Abstract
Heat shock protein 70 (Hsp70) is a molecular chaperone that plays an important role in cellular proteostasis. Hsp70s are also implicated in the survival and pathogenicity of malaria parasites. The main agent of malaria, Plasmodium falciparum, expresses six Hsp70s. Of these, two (PfHsp70-1 and PfHsp70-z) localize to the parasite cytosol. Previously conducted gene knockout studies suggested that PfHsp70-z is essential, and it has been demonstrated that small-molecule inhibitors targeting PfHsp70-1 cause parasite death. For this reason, both PfHsp70-1 and PfHsp70-z are potential antimalarial targets. Two cyclic lipopeptides, colistin and polymyxin B (PMB), have been shown to bind another heat shock protein, Hsp90, inhibiting its chaperone function. In the current study, we investigated the effect of PMB on the structure–function features of PfHsp70-1 and PfHsp70-z. Using surface plasmon resonance analysis, we observed that PMB directly interacts with both PfHsp70-1 and PfHsp70-z. In addition, using circular dichroism spectrometric analysis combined with tryptophan fluorescence measurements, we observed that PMB modulated the secondary and tertiary structures of Hsp70. Furthermore, PMB inhibited the basal ATPase activity and chaperone function of the two Hsp70s. Our findings suggest that PMB associates with Hsp70 to inhibit its function. In light of the central role of Hsp70 in cellular proteostasis and its essential role in the development of malaria parasites in particular, our findings expand the library of small-molecule inhibitors that target this medically important class of molecular chaperones.
Keywords: Heat shock protein, Polymyxin B, Chaperone, Aggregation, Heat stress, Inhibitor
Background
Heat shock proteins (Hsps) function as molecular chaperones (molecules that facilitate protein folding) (Hartl 1991). In light of their central role in proteostasis, Hsps have been proposed as potential drug targets against infectious diseases, among them, malaria (Shonhai 2010). Hsp70 members constitute one of the most distinct molecular chaperone families. They are generally made up of proteins with an average size of 70 kDa. Hsp70s are constituted by a nucleotide binding (ATPase) domain which is situated on the N-terminal segment of the protein and a C-terminally located substrate binding domain (SBD). Hsp70s exhibit low basal ATPase activity. Hsp40 proteins serve as co-chaperones of Hsp70. In this respect, their roles include stimulating the ATPase activity of Hsp70 as well as recruiting peptide substrates for Hsp70 to refold. Hsp40 co-chaperones are endowed with a conserved J domain which facilitates interaction with Hsp70, leading to stimulation of the ATPase activity of the latter (Hennessy et al. 2005). ATP-bound Hsp70 exhibits low affinity for substrate, leading to substrate release. On the other hand, ADP-bound Hsp70 exhibits high affinity for substrate (Kampinga and Craig 2010). Consequently, the nucleotide status of Hsp70 regulates substrate binding and release events.
The main agent of malaria, Plasmodium falciparum, expresses six members of the Hsp70 family (Shonhai 2014). Parasite Hsp70s are ubiquitously expressed proteins, and some of them have been proposed as potential antimalarial drug targets (Shonhai et al. 2007; Zininga and Shonhai 2014; Muralidharan et al. 2012). Two cytosol localized Hsp70s (PfHsp70-1 and PfHsp70-z) from P. falciparum, the main agent of malaria, have been previously described (Shonhai et al. 2008; Zininga et al. 2015a, b; 2016). PfHsp70-1 belongs to the category of canonical Hsp70s as it is closely related to the Escherichia coli Hsp70 homolog. On the other hand, PfHsp70-z belongs to the Hsp110 subgroup of Hsp70 proteins. The Hsp110 group exhibits independent chaperone activity and also serves as nucleotide exchange factors for canonical Hsp70s (Dragovic et al. 2006). For this reason, PfHsp70-z is thought to serve both as an independent molecular chaperone as well as a potential nucleotide exchange factor of PfHsp70-1 (Zininga et al. 2016). We previously demonstrated that PfHsp70-1 and PfHsp70-z interact in a nucleotide-dependent fashion (Zininga et al. 2016). The two proteins are thought to cooperate in facilitating protein folding in the malaria parasite. Protein quality control is important for the survival of malaria parasites under the physiologically divergent life stages that they encounter during their development. PfHsp70-z and PfHsp70-1 are particularly thought to play an important role at the blood stage of P. falciparum and are also implicated in the development of malaria pathology (Pallavi et al. 2010; Shonhai et al. 2011).
Hsp90 constitutes another group of molecular chaperones (Prodromou et al. 1997). Hsp70 and Hsp90 cooperate to facilitate the folding of proteins such as kinases and steroid hormone receptors (Jackson 2013). Hsp70 and Hsp90 are known to associate in order to facilitate substrate exchange via an adapter protein known as Hsp70–Hsp90 organizing protein (Hop; Lässle et al. 1997). We previously demonstrated that PfHsp70-1 and P. falciparum Hsp90 (PfHsp90) similarly interact through a Hop (PfHop) mediated partnership (Gitau et al. 2012; Zininga et al. 2015b). PfHsp70-1 is thought to form functional networks with several chaperones and co-chaperone partners. For this reason, the possible targeting of this protein by inhibitors would impact on a myriad of downstream pathways in which it is implicated (Shonhai 2010).
