Summary
Glucocorticoids (GCs) are hormones that are widely used in medicine; but although side effects are generally recognised, little is known about the precise mechanisms that is implicated in many of these side effects. Furthermore, GCs are highly correlated with stress and behaviour disorders. This study evaluated the effects of the glucocorticoid corticosterone on the ventral prostate of the Mongolian gerbil. Male gerbils (Meriones unguiculatus) (n = 5) received intraperitoneal injections of saline or corticosterone in doses of 0.5 mg/kg/day and 1.5 mg/kg/day for 5 days; while some of the animals were killed immediately after the treatment, the others were killed 5 days after the treatment period. The data show that corticosterone influences the structure and functionality of this organ. This hormone has anti‐proliferative and anti‐apoptotic properties in the prostate. In addition, the frequencies of the androgen (AR), oestrogen (ERα, ERβ) and glucocorticoid (GR) receptors changed. The frequencies of AR, GR and ERβ decreased in the Ct1/5 group; in the groups with rest period, the frequencies of GR increased and ERβ decreased in the epithelium. Changes in the proliferative index, apoptotic index and receptor activity may have contributed to the emergence of prostatic morphological alterations, such as the presence of cellular debris and inflammatory cells. Different doses of corticosterone had variable effects on the prostate, with a higher dose showing subtler effects and a lower dose showing more striking effects. The corticosterone effects on nuclear receptors were reverted or attenuated after a rest period, which was not observed for proliferation and apoptosis. In summary, we have demonstrated that corticosterone might influence the prostatic morphophysiology and that these changes may be linked in some way to the altered receptor distribution.
Keywords: corticosterone, gerbil, glucocorticoids, ventral prostate
There is a high incidence of prostate gland disease, and therefore, analysis of the morphophysiology of this gland is an important topic of study. Prostate cancer is the most common non‐cutaneous cancer in men in the United States, with one in six men having prostate cancer at some point in their life; this kind of cancer is a major cause of cancer‐related death (Ross & Kantoff 2007; Thomas et al. 2011).
The prostate is an androgen‐dependent gland; these hormones are important for cell differentiation, morphology maintenance and the regulation of secretory activity (Davies & Eaton 1991). The action of androgens also controls the interaction between the epithelium and stroma (Cunha et al. 2004a,b). During prostate development, signalling through the AR is necessary to induce stromal prostatic epithelial gland ductal morphogenesis and differentiation. The prostatic stromal compartment can be understood as a complex arrangement of smooth muscle cells and fibroblasts immersed in an extracellular matrix. In addition the stroma contains growth factors, regulatory molecules, remodelling enzymes, blood vessels, nerves and immune cells. Therefore, the stromal components act to regulate cell function and maintain overall prostatic tissue homeostasis (Vilamaior et al. 2000; Bruni‐Cardoso et al. 2008). Other non‐androgenic hormones can interfere with prostatic homeostasis, such as oestrogen that modulates the androgenic effects and increases tissue sensitivity to other hormones (Timms et al. 1999; García‐Flórez et al. 2005; Scarano et al. 2008, Da Silva et al. 2013); and progesterone that shows an anabolic capacity in castrated animals (Fochi et al. 2013; Shinohara et al. 2013; Zanatelli et al. 2013).
Glucocorticoids (GCs) affect prostate activity via interaction with their intracellular receptors. These steroid hormones are produced by the adrenal glands and regulated by the hypothalamic–pituitary–adrenal axis (HPA), which plays a fundamental role in the response to external and internal stimulus (Juruena et al. 2006). Cortisol in humans and corticosterone in rodents control several physiological processes, including development, metabolism, homeostasis, inflammation and stress responses (Marieb & Hoehn 2007). Most of the effects of GCs are mediated through activation of the glucocorticoid receptor (GR) (Chang et al. 1987; Mohler et al. 1996), a member of the nuclear receptor superfamily of ligand‐dependent transcription factors (Labeur & Holsboer 2010). Regulation of expression of the GR target gene can be either by transcriptional activation or repression. GR can bind to glucocorticoid response elements (GREs), activating transcription, or interact physically with other transcription factors, thereby inhibiting transcription (Ramamoorthy & Cidlowski 2013).
GCs are secreted at high levels in stressful situations and, once in circulation, these hormones can trigger a variety of specifics response for each tissue (Fleshner et al. 1995; Calvo & Volosin 2001; Cockrem 2013; Cruz‐Topete & Cidlowski 2015). Prolonged exposure to stress periods, however, may have adverse health effects, including on the prostate gland (Dinan 1994; Glaser & Kiecolt‐Glaser 2005; Zhu et al. 2005; Venâncio et al. 2012). The dynamics of present‐day life has generated a constantly stressful environment, gradually influencing the sleep–wake cycle, which has been a very active stress‐generating factor in modern society. Venâncio et al. (2012) showed that sleep restriction is a factor capable of promoting significant changes in the morphology of the ventral prostate, decreasing the concentration of testosterone and increasing the levels of corticosterone. In the case of sleep disruption, it was shown that after rest periods with equal duration to the deprivation, there is a normalization of hormone concentrations in treated animals (Hipólide et al. 2006; Andersen et al. 2009). Studies with rats have shown that administration of corticosterone (1–5 mg/kg) resulted in increased plasma corticosterone levels similar to the levels observed in stressed animals (Fleshner et al. 1995; Stöhr1 et al. 1999; Calvo & Volosin 2001; Venâncio et al. 2012). Furthermore, GCs are also widely used as drugs in clinical medicine, because of their broad activity spectrum and for anti‐inflammatory and immunosuppressive properties (Rhen & Cidlowski 2005; Nussinovitch et al. 2010). They can be used in almost all specialties. Dosages employed routinely in the treatment of inflammatory diseases, such as respiratory and joint disorders, range from 0.4 to 15 mg/day (Caldwell & Furst 1991; Gøtzsche & Johansen 2009). Although they are very widely used it is known that prolonged use can cause several adverse effects, especially the circulatory system and glucose homeostasis (Biddie et al. 2011). However, the effect of this hormone on the prostate specifically is relatively unexplored.
Some studies have shown that the application of glucocorticoids in rodents led to prostatic changes (Ribeiro et al. 2008; Simanainen et al. 2011) and can influence the hormonal regulation of the gland. Smith et al. (1984) demonstrated that GCs reduce the amount of androgen receptor (AR) in an androgen‐dependent cell line. They are also reported to have led to modification of the molecular mechanisms associated with programmed cell death in the prostate (Rennie et al. 1989), while Koutsilieris et al. (1992) showed that GCs can inhibit cell proliferation in androgen‐independent prostatic adenocarcinoma.
This study examined the effects of exogenous corticosterone administration on the morphophysiology of the ventral prostate in gerbils (Meriones unguiculatus), thereby seeking to understand the interference of GCs in prostate homeostasis, tracing a parallel with the use of the steroid‐based drugs widely used in medicine, as well as situations of stress. It was also analysed whether the effects of this hormone are persistent even after a period without corticosterone administration with same duration of treatment.
