Abstract
Chorioamnionitis is an inflammation in the fetal membranes or placenta. When chorioamnionitis develops, fetal lungs are exposed to inflammatory cytokines and mediators via amniotic fluid. Because inflammation plays a pivotal role in the development of bronchopulmonary dysplasia (BPD), a chronic lung disease of prematurity, fetal lung inflammation induced by chorioamnionitis has been considered to be one of the major pathogenetic factors for BPD. Although there have been a number of studies that demonstrated the relationship between chorioamnionitis and BPD, there are still controversies on this issue. The controversies on the relationship between chorioamnionitis and BPD arise from not-unified definitions of chorioamnionitis and BPD, different study populations, and the proportion of contribution between inflammation and infectious microorganisms. The publication bias also contributes to the controversies. Clinical trials targeting chorioamnionitis or microorganisms that cause chorioamnionitis will answer on the actual relationship between chorioamnionitis and BPD and provide a novel prophylactic strategy against BPD based on that relationship.
Keywords: Chorioamnionitis, Bronchopulmonary dysplasia, Ureaplasma
Introduction
Chorioamnionitis is a maternal inflammatory response that involves neutrophil infiltration in the fetal membranes or placenta with or without a fetal inflammatory response1). Chorioamnionitis has been known to be a major risk factor for preterm delivery and prematurity-associated morbidities2,3,4). Bronchopulmonary dysplasia (BPD) is one of the major morbidities of preterm infants and represents a chronic lung disease that develops exclusively inpreterm infants5). The association of chorioamnionitis and BPD has been reported in animal and human studies6,8,9). However, there are also not a few studies that reported conflicting results and the evidence of publication bias, which lead to continuing controversies on the relationship between chorioamnionitis and BPD9,10). In this review, the relationship between chorioamnionitis and BPD and the background for the controversy on this issue will be disussed. Because clinical chorioamnionitis can reflect other conditions than true inflammation in the fetal membranes, placenta, and fetal tissue, only histologic chorioamnionitis will be discussed in this review.
The effect of chorioamnionitis on the development of BPD
1. Animal studies
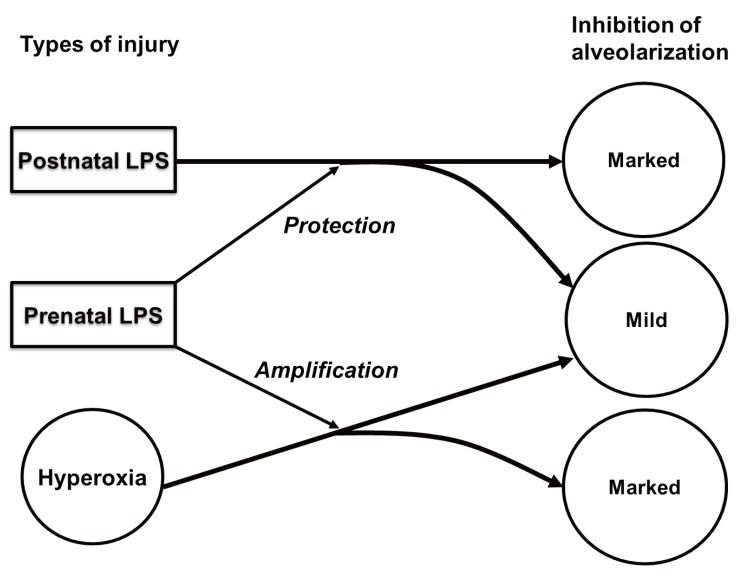
There have been a number of animal studies which demonstrated an association of chorioamnionitis and BPD11). The sheep and rodent models of BPD had been established and the pathogenetic role of chorioamnionitis on the development of BPD has been searched in these animal models12,13,14). The chorioamnionitis or intra-amniotic inflammation has been induced by intra-amniotic lipopolysaccharide (LPS) injection or direct Ureaplasma urealyticum inoculation into amniotic sacs. The experimental chorioamnionitis consistently resulted in fetal lung maturational responses which can be reflected by a reduction of the alveolar septal thickness, restructuring of the double capillary system to a single capillary system, or increased surfactant production15). However, these maturational responses were frequently followed by a disturbed lung development: fewer and larger distal airspaces resembling BPD of human preterm infants13). In a newborn rat model of BPD devised by the author, experimental chorioamnionitis induced by intra-amniotic LPS not only directly inhibited alveolarization but also amplified the inhibitory effect of hyperoxia on alveolarization14). However, in the same animal model, experimental chorioamnionitis protected the lungs against BPD triggered by postnatal systemic inflammation15), as shown in Fig. 1. In a preterm fetal sheep model, intra-amniotic Ureaplasma injection decreased elastic foci and increased smooth muscle around bronchioles and pulmonary arterioles which are distinctive pathologic findings seen in human BPD16,17,18). Taken together, these animal studies supports that chorioamnionitis accelerates lung maturation but eventually disrupt normal lung development resulting in BPD.
