Abstract
Although advances in chemotherapy have improved the prognosis for osteosarcoma, some patients do not respond sufficiently to treatment. Heat shock protein 70 (Hsp70) is expressed at high levels in cancer cells and attenuates the therapeutic efficacy of anticancer agents, resulting in a poorer prognosis. This study investigated whether small interfering RNA (siRNA)-mediated inhibition of Hsp70 expression in an osteosarcoma cell line would enhance sensitivity to cisplatin. The expression of Hsp70 with cisplatin treatment was observed by using Western blotting and real-time reverse transcription polymerase chain reaction (RT-PCR). Changes in the IC50 of cisplatin when Hsp70 was inhibited by siRNA were evaluated. Cisplatin’s effectiveness in inducing apoptosis was assessed by assay of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), caspase-3 activity, and mitochondrial membrane potential. Up-regulation of Hsp70 expression was dependent on the concentration of cisplatin. Inhibition of Hsp70 expression significantly reduced the IC50 of cisplatin. When cisplatin was added to osteosarcoma cells with Hsp70 expression inhibited, a significant increase in apoptosis was demonstrated in TUNEL, caspase-3, and mitochondrial membrane potential assays. Inhibition of Hsp70 expression induced apoptosis in cultured osteosarcoma cells, indicating that Hsp70 inhibition enhanced sensitivity to cisplatin. Inhibition of Hsp70 expression may provide a new adjuvant therapy for osteosarcoma.
Keywords: Heat shock protein 70 (Hsp70), Osteosarcoma, Cisplatin, Apoptosis
Introduction
Osteosarcoma is the most common histological form of primary malignant bone tumors and occurs mainly in young people (Damron et al. 2007). Therapeutic outcomes have been currently improved by treatment that comprises surgery and pre- and postoperative chemotherapy (Ferrari et al. 2007). Before the introduction of chemotherapy, 5-year survival was less than 20%, but this percentage has greatly increased thanks to the use of chemotherapy. Additionally, before chemotherapy was introduced, surgery mainly entailed amputation of the affected limb; however, thanks to effective chemotherapy and improved surgical procedures, limb-conserving surgery is now the standard treatment (Anderson et al. 2016; Ferrari et al. 2015).
First-line chemotherapy for osteosarcoma usually comprises combination therapy with cisplatin, doxorubicin, and methotrexate. Cisplatin is a platinum agent that is widely used in many cancer chemotherapy regimens. Cisplatin acts by blocking the synthesis of DNA, thereby inducing apoptosis by cancer cells (Dasari et al. 2014). Although cisplatin is a highly effective cancer treatment, its side effects are problematic. The dose of cisplatin required to treat osteosarcoma is particularly high, and this can easily damage the kidneys, peripheral nerves, and auditory nerve. A method is therefore required for obtaining an adequate therapeutic effect from a lower cisplatin dose. Caffeine, for example, has been shown to block the repair of DNA damage by tumor cells and thereby enhance their sensitivity to cisplatin, and its efficacy has been demonstrated clinically (Takeda et al. 2005). Temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, and genistein, an isoflavone, have also been shown to enhance sensitivity to cisplatin (Liu et al. 2014a; Fleuren et al. 2014). However, caffeine entails problems with cardiotoxicity, and the use of mTOR inhibitors for sarcomas has yet to be approved. Genistein has also yet to be proven to be effective against sarcoma.
Heat shock proteins (Hsps) are chaperone proteins that are induced by a range of different stresses such as heat stimulus and act to protect cells against stress. The expression and action of heat shock protein 70 (Hsp70) in cancer cells vary among different types of cancers; in breast cancer, bladder cancer, and osteosarcoma, it constitutes a negative prognostic factor, whereas in esophageal cancer and renal cancer, it reportedly acts as a positive prognostic factor (Ciocca et al. 2005; Uozaki et al. 2000; Goloudina et al. 2012). Since Pocaly et al. (2007) first reported that Hsp70 expression is involved in imatinib resistance in chronic myeloid leukemia (CML), several other studies have also identified the involvement of Hsp70 expression in resistance to anticancer agents in other forms of cancer (Yang et al. 2012; Behnsawy et al. 2013).