Material and methods
Experimental design
Thirty male gerbils (Meriones unguiculatus), aged from 90 days, were used in this study. All animals were maintained in the Bioterium of the Department of Biology, under appropriate light (12‐h light and 12‐h dark) and temperature (±25°C) conditions. Food and water were available ad libitum, in accordance with the internal rules of the Ethics Committee on Animal Use of UNESP (CEUA‐IBILCE/UNESP: 087/2013).
Animals received different doses of corticosterone (CORT), with or without a rest period. Gerbils were divided into six groups (n = 5, each group) according to their treatment (Figure 1). The groups were appointed as follows: CO5 – with saline solution for five days and killed in the sequence; Ct1/5 – treated with 0.5 mg/kg/day of CORT for five days and killed in the sequence; Ct2/5 – treated with 1.5 mg/kg/day of CORT for five days and killed in the sequence; CO10 – treated with saline solution for a period of five days and killed five days after the end of treatment; Ct1/10 – treated with 0.5 mg/kg/day of CORT for five days and killed five days after the end of treatment; and Ct2/10 – treated with 1.5 mg/kg/day of CORT for five days and killed five days after the end of treatment. Corticosterone (Sigma‐Aldrich, St. Louis, MO, USA) was diluted in saline solution. All administrations were performed by the intraperitoneal route. The animals were killed by CO2 anaesthesia and decapitation. Body weight was recorded immediately before anaesthesia.
Figure 1.
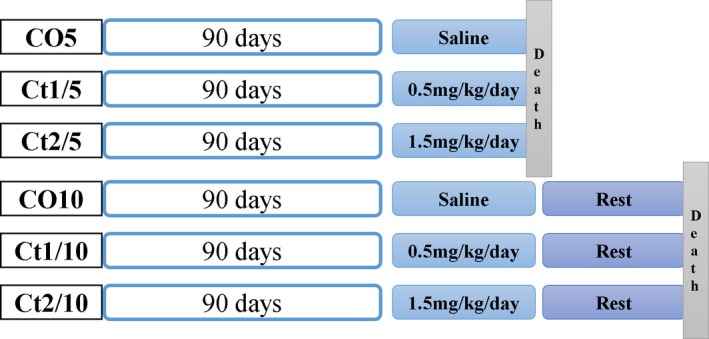
Schematic representation of treatments and experimental groups.
Doses were selected with the aim of simulating episodes of stress experienced daily; also the importance of rest periods after such events was analysed, and a parallel was traced with the use of the steroid‐based drugs widely used in medicine.
Serum hormone levels
Blood samples were collected immediately following decapitation, centrifuged at 3000 rpm to separate the plasma, frozen at −80°C and then subjected to quantification of testosterone and corticosterone levels. The hormone levels were assessed by ELISA with capture sandwich (antibody–antigen–antibody) tests using specific commercial kits (Enzo Life Sciences International Inc., PA, USA), with high sensitivity (5.67 pg/ml for testosterone and 27 pg/ml for corticosterone) and an interassay coefficient of variation of 11.3 pg/ml for testosterone and 8.2 pg/ml for corticosterone. The readings were obtained using an Epoch™ Multi‐Volume System spectrophotometer (Bio‐Tek Instruments, VT, USA).
Histologic analysis
The ventral prostate was removed, weighed and immediately fixed by immersion in paraformaldehyde (4%) diluted in 0.2M phosphate buffer, with a pH of 7.2, or Karnovsky fixative (0.1 M phosphate buffer, pH 7.2, containing 4% paraformaldehyde and 2.5% glutaraldehyde) for 24 h. Then the material was processed for inclusion in paraffin (Histosec MERCK), while others were embedded in historesin (Historesin embedding kit; Leica, Nussloch, Alemanha). Sections (1–5 μm thick) were cut using a rotating microtome and collected on glass slides. The slides were stained with haematoxylin and eosin (H&E) (Behmer et al. 1976) and used for general, stereological and morphometric analysis.
Stereology and morphometric analysis
The morphometric and stereological analyses were performed using slides stained with haematoxylin–eosin technique and an image analyser system with Image‐Pro Plus 6.0 software (Media Cybernetics Inc., Silver Spring, MD, USA).
For the morphometric study, measurements of epithelial cell height and stromal smooth muscle layer thickness were made. Forty fields per group were captured at magnification of 1000×. Measurements were made in each field with the Image‐Pro Plus 6.0 software; in the total were made 200 measurements per group.
Forty fields per group were captured at 200× magnification with Image‐Pro Plus 6.0 software. The stereological analysis was performed using the multipoint M130 test system proposed by Weibel (1978) and applied to the prostate as described by Huttunen et al. (1981). Through this method, the index of the ventral prostate tissue compartments was analysed (acinar lumen, glandular epithelium and stroma muscle).
Immunohistochemical analysis
To verify the expression of specific markers in the prostate, immunostainings were performed as follows: The deparaffinized and rehydrated sections were subjected to antigen retrieval in citrate buffer pH 6.0 at 100°C. The blocking of endogenous peroxidase was performed with 3% H2O2 in methanol or PBS, for 15–30 min. Slides were incubated with primary antibodies for glucocorticoid receptor (GR, rabbit polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA); androgen receptor (AR, rabbit polyclonal IgG, C‐20, Santa Cruz Biotechnology, Santa Cruz, CA, USA); oestrogen receptor alpha (ERα, rabbit polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA); oestrogen receptor beta (ERβ, rabbit polyclonal IgG, H‐150, Santa Cruz Biotechnology, Santa Cruz, CA, USA); and nuclear antigen of proliferating cells (PCNA monoclonal mouse IgG PC10, Santa Cruz Biotechnology, Santa Cruz, CA, USA). These were used at varied dilutions (1:50–1:100) for 60 min at 37°C or overnight at 4°C. After being washed in PBS and incubated with secondary antibodies marked with peroxidase for 45 min, the sections were passed by reaction with diaminobenzidine (DAB). The counterstaining of sections was performed with Harris haematoxylin. The slides were dehydrated and mounted in Canada balsam and evaluated by conventional light microscopy. The primary antibodies were replaced with the corresponding normal isotype serum in the negative control for each immunostaining.
For the detection of apoptotic cells, the TUNEL reaction was used; paraffin sections (5 μm thick) were immersed in TBS (20 mM, 140 mMNaCl, pH 7.6) and treated with proteinase K (1:1000 in 10 mM Tris, pH 8.0) for 23 min at room temperature. Endogenous peroxidases were blocked with H2O2 (30%) in methanol (1:10) for 5 min. Subsequently, the sections were incubated with biotinylated TdT and the enzyme terminal deoxynucleotidyl transferase (TdT) for 1 h at 37°C. Upon completion of the reaction, biotinylated nucleotides were detected using streptavidin peroxidase conjugate, and immunostaining was detected by diaminobenzidine (DAB) for 13 min in the dark. Subsequently, the sections were washed in water, counterstained with haematoxylin and mounted in Canada balsam. The primary antibodies were replaced with the corresponding normal isotype serum in the negative control for each immunostaining.