Polymyxin B (PMB) is a cyclic lipopeptide that comprises a polycationic peptide ring and a fatty acid 6-methyloctanoic acid. It is a potent inhibitor of Gram-negative bacteria as it binds to lipopolysaccharides (LPS) embedded in the outer membranes of the bacteria. Thus, PMB complexes with LPS to facilitate bacterial cell lysis (Hermsen et al. 2003). This leads to indiscriminate entry of a variety of compounds, among them, small peptides, including PMB itself into the cells (Hancock 1997). PMB is thus an effective antibiotic and is regarded as a potential tool for reversing the growing threat of multi-drug resistance (Zavascki et al. 2007).
It has been proposed that PMB and other cyclic lipopeptide-based antibiotics physically interact with Hsp90 to inhibit its chaperone function (Minagawa et al. 2012). However, the effect of PMB on the function of Hsp70 remains unknown. It has previously been proposed that Hsp70 binds to lipids during induction of liposome aggregation (Arispe et al. 2002). We therefore proposed that PMB may potentially bind and inhibit Hsp70 function. Our study investigated the effect of PMB on the structural and functional features of both PfHsp70-1 and PfHsp70-z. Data from this study demonstrate that PMB directly interacts with the both Hsp70 chaperones, inhibiting their function. Furthermore, our findings established that PMB abrogates the interaction of PfHsp70-1 with its partner proteins, PfHsp70-z and PfHop. We discuss the implications of our findings and the prospects of PMB as an inhibitor of Hsp70 in infectious diseases and other disease models.
Materials and methods
Materials
Unless otherwise specified, chemical reagents used in this study were purchased from Merck Chemicals (Darmstadt, Germany), Melford (Suffolk, UK), and Sigma-Aldrich (USA). Polymyxin B was purchased from Sigma-Aldrich (USA).
Expression and purification of recombinant proteins
A construct expressing PfHsp70-1 (pQE30/PfHsp70-1; Matambo et al. 2004) and PfHsp70-z (pQE30/PfHsp70-z; Zininga et al. 2015a) were used for the expression of recombinant PfHsp70-1 and PfHsp70-z. The proteins were expressed in E. coli XL1 Blue and JM109 cells, respectively, following a previously described method (Shonhai et al. 2008; Zininga et al. 2015a). The nucleotide-binding/ATPase domain of PfHsp70-1 (PfHsp70-1NBD) was expressed as previously described (Zininga et al. 2015b). The recombinant proteins were purified using affinity chromatography as previously described (Zininga et al. 2015a, b).
Determination of the binding affinities of polymyxin B for PfHsp70-1 and PfHsp70-z
The binding affinities of PMB for the P. falciparum Hsp70s were determined using a Bio-Rad ProteOn XPR36 system as previously described (Zininga et al. 2016). Briefly, PfHsp70-1, PfHsp70-1NBD, and PfHsp70-z (as ligands) were immobilized on the HTE chip at concentrations of 0.5 and 1 μg/ml, respectively. At these concentrations, we achieved 191 response units (RU) for PfHsp70-1, 193 RU for PfHsp70-z, and 198 RU for PfHsp70-1NBD per immobilization surface. As analytes, aliquots of PMB were prepared at final concentrations of 125, 250, 500, 1000, and 2000 nM, respectively, which were injected at 100 μl/min in each horizontal channel. Association was allowed for 2 min and dissociation was monitored for 8 min. Data collected were double referenced using a buffer blank (buffer without protein). A channel in which BSA was immobilized in place of the chaperones served a negative control. Steady-state equilibrium constant data was processed and analyzed using Bio-Rad ProteOn Manager version 3.1.0.6 and GE Healthcare Biosciences BIA evaluation version 4.1.1 software.
Analysis of the effect of PMB on the secondary and tertiary structure of PfHsp70-1 and PfHsp70-z
The secondary structure of the recombinant proteins was monitored using the Far-UV J-1500 CD spectrometer (JASCO Ltd., UK) as previously described (Zininga et al. 2015a). To determine the effects of PMB on the secondary structural orientation of the proteins, the assay was repeated in the presence of varying amounts (0.1–25 μM) of PMB, and the data were processed as a curve representing the general folded fraction of each protein as previously described (Zininga et al. 2015a).
In addition, the tertiary structural changes of the recombinant P. falciparum Hsp70s were investigated using both tryptophan and tyrosine fluorescence as previously described (Zininga et al. 2015a). Briefly, tyrosine and tryptophan fluorescence was monitored after initial excitation at 285 nm wavelength. The fluorescence spectrum was monitored between 300 and 400 nm using a JASCO FP-6300 spectrofluorometer. Several replicate reaction mixes were prepared and each preparation was scanned only once in order to avoid photobleaching. As controls, buffer containing PMB but lacking protein was used as the blank. As both proteins have been shown to be denatured by urea treatment before (Zininga et al. 2015a), the assays were repeated in its presence as a positive control.
Investigation of the effect of polymyxin B on the ATPase function of PfHsp70-1 and PfHsp70-z
The basal ATPase activities of PfHsp70-1 and PfHsp70-z were assessed by conducting a colorimetric method as previously described (Zininga et al. 2015a). In order to determine the effect of PMB on the ATPase activity of parasite Hsp70s, the assay was repeated in the presence of variable levels (0.1–25 μM) of PMB. As control, the respective boiled Hsp70 protein was used to cater for the spontaneous hydrolysis of ATP.