Against this backdrop, we hypothesized that inhibition of Hsp70 expression in osteosarcoma might improve the therapeutic efficacy of an anticancer agent (cisplatin). The objective of this study was to investigate if inhibition of Hsp70 expression in cultured osteosarcoma cells by small interfering RNA (siRNA) might enhance sensitivity of the cells to cisplatin.
Materials and methods
Cell lines and culture conditions
The human osteosarcoma cells MG63 (CRL1427) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Human osteosarcoma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX, USA), 100 units/ml penicillin, and 100 mg/ml streptomycin (complete DMEM; Nacalai Tesque) at 37 °C in 5% CO2/95% humidified air. The cells were trypsinized with trypsin/ethylenediaminetetraacetic acid (EDTA; Nacalai Tesque) and sub-cultured.
Reagents
Cisplatin was obtained from Wako (Tokyo, Japan) and was dissolved in a saline solution to give a stock solution of 1.0 mM and stored at −80 °C.
Transfection with small interfering RNA
MG63 cells were seeded into six-well plates at a density of 2.0 × 105 cells per well. The next day, the medium was changed and the cells were transfected with validated MISSION HSPA1A siRNA (SASI_Hs01_00051449; Sigma-Aldrich, St. Louis, MO, USA) or a negative control (MISSION siRNA Universal Negative Control; SIC-001; Sigma-Aldrich), each at a final concentration of 50 nM in cationic liposome (Lipofectamine RNAiMAX Reagent; Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. The plates were cultured for 48 h following which the cells were trypsinized and used in various experiments.
Western blot analysis
The cells were washed twice with PBS and then harvested and lysed in RIPA buffer (Nacalai Tesque) diluted tenfold in ultrapure water. Samples were frozen at −80 °C for 10 min, thawed at 37 °C, and then incubated on ice for 30 min. The samples were centrifuged at 20,600×g for 5 min and the supernatant was aspirated. Protein concentrations of the supernatants were measured using bicinchoninic acid (BCA) assays, and equal amounts of total proteins were loaded onto gels for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Samples containing 5 μg of protein were separated by 10% Bis-Tris Gel NuPAGE® electrophoresis using 5% MOPS SDS Running Buffer (Thermo Fisher Scientific, Waltham, MA, USA). Separated proteins were dry-blotted onto nitrocellulose iBlot®gel transfer stacks in iBlot Gel transfer devices (Thermo Fisher Scientific) for 7 min. The nitrocellulose membrane was blocked by shaking in Blocking One solution (Nacalai Tesque) for 60 min at room temperature. The blots were subsequently shaken overnight at 4 °C in this solution containing the following specific primary antibodies: anti-human Hsp70 mouse monoclonal antibody (1:1000 dilution; BioVision, Milpitas, CA, USA; catalog no. 3096–100) and mouse anti-human β-actin antibody (1:4000 dilution; Sigma-Aldrich; catalog no. A2228). β-Actin was assayed as a loading control. Blots were washed three times with TBST and shaken for 60 min at room temperature with peroxidase-conjugated anti-mouse IgG secondary antibodies (1:4000 dilution; Sigma-Aldrich; catalog no. A4416). The blots were then again washed three times with TBST, and chemiluminescence emission was visualized using Chemi-Lumi One Super (Nacalai Tesque). Protein band intensities were assessed using the ECL Select LAS500 (GE Healthcare, Buckinghamshire, England).
Total RNA extraction and real-time RT-PCR analysis
Cells were harvested and total RNA was extracted from those cells using ISOGEN (Nippon Gene, Osaka, Japan). Extracted RNAs were reverse transcribed using a PrimeScript™ RT Master Mix (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) was performed using the Biosystem 7300 (Applied Biosystems, Carlsbad, CA, USA). Sequences of the primers used were as follows: human Hsp70 (forward primer, 5′-CGGCAAGGTGGAGATCAT-3′; reverse primer, 5′-GGTGTTCTGCGGGTTCAG-3′) and 18S ribosomal RNA (forward primer, 5′-ATGAGTCCACTTTAAATCCTTTAACGA-3′; reverse primer, 5′-CTTTAATATACGCTATTGGAGCTGGAA-3′). Each 25 μl reaction mixture contained 2 μl of cDNA (100 ng) and 12.5 μl of the TaqMan Gene Expression PCR Master Mix (TOYOBO, Osaka, Japan) for the target gene. The amplification protocol consisted of 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. Relative changes in gene expression were normalized against the internal control gene, 18S ribosomal RNA.