For quantification of immunostainings and apoptotic cells, 30 prostatic acini per group (five animals each) were randomly selected and used for counting a minimum of 1000 cells, labelled and unlabelled, by Image‐Pro Plus software. The mean receptor frequency was calculated by dividing the positive cells by the total number of cells counted in each field, with the result being multiplied by 100. The labelling index of proliferation and apoptosis was measured by percentage (ratio between the numbers of labelled or unlabelled cells by the total number of cells obtained in each field).
Statistical analysis
The data used for the analysis are expressed as mean ± standard deviation or median. As the normality (Kolmogorov–Smirnov test) and homoscedasticity assumptions (Levene test) of the data appeared to be valid, data were analysed initially by two‐way analysis (anova) to examine the effects of treatment and rest period as the two factors and their interaction, post hoc multiple comparisons were carried out using the Bonferroni test, and values were considered to be statistically significant when P < 0.05. All statistical evaluations were performed using the Statistica 7.0 software (Copyright StatSoft, Inc., Tulsa, OK, USA).
Ethical approval statement
Animal handline and experiments were performed according to the Ethical Guidelines of the Univ. Estadual Paulista ‐ UNESP: Ethical Commitee Nr. 087/2013.
Results
Biometric analysis
Table 1 shows the animal weight, ventral prostate weight and relative weight (prostate weight / animal weight) for each group.
Table 1.
Biometric parameters of the animal and prostatic weights (mean±standard error) of the different experimental groups (n = 5). The values mentioned are the average and standard deviation
| CO5 | Ct1/5 | Ct2/5 | |
|---|---|---|---|
| Animal (g) | 61 ± 4.47a | 62.4 ± 8.29a | 51.6 ± 5.18b |
| Ventral Prostate (mg) | 19.9 ± 4.32a | 18.87 ± 7.39a | 9.72 ± 3.93b |
| Relative Weight (10−3) | 3.01 ± 0.85a | 2.43 ± 0.75ab | 1.83 ± 0.69b |
| CO10 | Ct1/10 | Ct2/10 | |
|---|---|---|---|
| Animal (g) | 56.8 ± 1.095c | 61.6 ± 6.229cd | 66 ± 5.831d |
| Ventral Prostate (mg) | 13.4 ± 3.97 | 9.92 ± 3.79 | 14.50 ± 4.84 |
| Relative Weight (10−3) | 2.96 ± 0.83 | 2.88 ± 0.88 | 2.53 ± 0.32 |
Two‐way anova showed a significant dose vs. rest period interaction for animal (P = 0.0025) and ventral prostate (P = 0.0104) weight.
The letters a and b are significant differences between the CO5, Ct1/5 and Ct2/5 groups (Bonferroni post‐tests, P ≤ 0.05). The letters c and d represent significant differences between the CO10, Ct1/10 and Ct2/10 groups (Bonferroni post‐tests, P ≤ 0.05).
The interaction of dose and rest period was significant for animal (P = 0.0025) and ventral prostate (P = 0.0104) weight. The Ct2/5 group showed a reduction in animal, ventral prostate and relative weight. The animal weight of the Ct2/10 group increased compared with the control.
Serum hormone levels
Figure 2 shows the serum hormone levels of testosterone and corticosterone in all groups. Serum testosterone levels did not significantly change with the treatment. As regards the corticosterone levels, the interaction of dose and rest period was significant (P = 0.0108) and the levels of the treated groups were not significantly different compared with the control group; however, the Ct1/5 group showed a significant increase compared with the Ct2/5 group.
Figure 2.
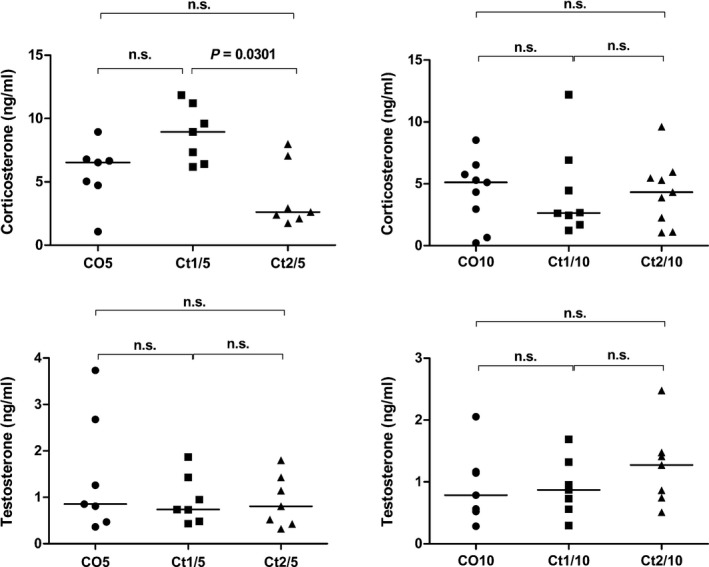
Serum testosterone (ng/ml) and corticosterone (ng/ml) levels. Different letters indicate significant differences between groups. Two‐way anova showed a significant dose vs. rest period interaction (P = 0.0108) for corticosterone levels (Bonferroni post‐tests, P ≤ 0.05).
Structural analysis of the prostate
The results of stereological analyses are shown in Table 2. The interaction of dose and rest period was significant for the percentage of muscle stroma percentage (P = 0.0003). All treated groups showed an increase in the luminal percentage and a reduction in stromal percentage. The epithelium percentage increased in the Ct1/10 group (Figure 4d–h) and decreased in the Ct2/5 group (11%) (Figure 3j–m).
Table 2.