Fig. 1. In a newborn rat model of bronchopulmonary dysplasia devised by the author, experimental chorioamnionitis induced by intra-amniotic lipopolysaccharide (LPS) amplified the inhibitory effect of hyperoxia on alveolarization. On the contrary, marked inhibition of alveolarization induced by postnatal systemic lipopolysaccharide administration was protected by the experimental chorioamnionitis in the same animal model.
2. Human studies
Despite not a few studies supporting the role of chorioamnionitis on the development of BPD, controversies continue on this issue2,3,4,19,20). The chorioamnionitis has been known to protect against respiratory distress syndrome (RDS). The beneficial effect of chorioamnionitis on the incidence of RDS has been shown under the exposure to antenatal corticosteroid3,4,21). However, this beneficial effect of chorioamnionitis on RDS may render the premature lungs more vulnerable to various postnatal injuries6,7,22,23,24). The chorioamnionitis increased the risk of BPD with a synergism with patent ductus arteriosus and mechanical ventilation8,24). On the contrary, funisitis, a type of chorioamnionitis primarily involving umbilical cord, has been shown to protect against BPD in a study10). A recent meta-analysis by Hartling et al.25) which included more than 15,000 infants demonstrated that chorioamnionitis is significantly associated with the development of BPD. In a retrospective cohort study by the author, while all types of chorioamnionitis decreased RDS, high grade amnionitis increased BPD26). However, in other cohort studies, chorioamnionitis was not associated with the development of BPD10,27). As in animal model of BPD, chorioamnionitis increased the development of BPD by adversely affecting various postnatal injuries including RDS, mechanical ventilation, and sepsis in the clinical observations8,23,28). This can also be expressed as chorioamnionitis may make the preterm lungs to be more susceptible to postnatal injuries resulting in amplified inflammation and ultimately BPD29). However, there are a number of confounding factors that can be involved between chorioamnionitis and BPD. The diversity and uncertainty of diagnostic criteria of chorioamnionitis and BPD and inhomogeneity of the study populations also matter in investigating the relationship between chorioamnionitis and BPD. All these factors make the research for the role of chorioamnionitis in the development of BPD challenging.
Background for the controversies on the role of chorioamnionitis in the development of BPD
1. Definition of chorioamnionitis
The diversity and uncertainty of the definition of chorioamnionitis may partly responsible for the controversies on the role of chorioamnionitis in the development of BPD. A recent systemic review of 59 studies reported that the definition of chorioamnionitis varied across the studies25). Different definitions of chorioamnionitis may confound the actual effect of chorioamnionitis on BPD. Furthermore, because chorioamnionitis can vary in location and extent, it is too insufficient to identify the relationship between chorioamnionitis and BPD, just to see if there is a chorioamnionitis or not. In this regard, the author investigated the relationship between chorioamnionitis on BPD using the definition of chorioamnionitis that reflects the location and extent of the inflammation26). In this study, the chorioamnionitis was divided into four categories according to the location of inflammation: any chorioamnionitis; amnionitis; funisitis; amnionitis plus funisitis. Each category was further divided into high and low grades according to the extent of inflammation. The results of the study revealed that high grade amnionitis increases BPD. Because amnion lines the amniotic cavity, high grade amnionitis may indicate strong chemotactic stimuli within the amiotic cavity that will consequently results in fetal pulmonary inflammation and fetal lung damage. In this regard, the relationship between high grade amnionitis and BPD may be biologically plausible. Large scale cohort study that uses unified but subdivided criteria of chorioamnionitis will be needed to clarify the actual relationship between chorioamnionitis and BPD.
2. Variations in the definition of BPD
It is important to have clear definition of BPD to compare outcomes in clinical trials or quality improvement and to identify risk factors in epidemiological studies. Which definition of BPD is used will also affect the result of the investigation for the relationship between chorioamnionitis and BPD. The requirement of supplemental oxygen has been used as a surrogate for pulmonary dysfunction seen in BPD. The duration of oxygen dependency and the extent of supplemental oxygen needed to maintain adequate arterial blood oxygen saturation have been used to define BPD and its severity. However, there has been a lack of uniformity in the definition of BPD among clinical trials and epidemiological studies on BPD30). Moreover, the indications for oxygen supplementation vary from center to center and from person to person in a center. Among the definitions of BPD, the need for supplemental oxygen at 36 weeks postmenstrual age without a physiologic test confirming the oxygen dependency is the most frequently used definition of BPD in clinical trials and epidemiologic studies31). However the incidence of BPD diagnosed by this definition can be influenced by different strategies for oxygen supplementation which will be different from center to center and from person to person in a center. Different arterial blood oxygen saturation targets, different ventilatory strategies, and different medication practice will lead to different oxygen supplementation strategies. In interpreting the results of the studies of the relationship between chorioamnionitis and BPD, the definition of BPD used in the individual study should be taken into account.