Cell viability assays
Cell viability was determined using the RealTime-Glo MT Cell Viability Assay Kit (Promega, Madison, WI, USA). Cells were plated in a poly-l-lysine (PLL)-coated 96-well plate at a density of 1000 cells per well in 100 μl of medium containing 10% FBS. After 24 h, MT cell viability substrate and Nanoluc® Enzyme was added to the medium and the cells were exposed to various concentrations of cisplatin. IC50 values (drug concentration causing 50% inhibition of cell viability) were determined after 48-h treatment with cisplatin. The CentroXS3 LB 960 system (Berthold Technologies, Oak Ridge, TN, USA) was used to measure light emission.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay
DNA fragmentation in apoptotic cells was assayed using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assays that were performed using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Mannheim, Germany). After exposure to cisplatin for 48 h, the cells were trypsinized and then recovered with culture medium. The cells were washed twice in PBS and fixed for 60 min with 2% paraformaldehyde. The cells were then immersed in permeabilizing solution (0.1% sodium citrate and 0.1% Triton X-100 with distilled deionized water) for 2 min. TUNEL staining was performed according to the manufacturer’s instructions. Samples were finally analyzed by flow cytometry using a FACS Vantage flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). For each sample, 10,000 events were recorded. The M1 domain was defined based on the peak of fluorescence intensity of the group of cells for which TUNEL staining was not performed. The percentage of cells in the M1 domain (considered as TUNEL-positive cells) was determined and compared between groups.
Caspase-3 activity
The activity of caspase-3, an apoptosis-related factor, was assessed using the Caspase-Glo 3/7 Assay (Promega). Cells were sub-cultured in a PLL-coated 96-well plate (1 × 104 cells/well) and were then treated with cisplatin for 24 h. After this treatment, the Caspase3/7 Reagent was added to the cells for 1 h at room temperature. A CentroXS3 LB 960 system was used to measure light emission.
Measurement of mitochondrial membrane potential
Mitochondrial membrane permeability was measured by assay of the uptake of rhodamine 123 (Rh123). The cationic fluorescent probe, Rh123, is preferentially retained in mitochondria in an amount proportional to the mitochondrial membrane potential (Δψ). The loss of mitochondrial integrity or the opening of the permeability transition pore channel results in leakage of the probe from the mitochondria and a consequent decrease in fluorescence (Ferlini et al. 2007). Cells were exposed to cisplatin for 24 h, collected with a cell scraper, washed in PBS, and incubated at 37 °C for 15 min with 10 μM Rh123. After centrifugation at 1500×g for 5 min, the cells were analyzed using flow cytometry. Cells with reduced fluorescence intensity compared to the control group were defined as Δψ low cells.
Statistical analysis
All duplicated and triplicated experiments yielded almost identical results. All experimental data are expressed as mean ± standard deviation (SD). Parametric one-way analysis of variance (ANOVA) was used to test for any differences among the groups. If the result was significant, the Tukey-Kramer test was used to determine specific differences between the groups. In all analyses, p < 0.05 was defined as statistically significant.
Results
Up-regulated expression of Hsp70 in cisplatin treatment
To investigate up-regulation of the expression of Hsp70 by the chemical stressing of osteosarcoma cells with cisplatin treatment, MG63 cells were treated for 48 h with cisplatin that was adjusted to a concentration of 10, 20, or 50 μM, and Hsp70 protein levels were then analyzed using Western blotting. Hsp70 protein expression levels increased as the concentration of cisplatin increased (Fig. 1).
Fig. 1.