Results of morphometric and stereological analysis, showing the percentage of the prostate gland tissue compartments (epithelium, lumen and muscle stroma), and the height of epithelial and stromal compartments. The values mentioned are the average and standard deviation
| Stereological analysis (%) | |||
|---|---|---|---|
| CO5 | Ct1/5 | Ct2/5 | |
| Epithelium | 46.61 ± 6.28a | 47.69 ± 9.41a | 41.43 ± 9.55b |
| Lumen | 20.88 ± 6.94a | 28.29 ± 9.55b | 34.44 ± 14.35c |
| Muscle stroma | 29.68 ± 6.04a | 23.99 ± 9.01b | 23.44 ± 8.84b |
| C010 | Ct1/10 | Ct2/10 | |
|---|---|---|---|
| Epithelium | 37.63 ± 9.84d | 42.95 ± 9.28e | 33.96 ± 9.43d |
| Lumen | 26.14 ± 10.74d | 33.09 ± 11.20e | 44.23 ± 15.63f |
| Muscle stroma | 38.84 ± 15.13d | 24.17 ± 9.01e | 21.10 ± 6.33e |
| Morphometric Analysis (μm) | |||
|---|---|---|---|
| CO5 | Ct1/5 | Ct2/5 | |
| Epithelium | 18.38 ± 4.91a | 21.2 ± 7.03b | 23.12 ± 7.40c |
| Muscle stroma | 15.39 ± 5.94a | 11.54 ± 4.55b | 11.82 ± 4.42b |
| C010 | Ct1/10 | Ct2/10 | |
|---|---|---|---|
| Epithelium | 12.21 ± 2.94d | 11.84 ± 3.74d | 18.02 ± 4.91e |
| Muscle stroma | 8.26 ± 2.87d | 8.65 ± 3.81d | 12.88 ± 4.64e |
Two‐way anova showed a significant dose vs. rest period interaction for percentage of muscle stroma (P = 0.0003) and height of epithelial (P ≤ 0.0001) and stromal (P ≤ 0.0001) compartments.
The letters a, b and c show significant differences between the CO5, Ct1/5 and Ct2/5 groups (Bonferroni post‐tests, P ≤ 0.05). The letters d, e and f represent significant differences between the CO10, Ct1/10 and Ct2/10 groups (Bonferroni post‐tests, P ≤ 0.05).
Figure 4.
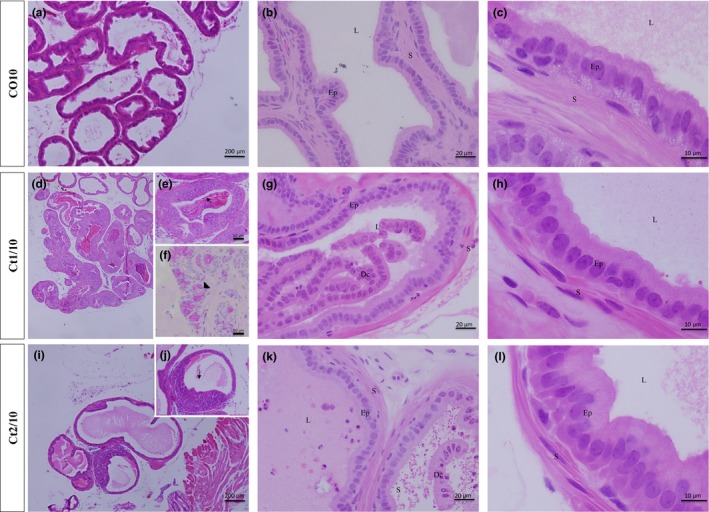
Histologic sections of prostate of gerbils, stained with haematoxylin and eosin. Epithelial compartment height increased in the Ct2/10 group (i–l) and the stromal compartment increased in the Ct2/10 group (i–l). Legend: Ep – epithelium; S – stroma; L – lumen; Dc – cellular debris; arrow – inflammatory cells; arrowhead – atypical cells.
Figure 3.
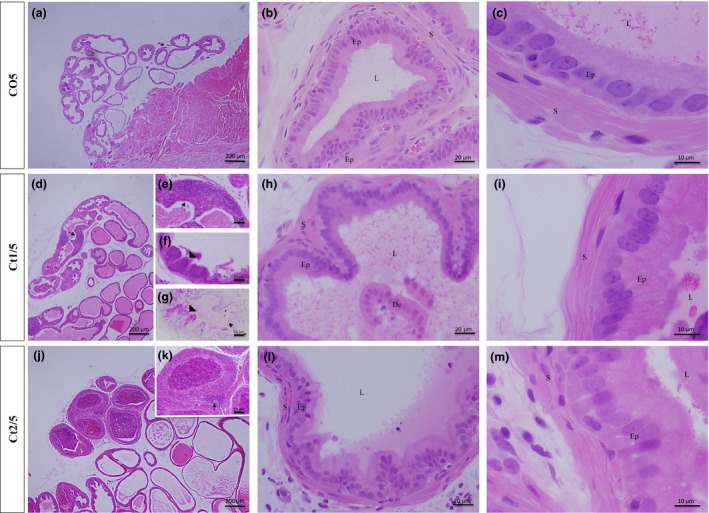
Histologic sections of prostate of gerbils, stained with haematoxylin and eosin. Morphological alterations in the treated groups can be observed, such as cell debris. Note an increase in epithelial compartment height in the Ct1/5 (d–i), Ct2/5 (j–m) groups and a decrease in stromal compartment height in the Ct1/5 (d–i) and Ct2/5 (j–m) groups. Legend: Ep – epithelium; S – stroma; L – lumen; Dc – cellular debris; arrow – inflammatory cells; arrowhead – atypical cells.
The results of morphometric analyses are shown in Table 2. The interaction of dose and rest period was significant for height of epithelial (P ≤ 0.0001) and stromal (P ≤ 0.0001) compartments. Hypertrophy of epithelial cells and a reduction in smooth muscle cell thickness were observed in the Ct1/5 (Figure 3d–i) and Ct2/5 (Figure 3j–m) groups; however, in the Ct2/10 group (Figure 4i–l), both compartments presented an increase.
The presence of some prostatic alterations in the treated groups was observed (Figure 3e and 4). In all treated groups, inflammatory foci were found in the epithelial compartment (Figure 3e and 4), with inflammatory cells inserted between the secretory cells. In the prostates of Ct2/5 animals, however, the inflammatory cells were also inserted within the glandular lumen (Figure 3j and k). The Ct1/5 and Ct1/10 groups showed some cells with atypical morphology in the epithelial compartment – large cells rich in secretory vesicles (Figures 3f,g and 4f). In some prostatic acini, epithelial cell debris were also observed within the glandular lumen (Figures 3h and 4g,k).
Immunohistochemical analysis
The interaction of dose and rest period was significant for GR‐positive cells in the epithelial (P ≤ 0.0001) and stromal (P ≤ 0.0001) compartments. There was a reduction in the frequency of GR‐positive cells in both prostatic compartments of the Ct1/5 group (Figures 5 and 6c), whereas in the Ct1/10 (Figures 5 and 6f) the staining increased in the epithelial compartment and it was similar to the control in the stromal compartment. In the Ct2/10 group (Figures 5 and 6 g), the staining for this receptor increased in both prostatic compartments compared with the control group.
Figure 5.