Assessment of the effect of polymyxin B on the chaperone function of P. falciparum Hsp70s
The chaperone function of P. falciparum Hsp70s was investigated by monitoring the heat-induced aggregation of a model protein, malate dehydrogenase (MDH), from porcine heart (Sigma-Aldrich) as previously described (Shonhai et al. 2008; Luthuli et al. 2013; Makumire et al. 2014; Zininga et al. 2016) with minor modifications. Briefly, the reaction was initiated by adding 0.25 μM MDH and 0.2 μM recombinant PfHsp70-1/PfHsp70-z in assay buffer TBS (50 mM Tris–HCl, pH 7.5, 100 mM NaCl), and the reaction mix was incubated at 48 °C for 1 h. In order to determine the effects of PMB on the chaperone function of parasite Hsp70, the assay was repeated in the presence of 12.5 μM PMB. We chose this concentration of PMB as it achieves saturation with respect to binding to the protein based on the kinetics data we generated. In addition, at this level (12.5 μM), PMB did not promote aggregation of PfHsp70-1/PfHsp70-z at the assay temperature (48 °C) we employed. After incubation, the samples were centrifuged for 10 min at 14,000×g to separate the supernatant and pellet fractions. The fractions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently quantified using densitometric analysis using the Bio-Rad ImageLab version 5.1 build 8 and ImageJ 1.47v software.
Analysis of the effect of polymyxin B on the association of PfHsp70-1 with PfHsp70-z and PfHop, respectively
The effect of PMB on the interaction of PfHsp70-1 with its functional partners, PfHsp70-z and PfHop, was investigated using the Bio-Rad ProteOn XPR36 surface plasmon resonance (SPR) system as previously described (Zininga et al. 2015a, b; 2016). Upon immobilizing the various proteins as ligands on a GLC sensor chip, the following response units (RU) were attained: PfHsp70-1, 178 RU; PfHsp70-1NBD, 188 RU; PfHsp70-z, 190 RU; and PfHop, 197 RU, respectively. The aliquots of PfHsp70-1, PfHsp70-1NBD, PfHsp70-z, and PfHop were prepared at 125, 250, 500, 1000, and 2000 nM and injected at 50 μl/min on each horizontal surface. To determine the effects of PMB on the interactions, the assays were repeated in the presence of nucleotide (5 mM ATP/ADP) and 12.5 μM PMB. The SPR data was analyzed as previously described (Zininga et al. 2015a; 2016).
Results
Polymyxin B binds to both PfHsp70-1 and PfHsp70-z
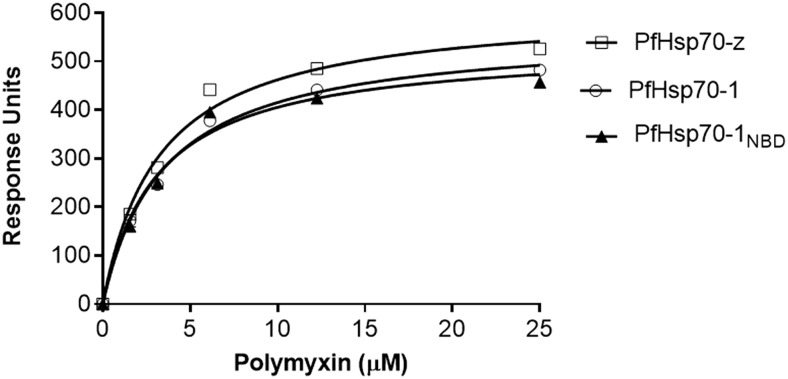
To confirm that PMB directly binds to the recombinant P. falciparum Hsp70s, we conducted equilibrium analysis using SPR. PMB interacted with the two Hsp70s (Fig. 1). The equilibrium binding affinities generated suggest that PMB binds to both PfHsp70-1 and PfHsp70-z in sub-micromolar ranges, suggesting high affinity interaction. In addition, data for PMB binding kinetics for either PfHsp70-1NBD or PfHsp70-1 were not statistically different (p > 0.05) (Table 1). This suggests that the nucleotide binding domain of PfHsp70-1 constitutes the minimum structural requirement for PMB binding. Furthermore, this reflects that in the absence of bound substrate, the SBD of PfHsp70-1 has little influence on the equilibrium PMB binding kinetics (Table 1). Altogether, PfHsp70-1 binds PMB with much higher affinity than PfHsp70-z (Table 1).
Fig. 1.
Polymyxin B is capable of binding both PfHsp70-1 and PfHsp70-z. The equilibrium analysis of the SPR data representing interaction of PfHsp70-1/PfHsp70-1NBD/PfHsp70-z and PMB are presented
Table 1.