Cisplatin (CDDP) enhanced the Hsp70 levels in MG63 cells. Cells were treated with CDDP for 48 h and Hsp70 protein expression was then analyzed by Western blotting. β-Actin protein was used as a loading control
Effects of Hsp70 siRNA transfection on cisplatin induction of Hsp70 expression
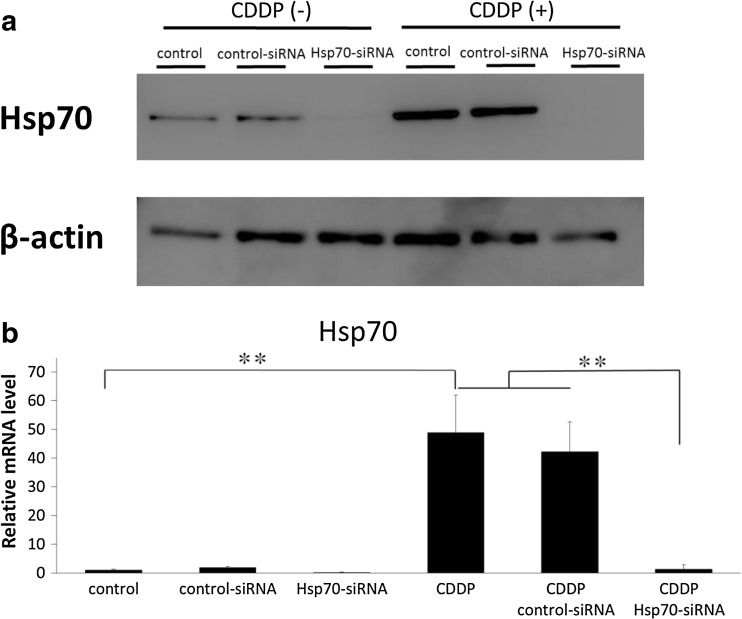
To evaluate whether Hsp70 siRNA can inhibit Hsp70 protein expression during treatment with cisplatin, MG63 cells were first transfected with Hsp70 siRNA for 48 h, following which they were treated with 50 μM cisplatin for 48 h. The dosage of cisplatin (50 μM) was selected as a high-dose administration example (Cummings et al. 2002; Liu et al. 2014b). The effect of Hsp70 siRNA transfection of MG63 cells on cisplatin induction of Hsp70 expression was then analyzed using Western blotting and real-time RT-PCR. Both the protein and mRNA expression of Hsp70 were clearly suppressed in the Hsp70 siRNA-transfected cells compared to that observed in control siRNA-transfected cells and in cisplatin-treated siRNA-transfected cells (Fig. 2a, b).
Fig. 2.
Effects of Hsp70 siRNA transfection on cisplatin induction of Hsp70 expression. MG63 cells were first transfected or not with Hsp70 or control siRNA for 48 h, following which they were treated with cisplatin (CDDP; 50 μM) for 48 h. Hsp70 protein expression was analyzed by Western blotting (a) and Hsp70 mRNA was analyzed using real-time RT-PCR (b). β-actin protein was used as a loading control. The data are expressed as mean ± standard deviation (n = 3). **p < 0.01
Suppression of Hsp70 expression in MG63 cells increased their sensitivity to cisplatin
To determine whether treatment with Hsp70 siRNA enhances the cytotoxic effect of cisplatin, MG63 cells were transfected with 50 nM Hsp70 siRNA and then treated with various doses of cisplatin, following which cell viability was assayed. Figure 3a shows that there was a decrease in cell viability as a function of drug concentration following treatment with cisplatin for 48 h. Hsp70 siRNA transfection enhanced the sensitivity of MG63 cells to cisplatin compared to control siRNA transfection. IC50 values (drug concentration causing 50% inhibition of cell viability) for cisplatin were 36.4 ± 1.94 μM in control siRNA-transfected cells and 23.4 ± 2.08 μM in Hsp70 siRNA-transfected cells (Fig. 3b).
Fig. 3.