3. Variations in the study population
The incidence of chorioamnionitis and BPD can vary according to the subject population studied. The lower the gestational age and birth weight, the higher the incidences of chorioamnionitis and BPD. Because the relationship between chorioamnionitis and BPD can also be influence by gestational age and birth weight, it is important to interpret the results of the studies on the relationship between chorioamnionitis and BPD considering the characteristics of the study population. The effects of chorioamnionitis on the development of BPD have been studied in different population groups11). Different study population may be one of the causes of controversy on the relationship between chorioamnionitis and BPD.
Which contributes more: inflammation or infectious microorganisms?
The chorioamnionitis can be caused by a variety of stimuli. Most cases of chorioamnionitis are associated with intrauterine infections caused by genital mycoplasma like U. urealyticum11). A recent systemic review reported that the exposure to Ureaplasma increased the incidence of BPD32). Ureaplasma has been believed to contribute to the development of BPD by dysregulating inflammatory responses in the immature lungs resulting in aberrant lung development and fibrosis33). Although intrauterine exposure to Ureaplasma is related to chorioamnionitis, the presence of Ureaplasma does not always overlap with chorioamnionitis34). A simple colonization of Ureaplasma in the fetal membranes or placenta can occur without inducing chorioamnionitis35). It is not yet known which of chorioamnionitis or Ureaplasma infection contributes more to the development of BPD. In studying the relationship between chorioamnionitis and BPD, simply examining whether or not the chorioamnionitis is present can lead to incorrect conclusions. Both the type of microorganisms causing chorioamnionitis and the extent of chorioamnionitis by those microorganisms should be evaluated to clarify the actual relationship between chorioamnionitis and BPD.
Ureaplasma and BPD
Microorganisms are found in approximately 75% of the placenta with chorioamnionitis36). Most common microorganism isolated from the placenta or amniotic fluid of pregnant women with chorioamnionitis is Ureaplasma species34). The association of Ureaplasma colonization with BPD has been demonstrated in human neonates by several meta-analyses that included more than 40 individual studies32,37,38). In animal studies including mice, sheep, and nonhuman primates, in utero Ureaplasma infection has been shown to induce a sustained dysregulated inflammatory response in the immature lungs that results in impaired alveolarization and excessive collagen and elastic deposition39). Disrupted alveolarization and airway and pulmonary vascular remodeling is a pathologic hallmark of BPD. The most definitive evidence for the role of Ureaplasma in the development of BPD will be obtained through the trial of anti-Ureaplasma agent in Ureaplasma-infected subjects. If the anti-Ureaplasma treatment can reduce the incidence of BPD in Ureaplasma-infected animals or human infants, the role of Ureaplasma in the development of BPD will be proven. In the Rhesus monkey intrauterine Ureaplasma infection model, azithromycin delayed onset of labor, eradicated the infection, and prevented fetal lung injury40,41). However, prolonged antenatal exposure to Ureaplasma infection resulted in persistent chorioamnionitis and fetal lung injury in a sheep model despite a successful eradication of Ureaplasma by azithromycin or solithromycin42). These results suggest that early detection of Ureaplasma infection will be essential for optimal timing of anti-Ureaplasma treatment to prevent fetal lung injury or BPD in the fetus or preterm infants infected with Ureaplasma. In human preterm infants, prophylactic azithromycin decreased the incidence of BPD. However, therapeutic macrolides did not decrease the incidence of BPD among Ureaplasma-positive preterm infants43). At the moment, there is not enough evidence that the eradication of Ureaplasma results in a reduction of BPD. A phase IIB, placebo-controlled, randomized trial of the multi-dose azithromycin regimen in preterm infants is currently underway. The result of this clinical trial will provide us with the information on the effect of azithromycin on the BPD and long-term neurodevelopmental outcomes39).
