.1016/j.rmed.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Sarna L, Cooley ME, Brown JK, et al. Women with lung cancer: quality of life after thoracotomy: a 6-month prospective study. Cancer nursing. 2010 Mar-Apr;33(2):85–92. doi: 10.1097/NCC.0b013e3181be5e51. [DOI] [PubMed] [Google Scholar]
- 22.Cykert S. Risk acceptance and risk aversion: patients’ perspectives on lung surgery. Thoracic surgery clinics. 2004 Aug;14(3):287–293. doi: 10.1016/S1547-4127(04)00016-7. [DOI] [PubMed] [Google Scholar]
- 23.Rocco G, Vaughan R. Outcome of lung surgery: what patients don’t like. Chest. 2000 Jun;117(6):1531–1532. doi: 10.1378/chest.117.6.1531. [DOI] [PubMed] [Google Scholar]
- 24.Carter-Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM. Lung cancer stigma predicts timing of medical help-seeking behavior. Oncology nursing forum. 2014 May;41(3):E203–210. doi: 10.1188/14.ONF.E203-E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. Bmj. 2004 Jun 19;328(7454):1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrell B, Koczywas M, Grannis F, Harrington A. Palliative care in lung cancer. The Surgical clinics of North America. 2011 Apr;91(2):403–417, ix. doi: 10.1016/j.suc.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maliski SL, Sarna L, Evangelista L, Padilla G. The aftermath of lung cancer: balancing the good and bad. Cancer nursing. 2003 Jun;26(3):237–244. doi: 10.1097/00002820-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Sarna L, Brown JK, Cooley ME, et al. Quality of life and meaning of illness of women with lung cancer. Oncology nursing forum. 2005 Jan;32(1):E9–19. doi: 10.1188/05.ONF.E9-E19. [DOI] [PubMed] [Google Scholar]
- 29.Koczywas M, Williams AC, Cristea M, et al. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Annals of surgical oncology. 2013 Jun;20(6):1788–1797. doi: 10.1245/s10434-012-2741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raz DJ, Sun V, Kim JY, et al. Long-Term Effect of an Interdisciplinary Supportive Care Intervention for Lung Cancer Survivors After Surgical Procedures. The Annals of thoracic surgery. 2015 Oct 3; doi: 10.1016/j.athoracsur.2015.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosher CE, Bakas T, Champion VL. Physical health, mental health, and life changes among family caregivers of patients with lung cancer. Oncology nursing forum. 2013 Jan;40(1):53–61. doi: 10.1188/13.ONF.53-61. [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, van Ryn M, Jensen RE, Griffin JM, Potosky A, Rowland J. Effects of gender and depressive symptoms on quality of life among colorectal and lung cancer patients and their family caregivers. Psycho-oncology. 2015 Jan;24(1):95–105. doi: 10.1002/pon.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Duberstein PR, Sorensen S, Larson MR. Levels of depressive symptoms in spouses of people with lung cancer: effects of personality, social support, and caregiving burden. Psychosomatics. 2005 Mar-Apr;46(2):123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- 34.Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013 Feb;21(2):431–437. doi: 10.1007/s00520-012-1532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosher CE, Champion VL, Azzoli CG, et al. Economic and social changes among distressed family caregivers of lung cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013 Mar;21(3):819–826. doi: 10.1007/s00520-012-1585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant M, Sun V, Fujinami R, et al. Family caregiver burden, skills preparedness, and quality of life in non-small cell lung cancer. Oncology nursing forum. 2013 Jul;40(4):337–346. doi: 10.1188/13.ONF.337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujinami R, Sun V, Zachariah F, Uman G, Grant M, Ferrell B. Family caregivers’ distress levels related to quality of life, burden, and preparedness. Psycho-oncology. 2015 Jan;24(1):54–62. doi: 10.1002/pon.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health affairs. 2001 Nov-Dec;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 39.Lorig KR, Mazonson PD, Holman HR. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis and rheumatism. 1993 Apr;36(4):439–446. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- 40.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. Journal of alternative and complementary medicine. 2005;11(Suppl 1):S7–15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- 41.Coleman K, Austin BT, Brach C, Wagner EH. Evidence on the Chronic Care Model in the new millennium. Health affairs. 2009 Jan-Feb;28(1):75–85. doi: 10.1377/hlthaff.28.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health services research. 2004 Aug;39(4 Pt 1):1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychological review. 1977 Mar;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 44.Gysels M, Higginson IJ. Interactive technologies and videotapes for patient education in cancer care: systematic review and meta-analysis of randomised trials. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2007 Jan;15(1):7–20. doi: 10.1007/s00520-006-0112-z. [DOI] [PubMed] [Google Scholar]
- 45.Tsimopoulou I, Pasquali S, Howard R, et al. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Annals of surgical oncology. 2015 Dec;22(13):4117–4123. doi: 10.1245/s10434-015-4550-z. [DOI] [PubMed] [Google Scholar]
- 46.Rajaram R, Ju MH, Bilimoria KY, Ko CY, DeCamp MM. National evaluation of hospital readmission after pulmonary resection. The Journal of thoracic and cardiovascular surgery. 2015 May 21; doi: 10.1016/j.jtcvs.2015.05.047. [DOI] [PubMed] [Google Scholar]
- 47.Puri V, Patel AP, Crabtree TD, et al. Unexpected readmission after lung cancer surgery: A benign event? The Journal of thoracic and cardiovascular surgery. 2015 Aug 28;150(6):1496–1505 e1495. doi: 10.1016/j.jtcvs.2015.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puri V. Decreasing readmissions after lung resection: The carrot or stick approach. The Journal of thoracic and cardiovascular surgery. 2015 Dec;150(6):1515–1516. doi: 10.1016/j.jtcvs.2015.06.037. [DOI] [PubMed] [Google Scholar]