Effects of treatment with Hsp70 siRNA and cisplatin on cell viability. The cells were treated with control or Hsp70 siRNA for 48 h and were then treated with cisplatin (CDDP) at the indicated concentrations for 48 h. Cell viability was determined using the RealTime-Glo MT Cell Viability Assay (a). IC50 values were determined after 48-h exposure to cisplatin (b). Values for Hsp70-silenced cells were compared to those of control siRNA-administered cells treated with the same concentration of cisplatin. The data are expressed as mean ± standard deviation (n = 5). *p < 0.05; **p < 0.01
Suppression of Hsp70 expression promotes cisplatin-induced apoptosis
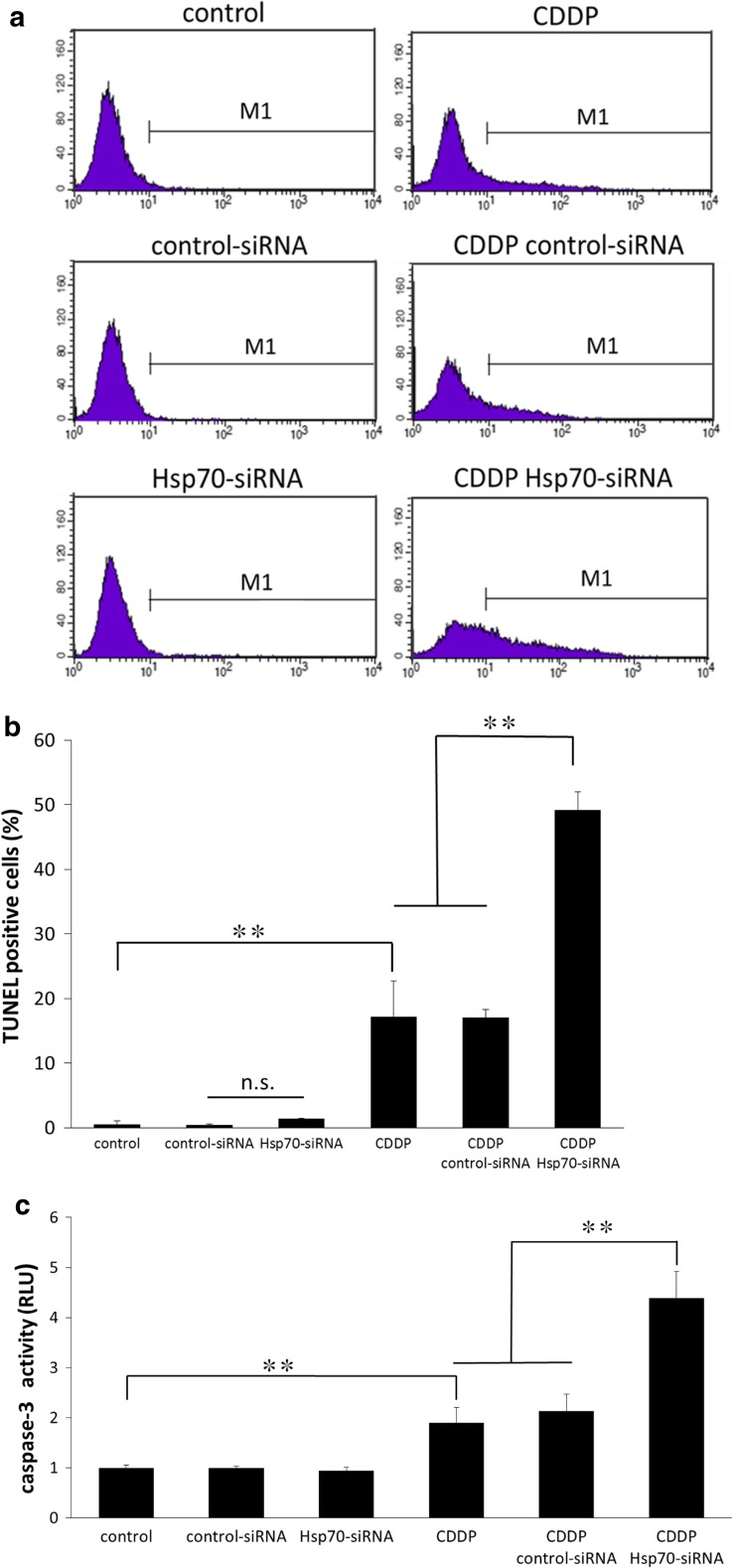
We next assessed the effect of Hsp70 siRNA transfection alone, cisplatin treatment alone, and combined treatment with Hsp70 siRNA and cisplatin on apoptosis of MG63 cells, using TUNEL staining. The TUNEL assay was performed after 48-h exposure of the siRNA-transfected or siRNA-non-transfected cells to 50 μM cisplatin, and stained cells were measured using flow cytometry. The percentage of apoptotic cells in cells transfected with Hsp70 siRNA alone did not differ in comparison with control or control siRNA-transfected cells. Cisplatin treatment significantly increased the percentage of TUNEL-positive cells compared to cells not treated with cisplatin. The highest increase in TUNEL-positive cells was observed in cells that were treated with both Hsp70 siRNA and cisplatin (Fig. 4a, b).