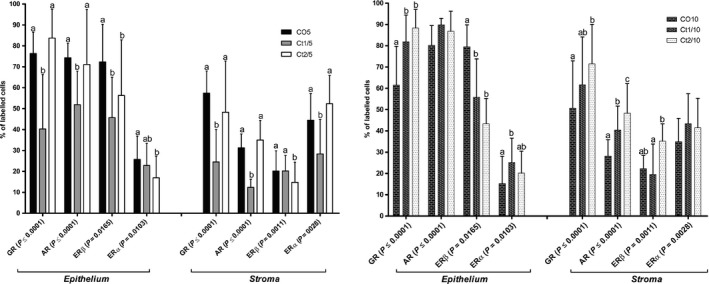
Quantification of cells immunostained for GR, AR, ERβ and ERα of the different experimental groups (n = 5). The values shown are the average and standard deviation. Two‐way anova showed a significant dose vs. rest period interaction for all receptors analysed in the epithelial and stromal compartments (P value presented on figure). The letters a and b represent the significant differences between the groups (Bonferroni post‐tests, P ≤ 0.05).
Figure 6.
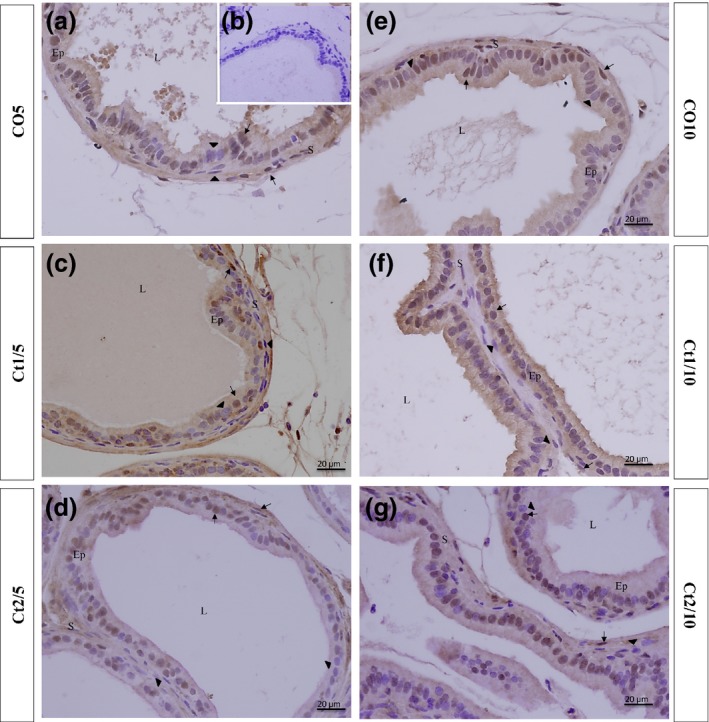
Histologic sections of the prostate of gerbils subjected to immunostaining for anti‐GR. Counterstaining: Harris haematoxylin. B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: GR‐positive cell; arrowhead: GR‐negative cell.
The interaction of dose and rest period was significant for AR‐positive cells in the epithelial (P ≤ 0.0001) and stromal (P ≤ 0.0001) compartments. The Ct1/5 group showed a significant reduction in AR‐positive cells in both the epithelial and stromal compartments (Figures 5 and 7c). There was a significant increase in the immunostaining of AR‐positive cells in the stroma of Ct1/10 (Figures 5 and 7f) and Ct2/10 (Figures 5 and 7 g) groups.
Figure 7.
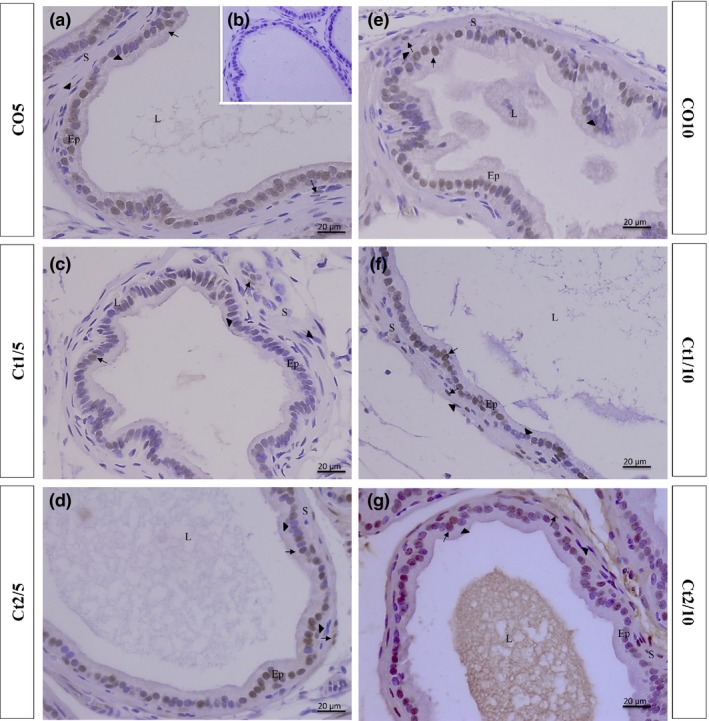
Histologic sections of the prostate of gerbils subjected to immunostaining for anti‐AR. Counterstaining: Harris haematoxylin. B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: AR‐positive cell; arrowhead: AR‐negative cell.
With regard to the immunostaining for ERβ, the interaction of dose and rest period was significant in the epithelial (P = 0.0165) and stromal (P ≤ 0.0011) compartments. The Ct1/5 (Figures 5 and 8c), Ct2/5 (Figures 5 and 8d), Ct1/10 (Figures 5 and 8f) and Ct2/10 (Figures 5 and 8g) groups showed a reduction in the frequency of epithelial stained cells compared with the controls. The Ct2/5 (Figures 5 and 8d) group showed a reduction in labelled stroma cells.
Figure 8.
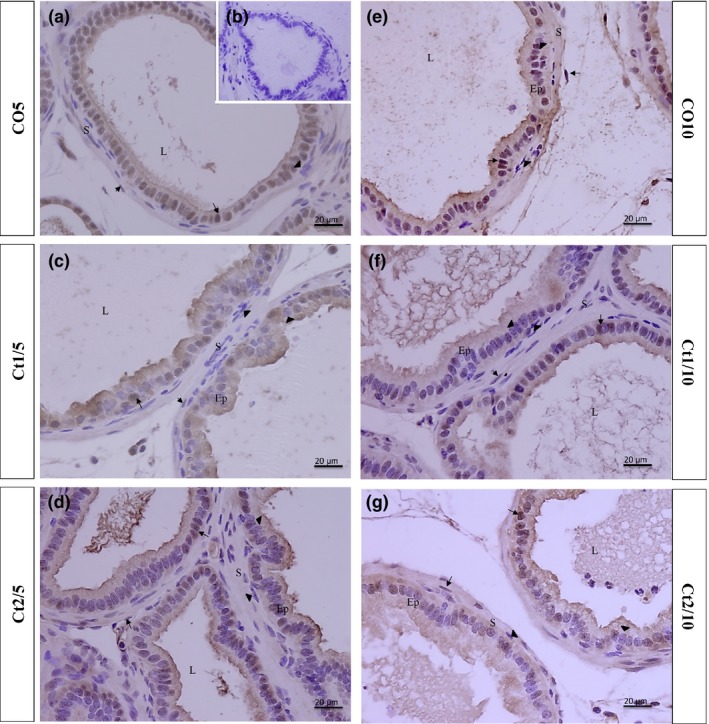
Histologic sections of the prostate of gerbils subjected to immunostaining for anti‐ERβ. Counterstaining: Harris haematoxylin. B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: ERβ‐positive cell; arrowhead: ERβ‐negative cell.