Comparative affinities for PMB binding to PfHsp70-z and PfHsp70-1
| Protein | K D (μM) [± standard deviation] |
|---|---|
| PfHsp70-1 | 0.38 [± 0.07] |
| PfHsp70-1NBD | 0.29 [± 0.09] |
| PfHsp70-z | 0.94 [± 0.03] |
The standard deviations shown in parentheses were obtained from at least three independent assays
Binding of polymyxin B by PfHsp70-1 and PfHsp70-z is associated with conformational changes
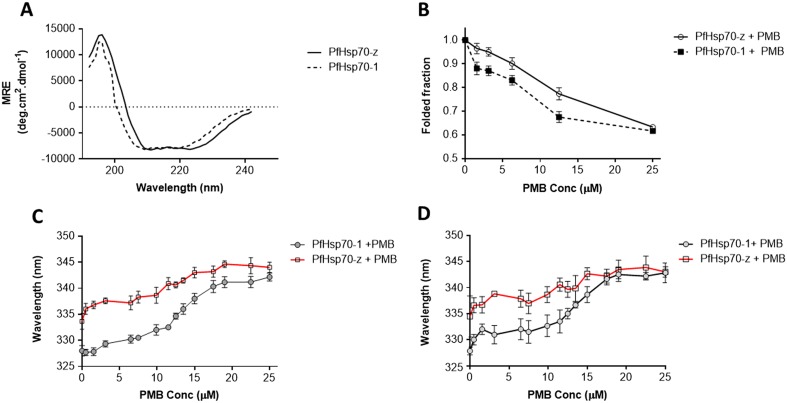
Analysis of the secondary and tertiary structures of the Hsp70s was conducted using CD and fluorescence spectroscopy (Fig. 2). In the absence of PMB, PfHsp70-1 and PfHsp70-z generally exhibited fairly similar folds with minor variations on the subdomain compositions as previously observed (Zininga et al. 2016; Fig. 2a). Analysis of the effect of PMB on the secondary structure of the Hsp70s was conducted by exposing the respective protein to various amounts of PMB and monitoring the changes in ellipticity of folded fraction at 222 nm (Fig. 2b). The changes in ellipticity represented loss of folded fraction and hence served as a measure of denaturation of the respective protein. Generally, the CD data suggest that PfHsp70-z was more resilient to structural perturbation by PMB binding in comparison to PfHsp70-1 (Fig. 2b). This is in agreement with our previous findings that PfHsp70-z is more stable to heat stress than PfHsp70-1 (Zininga et al. 2015b). However, at the highest concentration of PMB used (25 μM), both proteins had lost approximately 35% of their folded fractions.
Fig. 2.
Polymyxin B interacts with Hsp70 to induce conformational changes. Analysis of the stability of PfHsp70-1 and PfHsp70-z in the presence of PMB. a CD spectrum representing the secondary structural fold of PfHsp70-1 and PfHsp70-z; b comparative folded fractions of PfHsp70-1 and PfHsp70-z in response to 25 μM PMB exposure; the respective red shifts for c tryptophan fluorescence and d tyrosine/tryptophan fluorescence are shown
The tertiary conformational changes were monitored by fluorescence of tryptophan and tyrosine residues as both proteins possess tryptophan and tyrosine residues. A red shift representing protein denaturation was observed when PMB was introduced (Fig. 2c). The same phenomenon was also reflected by the tyrosine and tryptophan fluorescence data (Fig. 2d). Consistent with our previous observations, PMB causes structural changes to both PfHsp70-1 and PfHsp70-z synonymous to the effect of heat and urea (Zininga et al. 2015b).
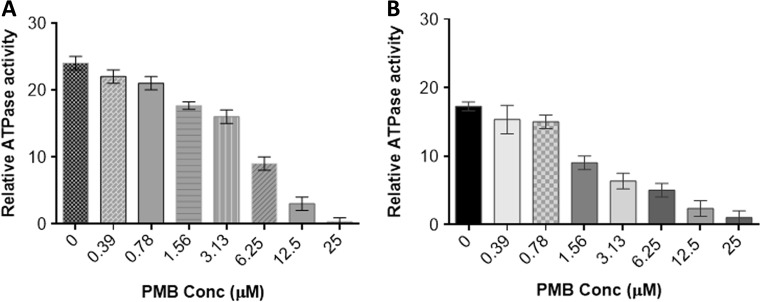
Polymyxin B inhibits the ATPase activities of PfHsp70-1 and PfHsp70-z
We previously observed that PfHsp70-z possesses slightly higher affinity for ATP than PfHsp70-1 (Zininga et al. 2015a). However, in the same study, we noted that the two proteins exhibit basal ATPase activities within the same range of magnitude. In the current study, the basal ATPase activities of both Hsp70s were determined under saturating ATP levels (5 mM) while the concentration of PMB was varied up to a maximum of 25 μM (Fig. 3). We noted that the rate of ATPase activity decreased with increase in PMB concentration (Fig. 3a, b), suggesting that PMB specifically interfered with the ATPase function of both PfHsp70-1 and PfHsp70-z. Since our current findings showed that the minimum structural component of Hsp70 that PMB binds to is the N-terminal ATPase domain, it is plausible that PMB binds to the same cleft that ATP binds to.
Fig. 3.