Fig. 4.
Hsp70 regulates cisplatin-induced apoptosis. Apoptosis of the indicated cells following exposure to cisplatin (CDDP; 50 μM) was measured using the TUNEL assay and subsequent flow cytometry. The percentage of TUNEL-positive cells is indicated within the M1 domain in (a) and is quantified in (b). The highest increase in TUNEL-positive cells was seen in cells treated with both Hsp70 siRNA and cisplatin. Caspase-3 activity was examined after 24-h exposure to cisplatin (CDDP; 50 μM). Inhibition of Hsp70 increases caspase-3 activity induced by cisplatin (c). The data are expressed as mean ± standard deviation (n = 4). **p < 0.01. n.s. not significant
Analysis of caspase-3 activity, which also reflects apoptosis, in cells treated as described in Fig. 4a, b, displayed similar results of apoptosis to those obtained in the TUNEL assay (Fig. 4c).
Combined Hsp70 siRNA and cisplatin treatment affects the intrinsic pathway of apoptosis
We examined the mitochondrial membrane potential (Δψ) of cells treated as described in Fig. 5 to check the apoptotic pathway involved in the induced apoptosis. The mitochondrial membrane potential (Δψ) was assayed by determination of the reduction of rhodamine 123 using flow cytometry. The percentage of cells with decreased mitochondrial membrane potential increased following treatment of cells with cisplatin alone compared to cells not treated with cisplatin. However, the highest increase in the percentage of cells with a low Δψ was detected in cells treated with a combination of Hsp70 siRNA plus cisplatin (Fig. 5).
Fig. 5.
Combined treatment with cisplatin and Hsp70 siRNA induces depolarization of the mitochondrial membrane potential (Δψ). After treatment with cisplatin for 48 h, the mitochondrial membrane potential, Δψ, was assessed by flow cytometry using rhodamine 123. Data are shown as mean ± SE (n = 3). **p < 0.01
Discussion
Hsps are proteins that are induced in cells as a result of physical, chemical, or other forms of stress (Zhang et al. 2002). They are classified by molecular weight, and those so far identified include Hsp27, 60, 70, and 90. Although their expression and function vary, many Hsps have been reported not only to protect normal cells against stress but also to promote their recovery from damage (Jaattela et al. 1992, 1993; Li et al. 1992). Studies are thus ongoing for the prevention or treatment of a variety of diseases by promoting the expression of Hsps.
The expression of Hsps has also been observed in a range of different cancer cells (Gimenez et al. 2010). Aghdassi et al. (2007) found that Hsp70 is strongly expressed in pancreatic cancer cells. Uozaki (2000) and Moon et al. (2010) found that Hsps are expressed in pathological tissue from osteosarcoma patients, with the level of expression corresponding to the degree of malignancy of the cells concerned. We also observed Hsp70 expression in the cultured MG63 osteosarcoma cells used in this study.
Hsps may also protect tumor cells. In particular, if Hsps protect tumor cells against anticancer agents, this may weaken the effectiveness of chemotherapy, posing a problem for treatment (Guttmann and Koumenis 2011). Drugs such as alkylating agents, antimetabolites, anticancer antibiotics, and platinum agents have conventionally been used as anticancer agents. Among these drugs, cisplatin is a platinum agent that is widely used in clinical practice to treat a variety of cancer cells. It has been used in the treatment of osteosarcoma since the 1970s and is one of the basic drugs for treating this cancer (Yamamoto et al. 2013). Here, we found that Hsp70 up-regulation in osteosarcoma cells corresponded to the cisplatin dose, suggesting that Hsp70 may act to protect MG63 cells against the antitumor action of cisplatin.
Cisplatin is administered in large doses in the treatment of osteosarcoma and its side effects are correspondingly severe, meaning that it may be difficult to continue the administration of the amount of cisplatin required (Rabik et al. 2007). If the up-regulation of Hsp70 expression induced by cisplatin administration could be inhibited, this might not only prevent the attenuation of antitumor effect but also enable a sufficient antitumor effect to be obtained with a lower dose. In this study, we reduced the IC50 of cisplatin in MG63 cells by using siRNA to specifically inhibit Hsp70 expression, showing the possibility that an equivalent antitumor effect to that of the usual dose can be obtained with low dose cisplatin. Figure 3 illustrates the results being described.