As regards ERα, the interaction of dose and rest period was significant in the epithelial (P = 0.0103) and stromal (P = 0.0028) compartments. A reduction in the amount of immunopositive cells in the epithelium was observed in the Ct2/5 group (Figures 5 and 9d); there was also a reduction in the Ct1/5 group (Figures 5 and 9c) in the frequency of stromal stained cells compared with the controls.
Figure 9.
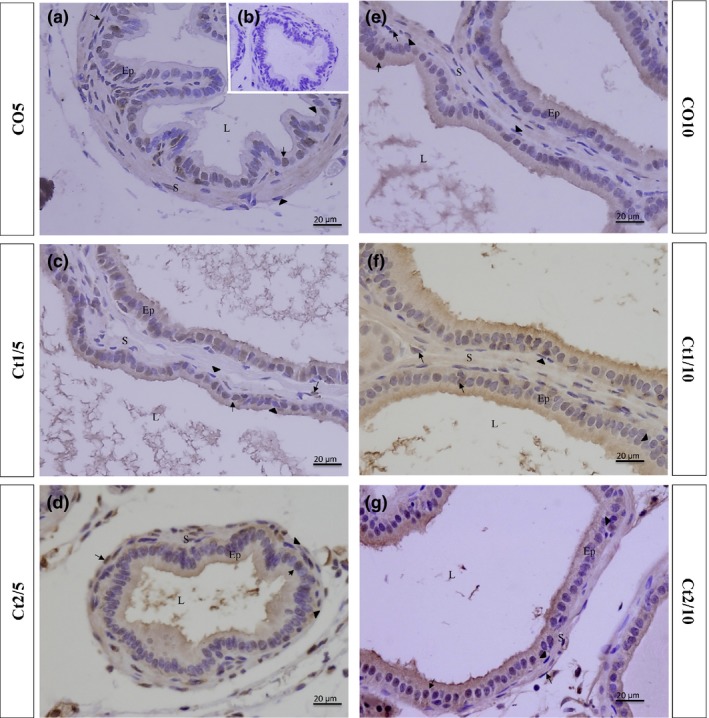
Histologic sections of the prostate of gerbils subjected to immunostaining for anti‐ERα. Counterstaining: Harris haematoxylin. B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: ERα‐positive cell; arrowhead: ERα‐negative cell.
The interaction of dose and rest period was significant for proliferation in the epithelial (P = 0.0012) and stromal (P ≤ 0.0001) compartments. All animals showed reductions in the rate of proliferation. In groups without a rest period (Ct1/5 and Ct2/5) (Figures 10 and 11a–d), this reduction occurred in the stroma; in groups with rest, variation occurred in the epithelium (Figures 10 and 11e–g). However, the Ct2/10 group (Figure 10 and 11 g) was observed an increased in the stromal compartment.
Figure 10.
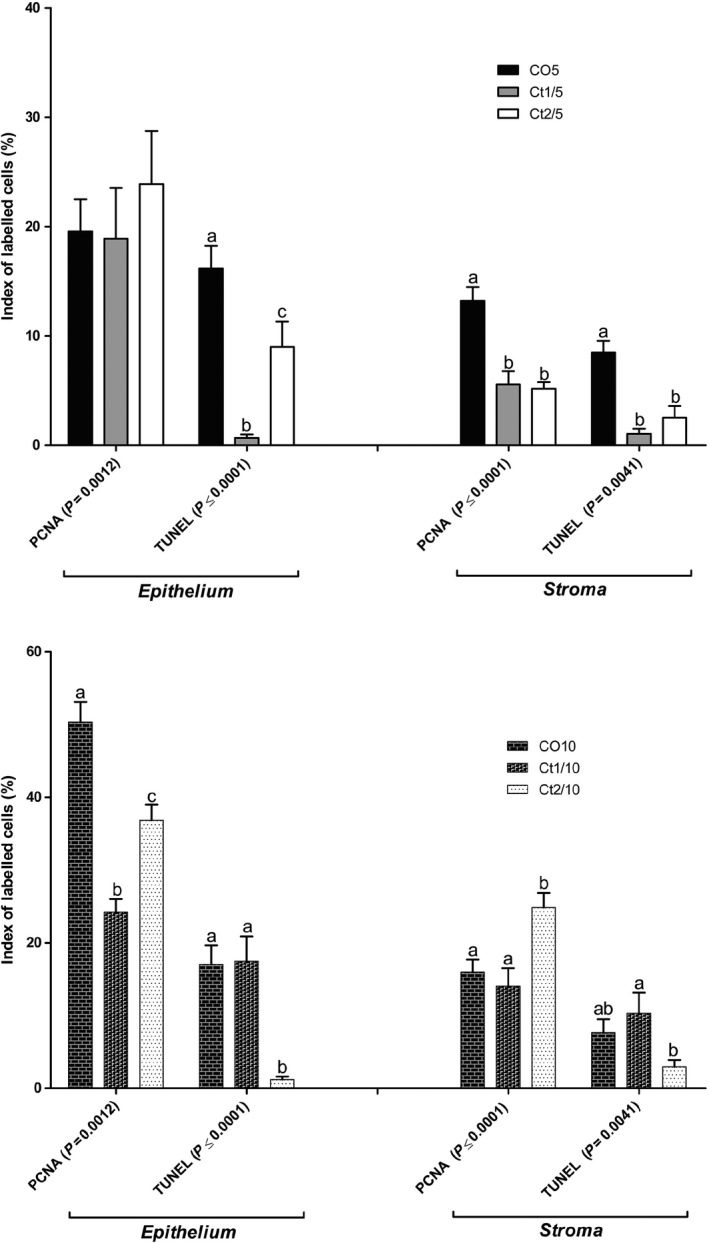
Quantification of cells immunostained for proliferation and apoptosis of the different experimental groups (n = 5). The values shown are the average and standard error. Two‐way anova showed a significant dose vs. rest period interaction (P value presented on figure) for proliferation and apoptosis. The letters a, b and c represent the significant differences between the groups (Bonferroni post‐tests, P ≤ 0.05).
Figure 11.