Polymyxin B inhibits the intrinsic ATPase activity of Hsp70. The effect of PMB on the basal ATPase activities of a PfHsp70-1 and b PfHsp70-z was investigated. Released Pi was monitored at 595 nm using a direct colorimetric assay
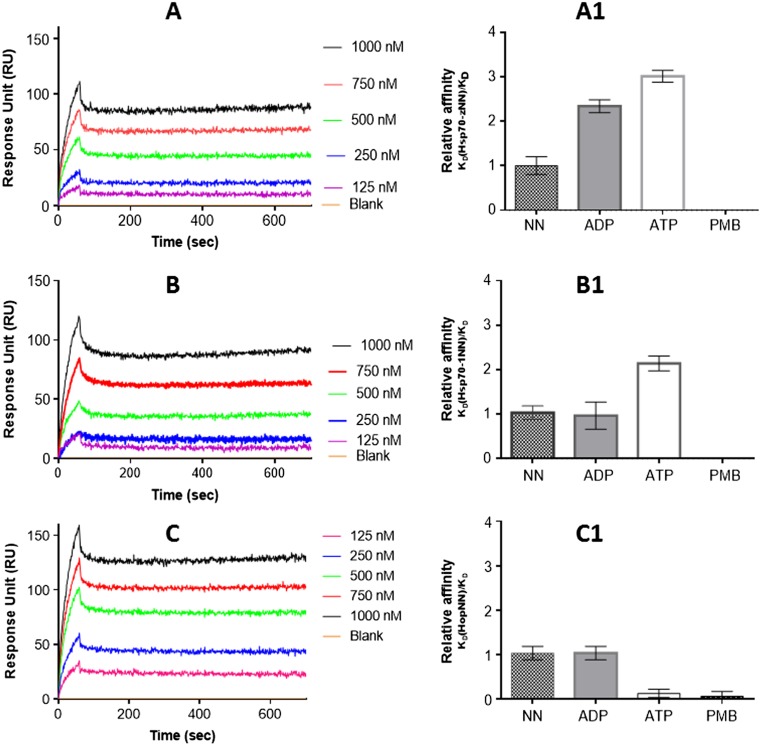
Polymyxin B inhibits the on and off rates of PfHsp70-1 with its functional associates
The effect of PMB on the interaction of PfHsp70-1 with PfHsp70-z (Zininga et al. 2016) was investigated using SPR analysis. Our previous studies demonstrated that PfHsp70-1 associates with PfHsp70-z both in the presence and absence of nucleotide (Zininga et al. 2016). However, ATP was the most effective in promoting the association of PfHsp70-1 with PfHsp70-z (Zininga et al. 2016; Table 2). For this reason, we investigated the effect of PMB on the association of the protein pairs in the absence and presence of nucleotides. As expected, ATP promoted the interaction of PfHsp70-z with both full-length PfHsp70-1 (Fig. 4a; Table 2) and its nucleotide-binding domain (PfHsp70-1NBD) as previously observed (Zininga et al. 2016; Fig. 4b). However, addition of PMB to the reaction mix resulted in suppression of the interaction between PfHsp70-z and PfHsp70-1/PfHsp70-1NBD (Fig. 4a1, b1).
Table 2.
Relative affinities for association of PfHsp70-1 with its interactors in the presence of nucleotide and/or PMB
| Analyte, ligand | Nucleotide | K D (M) | χ 2 | Reference |
|---|---|---|---|---|
| PfHsp70-1, PfHsp70-z | ATP | 2.41 (± 0.2) e−08 | 1.86 | Zininga et al. 2016 |
| ADP | 1.21 (± 0.1) e−06 | 2.12 | Zininga et al. 2016 | |
| NN | 5.98 (± 0.5) e−05 | 3.32 | Zininga et al. 2016 | |
| PMB | 2.50 (± 0.8) e−04* | 2.65 | This study | |
| PfHsp70-1NBD, PfHsp70-z | ATP | 2.12 (± 0.2) e−09 | 2.65 | Zininga et al. 2016 |
| ADP | 9.86 (± 0.9) e−08 | 6.52 | Zininga et al. 2016 | |
| NN | 1.57 (± 0.6) e−08 | 2.44 | Zininga et al. 2016 | |
| PMB | 8.41 (± 0.7) e−05** | 4.72 | This study | |
| PfHsp70-1, PfHop | ATP | 1.13 (± 0.5) e−08 | 2.15 | Zininga et al. 2015b |
| ADP | 1.72 (± 0.5) e−09 | 2.30 | Zininga et al. 2015b | |
| NN | 1.91 (± 0.01) e−09 | 2.17 | Zininga et al. 2015b | |
| PMB | 1.60 (± 0.08) e−06** | 1.22 | This study |
The interaction kinetics represented by the equilibrium constant (K D) were determined by SPR analysis alternating the status of PfHsp70-z/PfHop and PfHsp70-1/PfHsp70-1NBD as ligand and analyte, respectively. The ligand was the respective immobilized protein on the GLC chip surface and the analyte was the respective protein injected at a flow rate of 50 μl/min. Data were analyzed by using readings obtained for the running buffer (in the presence of nucleotides, but in the absence of protein analyte) as baseline (Fig. 4). Data are represented as mean plus/minus standard deviation. Chi-square (χ 2) values represent the Langmuir curve fitting residuals. Statistical analysis was conducted using one-way ANOVA. *p < 0.005, **p < 0.001 represents statistically significant differences in affinities noted under the variable experimental conditions
Fig. 4.