Cisplatin is known to induce cell death by activating the apoptosis pathway (Zhang et al. 2015; Seki et al. 2000), and this constitutes the main mechanism of its antitumor action. In cells that express Hsp70, however, Hsp70 expression has been shown to exert a strongly anti-apoptotic effect (Terauchi et al. 2003; Saleh et al. 2000). In this study, we used three different types of assessments to show that apoptosis is significantly increased: the changes in mitochondrial membrane potential that occur at an early stage of apoptosis; activity of caspase-3, an enzyme in the caspase family that is activated downstream; and DNA fragmentation, the final stage of cell death. Enhanced sensitivity to cisplatin may be induced by inhibiting the anti-apoptotic effect exerted by Hsp70.
In order to translate this study into clinical use, it is necessary to develop means for safely and selectively suppressing Hsp70. Treatment with siRNA has a specific inhibitory effect; however, there are problems of in vivo stability and of delivery to locations (Inoue et al. 2014; Barba et al. 2017). Hsp70 inhibitors such as VER155008 may not obtain the expected inhibiting effects in some cases because of the influence of other chaperones and co-chaperones (Asling et al. 2016). Some natural products can suppress Hsp70 and these agents are said to have a smaller adverse effect on the human body, and future clinical application can be expected (Zhao et al. 2016). The mechanism of Hsps in tumor growth has been clarified in recent years and it is important to find a new treatment for the regulation of Hsps expression (Bruning et al. 2015; Calderwood and Gong 2016).
Our results showed that inhibition of Hsp70 expression in osteosarcoma cells enhanced their sensitivity to cisplatin. This finding suggested that inhibition of Hsp70 expression might have the potential to provide a new adjuvant therapy for chemotherapy in the treatment of osteosarcoma.
This study was limited by its nature as an in vitro study of a single human osteosarcoma cell line and by the fact that it selectively knocked out only Hsp70-1 in the Hsp70 family.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 26462273 and 16H05452.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aghdassi A, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Update on survival in osteosarcoma. The Orthopedic clinics of North America. 2016;47:283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Asling J, Morrison J, Mutsaers AJ. Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy. Cell Stress Chaperones. 2016;21:1065–1076. doi: 10.1007/s12192-016-0730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba AA, et al. Engineering approaches in siRNA delivery. Int J Pharm. 2017 doi: 10.1016/j.ijpharm.2017.02.032. [DOI] [PubMed] [Google Scholar]
- Behnsawy HM, Miyake H, Kusuda Y, Fujisawa M. Small interfering RNA targeting heat shock protein 70 enhances chemosensitivity in human bladder cancer cells. Urol Oncol. 2013;31:843–848. doi: 10.1016/j.urolonc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Bruning A, Juckstock J. Misfolded proteins: from little villains to little helpers in the fight against cancer. Front Oncol. 2015;5:47. doi: 10.3389/fonc.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Gong J. Heat shock proteins promote cancer: it’s a protection racket. Trends Biochem Sci. 2016;41:311–323. doi: 10.1016/j.tibs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007;459:40–47. doi: 10.1097/BLO.0b013e318059b8c9. [DOI] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlini C, Scambia G. Assay for apoptosis using the mitochondrial probes, rhodamine123 and 10-N-nonyl acridine orange. Nat Protoc. 2007;2:3111–3114. doi: 10.1038/nprot.2007.397. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19:341–346. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- Ferrari S, Serra M (2015) An update on chemotherapy for osteosarcoma Expert Opin Pharmacother 16:2727–2736. doi:10.1517/14656566.2015.1102226 [DOI] [PubMed]
- Fleuren ED, et al. Temsirolimus combined with cisplatin or bevacizumab is active in osteosarcoma models. International journal of cancer Journal international du cancer. 2014;135:2770–2782. doi: 10.1002/ijc.28933. [DOI] [PubMed] [Google Scholar]