Conclusions
The results of a number of animal experimental and human epidemiologic studies suggest that chorioamnionitis affects the development of BPD directly or indirectly. However, there are not a few limitations in the individual study. Not-unified definitions of the chorioamnionitis and BPD, differences in the study populations, and the proportion of contribution between inflammation and infectious microorganisms should be considered in interpreting the results of these studies. The results of the clinical trials of anti-Ureaplasma or anti-inflammatory treatment for pregnant women with chorioamnionitis or preterm infants who have been exposed chorioamnionitis will provide answer to the controversies on causal relationship between chorioamnionitis and BPD and a novel prophylactic strategy against BPD based on that relationship.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:1162–1166. doi: 10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol. 2005;22:155–159. doi: 10.1055/s-2005-865020. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 5.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25(Suppl 2):S31–S35. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 6.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 7.Plakkal N, Soraisham AS, Trevenen C, Freiheit EA, Sauve R. Histological chorioamnionitis and bronchopulmonary dysplasia: a retrospective cohort study. J Perinatol. 2013;33:441–445. doi: 10.1038/jp.2012.154. [DOI] [PubMed] [Google Scholar]
- 8.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 9.Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994;93:712–718. [PubMed] [Google Scholar]
- 10.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–1319. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 11.Glaser K, Speer CP. Pre and postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. In: Bhandari V, editor. Bronchopulmonary dysplasia. Philadephia (PA): Humana Press; 2016. pp. 55–77. [Google Scholar]
- 12.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer BW, Ladenburger A, Kunzmann S, Speer CP, Been JV, van Iwaarden JF, et al. Intravenous lipopolysaccharide-induced pulmonary maturation and structural changes in fetal sheep. Am J Obstet Gynecol. 2009;200:195.e1–195.e10. doi: 10.1016/j.ajog.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res. 2009;65:323–327. doi: 10.1203/PDR.0b013e318193f165. [DOI] [PubMed] [Google Scholar]
- 15.Choi CW, Lee J, Oh JY, Lee SH, Lee HJ, Kim BI. Protective effect of chorioamnionitis on the development of bronchopulmonary dysplasia triggered by postnatal systemic inflammation in neonatal rats. Pediatr Res. 2016;79:287–294. doi: 10.1038/pr.2015.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 17.Viscardi RM, Manimtim WM, Sun CC, Duffy L, Cassell GH. Lung pathology in premature infants with Ureaplasma urealyticum infection. Pediatr Dev Pathol. 2002;5:141–150. doi: 10.1007/s10024001-0134-y. [DOI] [PubMed] [Google Scholar]
- 18.Viscardi R, Manimtim W, He JR, Hasday JD, Sun CC, Joyce B, et al. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol. 2006;9:143–151. doi: 10.2350/10-05-0112.1. [DOI] [PubMed] [Google Scholar]
- 19.Erdemir G, Kultursay N, Calkavur S, Zekioğlu O, Koroglu OA, Cakmak B, et al. Histological chorioamnionitis: effects on premature delivery and neonatal prognosis. Pediatr Neonatol. 2013;54:267–274. doi: 10.1016/j.pedneo.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Soraisham AS, Trevenen C, Wood S, Singhal N, Sauve R. Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J Perinatol. 2013;33:70–75. doi: 10.1038/jp.2012.49. [DOI] [PubMed] [Google Scholar]
- 21.Lahra MM, Beeby PJ, Jeffery HE. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F13–F16. doi: 10.1136/adc.2007.135889. [DOI] [PubMed] [Google Scholar]
- 22.Speer CP. Neonatal respiratory distress syndrome: an inflammatory disease? Neonatology. 2011;99:316–319. doi: 10.1159/000326619. [DOI] [PubMed] [Google Scholar]
- 23.Been JV, Rours IG, Kornelisse RF, Jonkers F, de Krijger RR, Zimmermann LJ. Chorioamnionitis alters the response to surfactant in preterm infants. J Pediatr. 2010;156:10–15.e1. doi: 10.1016/j.jpeds.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Choi CW, Kim BI, Kim HS, Park JD, Choi JH, Son DW. Increase of interleukin-6 in tracheal aspirate at birth: a predictor of subsequent bronchopulmonary dysplasia in preterm infants. Acta Paediatr. 2006;95:38–43. doi: 10.1080/08035250500404085. [DOI] [PubMed] [Google Scholar]