PMB abrogates the interaction of PfHsp70-1 with both PfHsp70-z and PfHop. Shown are the representative SPR sensorgrams representing interaction of PfHsp70-1 with PfHsp70-z (a), PfHsp70-1NBD with PfHsp70-z (b), and PfHsp70-1 with PfHop (c). The relative affinities for the interaction of the following protein pairs are shown: PfHsp70-1-PfHsp70-z (a1); PfHsp70-1 NBD-PfHsp70-z (b1); PfHsp70-1-PfHop (c1). The assays were conducted under the following conditions: absence of nucleotides (NN); in the presence of nucleotides (ATP/ADP) or PMB, respectively
PfHsp70-1 is known to associate with its co-chaperone partner, PfHop (Gitau et al. 2012; Zininga et al. 2015b). Thus, we further investigated the effect of PMB on this association. As previously observed (Zininga et al. 2015a), the association of PfHsp70-1 and PfHop occurred in the absence and in the presence of ADP/ATP (Fig. 4c). Altogether, ADP promotes the association most effectively (Zininga et al. 2016; Fig. 4c1). However, the relative affinities of the association of PfHsp70-1 and PfHop in the absence and presence of ADP/ATP were generally reduced in the presence of PMB (Fig. 4c). This suggests that PMB interferes with the association of PfHsp70-1 with PfHop.
Polymyxin B suppresses the chaperone activities of both PfHsp70-1 and PfHsp70-z in vitro
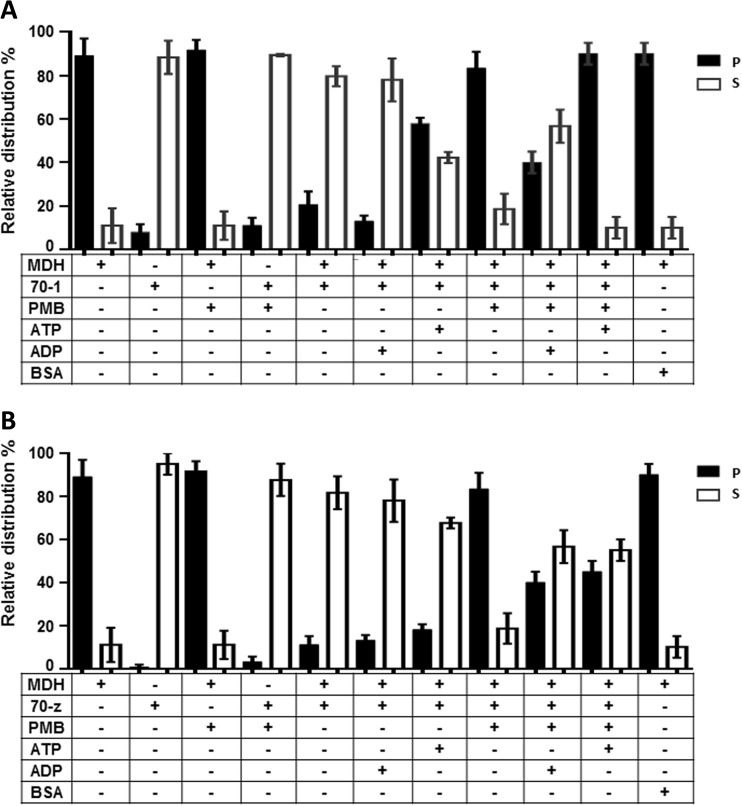
Previous studies have shown that both PfHsp70-1 and PfHsp70-z are heat stable, although PfHsp70-z was more resilient to stress than PfHsp70-1 (Shonhai et al. 2008; Luthuli et al. 2013; Zininga et al. 2016). In addition, both proteins have been shown to be capable of suppressing the heat-induced aggregation of model proteins, such as MDH (Shonhai et al. 2008; Luthuli et al. 2013; Zininga et al. 2016). In the current study, MDH was subjected to heat stress at 48 °C and the protein aggregated in the absence of the chaperones as expected (Fig. 5a, b). In the presence of a non-chaperone (BSA), MDH aggregated in response to heat stress. However, the introduction of either PfHsp70-1 or PfHsp70-z led to the suppression of the heat-induced aggregation of MDH. The introduction of ADP to the reaction of either PfHsp70-1 or PfHsp70-z did not alter the chaperone activity of the respective proteins (Fig. 5a, b; Zininga et al. 2016). On the other hand, as previously reported, the addition of ATP, while not affecting the activity of PfHsp70-z, did inhibit the activity of PfHsp70-1 (Fig. 5a, b; Shonhai et al. 2008; Zininga et al. 2016). Next, we investigated the effect of PMB on the chaperone activity of the two proteins. When the chaperones were excluded from the reaction mixes, MDH fully aggregated both in the absence and presence of 12.5 μM of PMB (Fig. 5). However, 12.5 μM of PMB did not influence the solubility profiles of either PfHsp70-1 or PfHsp70-z at 48 °C. Only MDH aggregated upon being exposed to heat stress at the same level (12.5 μM) of PMB. In the presence of PMB, the chaperone activities of both chaperones were abrogated. The effect of PMB on the chaperone activity of PfHsp70-1 was similar to the effect of ATP as the nucleotide is known to suppress the chaperone activity of PfHsp70-1 (Shonhai et al. 2008). It should be noted that although PMB inhibited the chaperone activity of PfHsp70-1/PfHsp70-z, the addition of ADP to the reaction slightly rescued the aggregation of MDH.
Fig. 5.