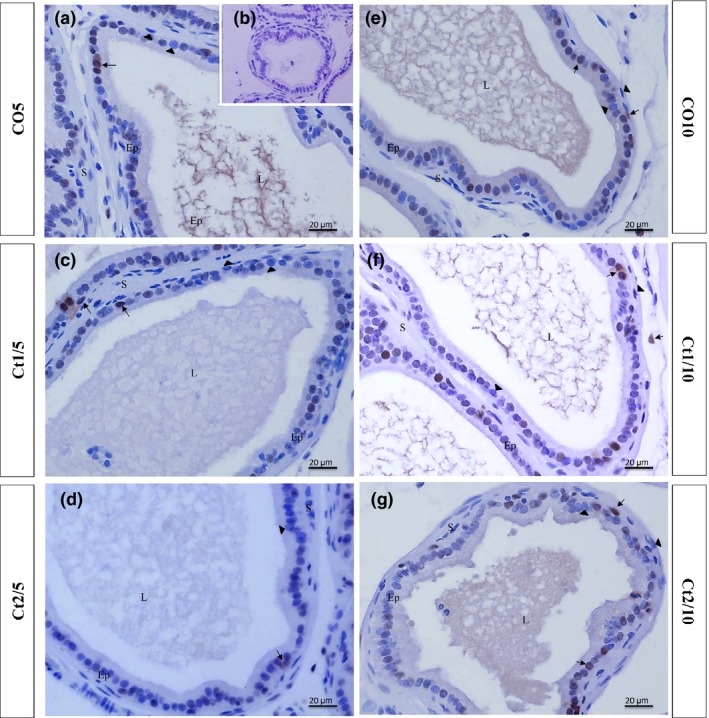
Histologic sections of the prostate of gerbils subjected to immunostaining for proliferating cells (PCNA). B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: immunostained cell; arrowhead: unstained cell.
In terms of variations in the markings for apoptosis, the interaction of dose and rest period was significant in the epithelial (P ≤ 0.0001) and stromal (P = 0.0041) compartments. The Ct1/5 (Figures 10 and 12c) and Ct2/5 (Figures 10 and 12d) groups had decreased epithelial and stromal markers, whereas the Ct2/10 (Figures 10 and 12g) group showed lower marking cells than the control group, only in the epithelial compartment.
Figure 12.
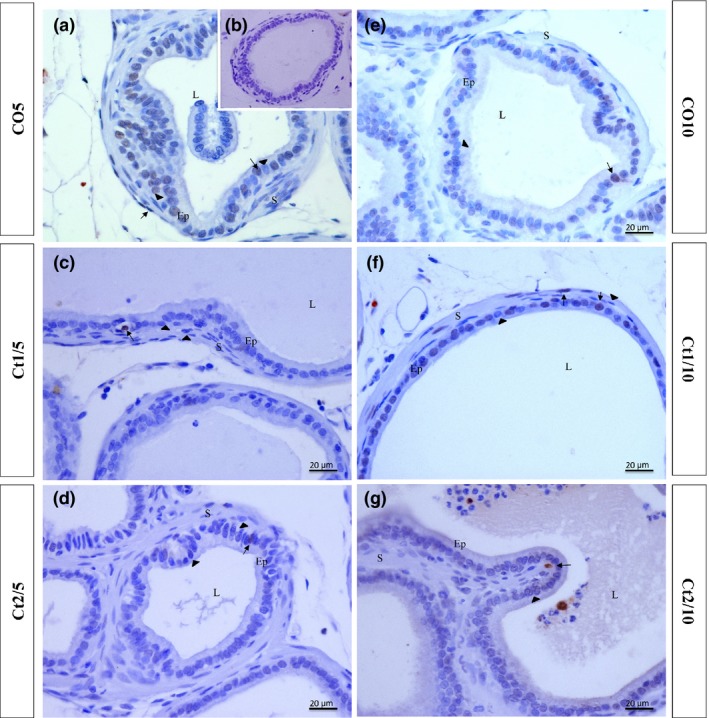
Histologic sections of the prostate of gerbils subjected to immunostaining for apoptotic cells (TUNEL). Counterstaining: Harris haematoxylin. B: negative control. EP: alveolar epithelium; L: alveolar lumen; S: prostatic stroma. Arrow: immunostained cell; arrowhead: unstained cell.
Discussion
In the present study, we analysed the influence of corticosterone in different doses on the gerbil prostate and the recovery from its effects after a rest period of five days. This hormone showed anti‐proliferative and anti‐apoptotic properties and was able to influence the signalling pathways of other hormones that are important for prostate homeostasis, such as androgens and oestrogens. Furthermore, the application of corticosterone led to significant morphological changes in the prostate.
The elevated corticosterone concentration in the blood serum of animals treated with a lower dose compared with that observed in group treated with a higher dose may be due to the activation of the negative feedback process. After the rest period, corticosterone levels in the blood were similar to those of control animals, showing that this period was sufficient for the metabolism of the exogenous hormone, allowing the stabilization of endogenous concentrations of this hormone. Furthermore, it is known that the lifetime of corticosterone in the blood is only 4 h and becomes undetectable after a period of 24 h without administration of this hormone (Fortier 1959; Tornello et al. 1982). Thus, it is reasonable to propose that in the animals receiving higher doses of corticosterone the activation of a negative feedback occurred. The levels of serum hormone were maintained, as the period between the last exogenous administration and blood collection was sufficient to allow for its metabolism. Although after the exogenous application of corticosterone the plasma levels remained similar to that observed in control animals, studies show that two isoforms of the 11β‐hydroxysteroid dehydrogenase enzyme act reversibly synthesized together to prevent prolonged and intense effect of GCs. The HSD11B2 isoform oxidizes corticosterone forming 11‐dehydrocorticosterone; this inactive metabolite can be reduced by the HSD11B1 isoform forming an active corticosterone again (Witorsch 2016). The application of corticosterone did not affect testosterone levels; based on this, it can be assumed that the responses observed in the prostate were not triggered by systemic changes in androgens.
The anti‐inflammatory and immunosuppressive effects of GCs are widely known; however, studies show that two types of responses can be triggered and may exert anti‐ and pro‐inflammatory effects, depending on the type of exposure to GCs and the baseline of the immune system (Busillo & Cidlowski 2013; Cruz‐Topete & Cidlowski 2015). A pro‐inflammatory action of corticosterone was observed in this study after applying both doses for a short period; moreover, the rest period did not lead to reversal of this feature. The mechanism by which GCs may increase inflammation is not fully understood. Currently, the exclusively anti‐inflammatory role of glucocorticoids is being questioned.