- 25.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2012;97:F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 26.Kim SY, Choi CW, Jung E, Lee J, Lee JA, Kim H, et al. Neonatal morbidities associated with histologic chorioamnionitis defined based on the site and extent of inflammation in very low birth weight infants. J Korean Med Sci. 2015;30:1476–1482. doi: 10.3346/jkms.2015.30.10.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg RL, Andrews WW, Goepfert AR, Faye-Petersen O, Cliver SP, Carlo WA, et al. The Alabama Preterm Birth Study: umbilical cord blood Ureaplasma urealyticum and Mycoplasma hominis cultures in very preterm newborn infants. Am J Obstet Gynecol. 2008;198:43.e1–43.e5. doi: 10.1016/j.ajog.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inatomi T, Oue S, Ogihara T, Hira S, Hasegawa M, Yamaoka S, et al. Antenatal exposure to Ureaplasma species exacerbates bronchopulmonary dysplasia synergistically with subsequent prolonged mechanical ventilation in preterm infants. Pediatr Res. 2012;71:267–273. doi: 10.1038/pr.2011.47. [DOI] [PubMed] [Google Scholar]
- 29.Thome U, Götze-Speer B, Speer CP, Pohlandt F. Comparison of pulmonary inflammatory mediators in preterm infants treated with intermittent positive pressure ventilation or high frequency oscillatory ventilation. Pediatr Res. 1998;44:330–337. doi: 10.1203/00006450-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Bancalari E, Claure N. Bronchopulmonary dysplasia: definitions and epidemiology. In: Bhandari V, editor. Bronchopulmonary dysplasia. Philadephia (PA): Humana Press; 2016. pp. 167–182. [Google Scholar]
- 31.Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. 2014;34:705–710. doi: 10.1038/jp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, et al. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. Pediatr Infect Dis J. 2014;33:697–702. doi: 10.1097/INF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 33.Viscardi RM, Hasday JD. Role of Ureaplasma species in neonatal chronic lung disease: epidemiologic and experimental evidence. Pediatr Res. 2009;65(5 Pt 2):84R–90R. doi: 10.1203/PDR.0b013e31819dc2f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namba F, Hasegawa T, Nakayama M, Hamanaka T, Yamashita T, Nakahira K, et al. Placental features of chorioamnionitis colonized with Ureaplasma species in preterm delivery. Pediatr Res. 2010;67:166–172. doi: 10.1203/PDR.0b013e3181c6e58e. [DOI] [PubMed] [Google Scholar]
- 35.Jung E, Choi CW, Kim SY, Sung TJ, Kim H, Park KU, et al. Coexistence of Ureaplasma and chorioamnionitis is associated with prolonged mechanical ventilation. Pediatr Int. 2017;59:34–40. doi: 10.1111/ped.13072. [DOI] [PubMed] [Google Scholar]
- 36.Hermansen MC, Hermansen MG. Perinatal infections and cerebral palsy. Clin Perinatol. 2006;33:315–333. doi: 10.1016/j.clp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Wang EE, Cassell GH, Sánchez PJ, Regan JA, Payne NR, Liu PP. Ureaplasma urealyticum and chronic lung disease of prematurity: critical appraisal of the literature on causation. Clin Infect Dis. 1993;17(Suppl 1):S112–S116. doi: 10.1093/clinids/17.supplement_1.s112. [DOI] [PubMed] [Google Scholar]
- 38.Schelonka RL, Katz B, Waites KB, Benjamin DK., Jr Critical appraisal of the role of Ureaplasma in the development of bronchopulmonary dysplasia with metaanalytic techniques. Pediatr Infect Dis J. 2005;24:1033–1039. doi: 10.1097/01.inf.0000190632.31565.83. [DOI] [PubMed] [Google Scholar]
- 39.Viscardi RM, Kallapur SG. Role of ureaplasma respiratory tract colonization in bronchopulmonary dysplasia pathogenesis: current concepts and update. Clin Perinatol. 2015;42:719–738. doi: 10.1016/j.clp.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol. 2012;207:475.e1–475.e14. doi: 10.1016/j.ajog.2012.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acosta EP, Grigsby PL, Larson KB, James AM, Long MC, Duffy LB, et al. Transplacental transfer of Azithromycin and its use for eradicating intra-amniotic ureaplasma infection in a primate model. J Infect Dis. 2014;209:898–904. doi: 10.1093/infdis/jit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura Y, Payne MS, Keelan JA, Noe A, Carter S, Watts R, et al. Maternal intravenous treatment with either azithromycin or solithromycin clears Ureaplasma parvum from the amniotic fluid in an ovine model of intrauterine infection. Antimicrob Agents Chemother. 2014;58:5413–5420. doi: 10.1128/AAC.03187-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair V, Loganathan P, Soraisham AS. Azithromycin and other macrolides for prevention of bronchopulmonary dysplasia: a systematic review and meta-analysis. Neonatology. 2014;106:337–347. doi: 10.1159/000363493. [DOI] [PubMed] [Google Scholar]