Polymyxin B suppresses chaperone function of PfHsp70-1and PfHsp70-z. The chaperone function of PfHsp70-1/PfHsp70-z was conducted by monitoring the heat-induced aggregation of MDH in vitro at 48 °C and quantitating the pellet (P) and soluble (S) fractions after heat exposure. The chaperone capabilities of PfHsp70-1 (a) and PfHsp70-z (b) were assessed by assessing the heat-induced aggregation of MDH in the presence of nucleotides and PMB, respectively. The activity of PfHsp70-1 and PfHsp70-z in the presence of PMB compared to the nucleotide-free state and ADP-bound state was statistically significant (p < 0.005), respectively. Standard deviations obtained from three replicate assays are shown
Discussion
PMB is a well-established antibiotic which has been previously proposed as a viable choice against the growing threat of multi-drug-resistant Gram-negative bacteria (Zavascki et al. 2007). While it is known that PMB primarily binds to bacterial LPS, thus effecting death, its mechanism of action is not fully understood. To the best of our knowledge, this is the first study to demonstrate that polymyxin B binds to Hsp70, inhibiting its chaperone function. The molecular chaperone Hsp70 plays a central role in cellular proteostasis. For this reason, Hsp70 is implicated in the survival and pathogenicity of parasites, among them, the main agent of malaria, P. falciparum (Shonhai et al. 2007; 2008). Our study established that PMB is capable of making direct contact with two cytosol isoforms of Hsp70 (PfHsp70-1 and PfHsp70-z) from P. falciparum, thereby inhibiting their function in vitro.
The molecular chaperone Hsp70 is known to interact with lipids (Arispe et al. 2002). Since PMB is a cyclic lipopeptide, we hypothesized that it would potentially associate with Hsp70. Using SPR-based assays, we established that PMB binds primarily to the ATPase domain of Hsp70 (Fig. 1). For this reason, we speculated that PMB may compete with ATP to bind the nucleotide-binding domain of Hsp70. This was further validated by our findings that PMB inhibits the ATPase activities of both proteins (Fig. 3). We also established that PMB has higher affinity for PfHsp70-1 compared to PfHsp70-z. We previously established that ATP binds to PfHsp70-1 to inhibit its chaperone function, while on the other hand the chaperone activity of PfHsp70-z is nucleotide independent. This further suggests that the two molecular chaperones are uniquely modulated by ligands and co-chaperones that regulate their function. We further established that both PfHsp70-1 and PfHsp70-z exhibited folds that mirror conformations that they assume when subjected to either heat stress or urea treatment (Zininga et al. 2015a). This indicates that PMB binds Hsp70 to destabilize its conformation. However, the effect of PMB was more enhanced on PfHsp70-1 than it was on PfHsp70-z. Incidentally, we previously established that of the two chaperones, PfHsp70-z was more resilient to heat stress and urea denaturation than PfHsp70-1 (Zininga et al. 2016).
Hsp70 is perceived as constituting a hub of the cell’s protein folding system. This is largely because of the central position that it holds through its association with several chaperones and co-chaperone partners. In the current study, we investigated the effect of PMB on the direct association of PfHsp70-1 with PfHsp70-z. We demonstrated that PMB abrogates the interaction of PfHsp70-1 with both PfHsp70-z and PfHop (Fig. 4). Thus, PMB is not only important through its direct role in inhibiting Hsp70 but may have adverse effects on its downstream network partners. Interestingly, a previous independent study proposed that PMB interacts and inhibits another chaperone, Hsp90 (Minagawa et al. 2012). Hsp70 and Hs90 cooperate to facilitate fold of a special group of proteins such as kinases and steroid hormone receptors (Jackson 2013). The association of the two chaperones is mediated by Hop which serves as a module (Lässle et al. 1997). As the current study has established that PMB inhibits Hsp70 directly and also abrogates its association with Hop, the data suggest that PMB may be a versatile inhibitor of the molecular chaperone system of the cell.
We further established that PMB inhibits the chaperone function of both PfHsp70-1 and PfHsp70-z in vitro (Fig. 5). Whereas PfHsp70-z exhibits nucleotide-independent chaperone activity (suppression of heat-induced aggregation of protein), the chaperone activity of PfHsp70-1 is inhibited by ATP (Shonhai et al. 2008; Zininga et al. 2016). Our current data further suggest that PMB inhibits the chaperone activity of Hsp70. However, only ADP was capable of slightly reversing the effect of PMB on MDH aggregation, while ATP has the same effect as PMB.
Altogether, our findings confirm that PMB binds to Hsp70. The findings further demonstrated that PMB inhibits the chaperone function of Hsp70. Furthermore, PMB disrupts the association of Hsp70 with its functional partners. Thus, the effect of PMB has broader downstream effects on the function of Hsp70. For this reason, it is important to further investigate if PMB is capable of selectively inhibiting parasite Hsp70 without adversely affecting the host chaperone system. In any event, the findings from this study broadens the horizon in the search for small molecule inhibitors of Hsp70 towards targeting its function in parasite survival.
Acknowledgements
This project was supported through a grant (L1/402/14-1) provided to A.S. by the Deutsche Forschungsgemeinschaft (DFG) under the theme, “German–African Cooperation Projects in Infectiology.” We are grateful to the Department of Science and Technology/National Research Foundation (NRF) of South Africa for providing an equipment grant (UID, 75464) and NRF mobility grant (UID, 92598) awarded to A.S., DST/NRF National Nanotechnology Equipment grant awarded to E.P., and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (grant 64788 to H.W.D.) which facilitated the study. T.Z. is a recipient of the Claude Leon fellowship and A.S. is a recipient of a Georg Foster research fellowship awarded by the Alexander von Humboldt Foundation, Germany.