- Gimenez Ortiz A, Montalar Salcedo J. Heat shock proteins as targets in oncology. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010;12:166–173. doi: 10.1007/s12094-010-0486-8. [DOI] [PubMed] [Google Scholar]
- Goloudina AR, Demidov ON, Garrido C. Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett. 2012;325:117–124. doi: 10.1016/j.canlet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Guttmann DM, Koumenis C. The heat shock proteins as targets for radiosensitization and chemosensitization in cancer. Cancer biology & therapy. 2011;12:1023–1031. doi: 10.4161/cbt.12.12.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, et al. Sonoporation-mediated transduction of siRNA ameliorated experimental arthritis using 3 MHz pulsed ultrasound. Ultrasonics. 2014;54:874–881. doi: 10.1016/j.ultras.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M, Wissing D. Heat-shock proteins protect cells from monocyte cytotoxicity: possible mechanism of self-protection. J Exp Med. 1993;177:231–236. doi: 10.1084/jem.177.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Li L, Liu RY, Rehman M, Lee WM. Heat shock protein hsp70 protects cells from thermal stress even after deletion of its ATP-binding domain. Proc Natl Acad Sci U S A. 1992;89:2036–2040. doi: 10.1073/pnas.89.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yan L, Wang L, Tai W, Wang W, Yang C. Genistein enhances the effect of cisplatin on the inhibition of non-small cell lung cancer A549 cell growth in vitro and in vivo. Oncol Lett. 2014;8:2806–2810. doi: 10.3892/ol.2014.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. A novel androstenedione derivative induces ROS-mediated autophagy and attenuates drug resistance in osteosarcoma by inhibiting macrophage migration inhibitory factor (MIF) Cell Death Dis. 2014;5:e1361. doi: 10.1038/cddis.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A, Bacchini P, Bertoni F, Olvi LG, Santini-Araujo E, Kim YW, Park YK. Expression of heat shock proteins in osteosarcomas. Pathology. 2010;42:421–425. doi: 10.3109/00313025.2010.493866. [DOI] [PubMed] [Google Scholar]
- Pocaly M, et al. Overexpression of the heat-shock protein 70 is associated to imatinib resistance in chronic myeloid leukemia. Leukemia. 2007;21:93–101. doi: 10.1038/sj.leu.2404463. [DOI] [PubMed] [Google Scholar]
- Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Seki K, Yoshikawa H, Shiiki K, Hamada Y, Akamatsu N, Tasaka K. Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcoma. Cancer Chemother Pharmacol. 2000;45:199–206. doi: 10.1007/s002800050030. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tsuchiya H, Tajino T, Kikuchi S. An experimental study on the therapeutic effect of consecutive low-dose cisplatin with caffeine in sarcoma-bearing mice. Anticancer Res. 2005;25:2777–2784. [PubMed] [Google Scholar]
- Terauchi R, et al. Hsp70 prevents nitric oxide-induced apoptosis in articular chondrocytes. Arthritis Rheum. 2003;48:1562–1568. doi: 10.1002/art.11040. [DOI] [PubMed] [Google Scholar]
- Uozaki H, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–673. doi: 10.1016/S0344-0338(00)80118-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tsuchiya H. Chemotherapy for osteosarcoma—where does it come from? What is it? Where is it going? Expert Opin Pharmacother. 2013;14:2183–2193. doi: 10.1517/14656566.2013.827171. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang J, Zhou Y, Wang Y, Wang S, Zhang W. Hsp70 promotes chemoresistance by blocking Bax mitochondrial translocation in ovarian cancer cells. Cancer Lett. 2012;321:137–143. doi: 10.1016/j.canlet.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huang L, Zhang J, Moskophidis D, Mivechi NF. Targeted disruption of hsf1 leads to lack of thermotolerance and defines tissue-specific regulation for stress-inducible Hsp molecular chaperones. J Cell Biochem. 2002;86:376–393. doi: 10.1002/jcb.10232. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao Z, Xiong L, Yang S. Inhibition of autophagy enhances cisplatin-induced apoptosis in the MG63 human osteosarcoma cell line. Oncol Lett. 2015;10:2941–2946. doi: 10.3892/ol.2015.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Jiang BO, Wang D, Liu W, Zhang H, Liu W, Qiu Z. Triptolide reduces the viability of osteosarcoma cells by reducing MKP-1 and Hsp70 expression. Experimental and therapeutic medicine. 2016;11:2005–2010. doi: 10.3892/etm.2016.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]