Compliance with ethical standards
Conflict of interest
All the authors declare no conflicts of interest.
References
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7(4):330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 2006;25:2519–2528. doi: 10.1038/sj.emboj.7601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitau GW, Mandal P, Blatch GL, Przyborski J, Shonhai A (2012) Characterisation of the plasmodium falciparum Hsp70-Hsp90 organising protein (PfHop). Cell Stress Chaperones 17(2):191–202 [DOI] [PMC free article] [PubMed]
- Hancock RE (1997) Peptide antibiotics. Lancet 349:418–422 [DOI] [PubMed]
- Hartl FU. Heat shock proteins in protein folding and membrane translocation. Semin Immunol. 1991;3:5–16. [PubMed] [Google Scholar]
- Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Not all J domains are created equal: implications for the specificity of Hsp40–Hsp70 interactions. Protein Sci. 2005;14:1697–1709. doi: 10.1110/ps.051406805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsen ED, Sullivan CJ, Rotschafer JC. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect Dis Clin N Am. 2003;17:545–562. doi: 10.1016/S0891-5520(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Jackson SE. Hsp90: structure and function. Top Curr Chem. 2013;328:155–240. doi: 10.1007/128_2012_356. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Craig E. The Hsp70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579–592. doi: 10.1038/nrm2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lässle N, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1 characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- Luthuli SD, Chili MM, Revaprasadu N, Shonhai A. Cysteine-capped gold nanoparticles suppress aggregation of proteins exposed to heat stress. IUBMB Life. 2013;65:454–461. doi: 10.1002/iub.1146. [DOI] [PubMed] [Google Scholar]
- Makumire S, Chakravadhanula VSK, Köllisch G, Redel E, Shonhai A (2014) Immunomodulatory activity of Zinc peroxide (ZnO2) and titanium dioxide (TiO2) nanoparticles and their effects on DNA and protein integrity. Toxicol Lett 227(1):56–64 [DOI] [PubMed]
- Matambo TS, Odununga OO, Boshoff A, Blatch GL. Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Prot Expr Purif. 2004;33:214–222. doi: 10.1016/j.pep.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Minagawa S, Kondoh Y, Sueoka K, Osada H, Nakamoto H. Cyclic lipopeptide antibiotics bind to the N-terminal domain of the prokaryotic Hsp90 to inhibit the chaperone activity. Biochem J. 2012;435:237–246. doi: 10.1042/BJ20100743. [DOI] [PubMed] [Google Scholar]
- Muralidharan V, Oksman A, Pal P, Lindquist S, Goldberg DE. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat Commun. 2012;3:1310. doi: 10.1038/ncomms2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavi R, Archarya P, Chandran S, Daily JP, Tatu U. Chaperone expression profiles correlate with distinct physiological states of Plasmodium falciparum in malaria patients. Malar J. 2010;9:236. doi: 10.1186/1475-2875-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C, Roe SM, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/S0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Shonhai A. Plasmodial heat shock proteins: targets for chemotherapy. FEMS Immunol Med Microbiol. 2010;58:61–74. doi: 10.1111/j.1574-695X.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- Shonhai A. Role of Hsp70s in development and pathogenicity of plasmodium species. In: Shonhai A, Blatch G, editors. Heat shock proteins of malaria. New York: Springer; 2014. pp. 47–70. [Google Scholar]
- Shonhai A, Boshoff A, Blatch GL. The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. 2007;16:1803–1818. doi: 10.1110/ps.072918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonhai A, Botha M, de Beer TAP, Boshoff A, Blatch GL. Structure-function study of Plasmodium falciparum Hsp70 using three dimensional modelling and in-vitro analyses. Protein Pept Lett. 2008;15:1117–1125. doi: 10.2174/092986608786071067. [DOI] [PubMed] [Google Scholar]
- Shonhai A, Maier AG, Przyborski JM, Blatch GL. Intracellular protozoan parasites of humans: the role of molecular chaperones in development and pathogenesis. Protein Pept Lett. 2011;18:143–157. doi: 10.2174/092986611794475002. [DOI] [PubMed] [Google Scholar]
- Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- Zininga T, Shonhai A. Are heat shock proteins druggable candidates? Am J Biochem Biotechnol. 2014;10:211–213. doi: 10.3844/ajbbsp.2014.208.210. [DOI] [Google Scholar]
- Zininga T, Achilonu I, Hoppe H, Prinsloo E, Dirr HW, Shonhai A. Overexpression, purification and characterisation of the Plasmodium falciparum Hsp70-z (PfHsp70-z) protein. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zininga T, Makumire S, Gitau GW, Njunge JM, Pooe OJ, Klimek H, Scheurr R, Raifer H, Prinsloo E, Przyborski JM, Hoppe H, Shonhai A. Plasmodium falciparum hop (PfHop) interacts with the Hsp70 chaperone in a nucleotide-dependent fashion and exhibits ligand selectivity. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zininga T, Achilonu I, Hoppe H, Prinsloo E, Dirr HW, Shonhai A. Plasmodium falciparum Hsp70-z (Hsp110c) exhibits independent chaperone activity and interacts with Hsp70-1 in a nucleotide dependent fashion. Cell Stress Chaperones. 2016;21(3):499–513. doi: 10.1007/s12192-016-0678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]