GCs act on cells through interactions with their receptors in the prostate; in the prostate of gerbils, the presence of GRs was observed in both the epithelium and stroma, with greater quantities in the first compartment. GRs are controlled by self‐regulating mechanisms, and the receptor can negatively coordinate transcription of the GR gene when there is an excess of its ligand, thereby leading to a reduction in the concentration of GRs. This downregulation mechanism of the GR caused a reduction in sensitivity to this hormone, providing a partial protective effect to excessive GCs (Faria & Loungui 2006). Ramamoorthy and Cidlowski (2013) described a molecular mechanism for the negative regulation of GRs, identifying an nGRE (functional negative glucocorticoid response element) that binds to exon 6 of the gene, thereby inhibiting transcription initiation. This nGRE is a complex formed by GR‐NCoR1‐histone deacetylase 3 (HDAC3). This study demonstrates that negative regulation is exerted by GCs, and in the group with higher serum corticosterone levels (Ct1/5), the concentration of GRs decreased; after the rest period and for standard corticosterone levels, there was an increase in GR levels. The downregulation of GR observed after treatment with exogenous corticosterone has already been demonstrated in the cerebral tissue (Tornello et al. 1982). The negative regulation of steroid hormones on its receptors was also observed on the peripheral tissues (Walters & Clark 1979). The fact that there is an increase in the quantity of GR after the period of rest corroborates their inversely proportional interaction with the corticosterone levels.
The data presented here emphasize the anti‐proliferative property of GCs. Other studies have shown that GCs may inhibit some signalling pathways and a number of transcription factors involved in cell proliferation, such as AP‐1, SRF, NF‐kB, p53 and STAT1 (Nishimura et al. 2000; Yemelyanov et al. 2007). Costa et al. (2012) showed that the reduction in inhibition rate in animals treated with GCs leads to a reduction in plasma concentration of AKT and mTOR, proteins involved in the regulation of cell growth and development.
ARs and GRs exert opposite effects on tissues in which they are co‐expressed, such as the prostate (Smith et al. 1984; Burstein et al. 1995). Androgens act via AR‐stimulating proliferation and GCs act via GR‐inhibiting proliferation (Smith et al. 1984). This antagonistic effect between AR and GR can be related to the effect of GCs on the AR and on the interaction between these two receptors. Studies have shown that the application of GCs leads to a reduction in AR levels in cells co‐expressing GR and AR, thereby reducing the sensitivity to androgens in cells co‐expressing AR and GR (Smith et al. 1984; Burstein et al. 1995). The results presented in this experiment confirm the ability of GCs to reduce the amount of AR‐positive cells. Chen et al. (1997) showed that in cells co‐expressing AR and GR, these two receptors interact at the transcriptional level and mutually inhibit their activities; this interaction is correlated with the capacity to form of a heterodimer in a common DNA site. The development and homeostasis of the prostate gland occurs through an intense paracrine relationship between the epithelium and stroma, mainly through the AR and ER. The AR and ERα present in the glandular stroma promote a proliferative response in the epithelial compartment, while the AR and ERβ assets in prostatic epithelium regulate such proliferation and also the glandular secretion (Marker et al. 2003; Cunha et al. 2004a,b). This epithelium–stroma interaction can be observed also in relation to glucocorticoids. Recently, it has been shown that prolonged exogenous corticosterone administration for 4 weeks is able to induce a prostatic hyperplasia by activation of GR, specifically in the glandular stroma. This response occurs apparently by paracrine action of FGF10 produced by fibroblasts and smooth muscle cells on the secretory epithelial cells (Simanainen et al. 2011; Zhao et al. 2014). The time of exposure to corticosterone employed in this study was significantly lower, triggering a likely immediate reduction in the supply of GR as a response to increased hormonal demand, enough to prevent a hyperplasia of epithelial cells but not their hypertrophy.
Pro‐apoptotic properties of GCs have been widely studied, and this is known to occur in different cell types. However, this study showed that corticosterone reduced apoptosis in the treated groups, confirming that GCs act in specific ways in different cell types. The mechanisms by which GCs induce apoptosis in lymphoid cells have been extensively studied and include depolarization of the mitochondrial membrane potential and expression of CD‐95 followed by caspase cascade (Kofler 2000; Distelhorst 2002; Herr et al. 2003). However, studies showed that in some cell types, such as carcinomas, human peripheral blood neutrophils, glomerular endothelial cells, lung epithelial cells and hepatoma cells, apoptosis may be suppressed (Pagliacci et al. 1993; Kato et al. 1995; Wen et al. 1997; Yamamoto et al. 1998; Messmer et al. 1999; Herr et al. 2003; Rutz & Herr 2004). Sasson and others showed that glucocorticoids can inhibit apoptosis induced by p53 and cAMP in a dose‐dependent manner in normal and immortalized granulosa cells (Bland et al. 1999; Sasson et al. 2001). In the mammary gland, the anti‐apoptotic effects of glucocorticoids were also observed. Feng et al. (1995) demonstrated the existence of a crosstalk between the steroid hormone receptors and the AP‐1 transcription factor, resulting in the impairment of its activity, triggering the inhibition of glandular involution and programed cell death. It is reasonable to suppose that this same mechanism found in the mammary gland may occur in the ventral prostate, explaining in part the reduction of apoptosis observed in the treated animals. Runnebaum and Brunning showed that treatment with dexamethasone might lead to the inhibition of cIAP2, a cytosolic caspase inhibitor, by interfering with the proteolytic activity of caspases 3 and 9, thus revealing the anti‐apoptotic property of glucocorticoids (Runnebaum & Bruning 2005). The mechanism by which the apoptotic pathway is affected by GCs is still an open question, and these hormones exert a pro‐apoptotic effect in specific cell types while leading to an anti‐apoptotic effect in other cell types. Numerous in vitro studies have been conducted emphasizing the anti‐proliferative ability of GCs. However, in this study, we can see the need to understand the apoptotic changes caused by these hormones, especially in the prostate. An imbalance between proliferation and death rates in the prostate may be responsible for the morphological changes observed in the experimental groups. Rodríguez‐Berriguete et al. (2010) showed that the emergence of prostate diseases, such as benign hyperplasia, intra‐epithelial neoplasia and cancer, is associated with the overexpression of inhibitors of apoptosis.
Other non‐androgenic hormones also affect the prostate homeostasis. Similarly to androgens, oestrogens play essential roles in the physiology and pathology of the prostate during development, and the balance of these hormones is critical for the maintenance of the normal gland (McPherson et al. 2008). Both oestrogen receptors (ERα and ERβ) are expressed in the stroma and epithelium. Treatment with GCs led to a reduction in the number of ERβ‐positive cells, showing an antagonistic effect of this hormone on these receptors. For ERα, an increase in the frequency of these receptors was observed in the Ct1/10 group. The relationship between GCs and oestrogen receptors has not been well studied, and the mechanism by which these hormones interfere with these receptors is not yet understood; it may be via a mechanism involving the GR, by the direct binding of GCs with ER or by interfering with other molecules that are present in these signalling pathways. Some studies have pointed out that oestrogen increases the response of corticosterone to stress, interfering in the negative feedback through their interaction with the ERα, while the opposite effect is observed by its ERβ interaction (Turner 1992; Burgess & Handa 1993; Lund et al. 2004).
In summary, this study showed that corticosterone affects the prostate by changing the frequency of the receptors of the major regulating hormones of this organ such as androgens and oestrogens, and the glucocorticoid receptor. Along with these changes, this hormone may lead to an imbalance in proliferation and death rates. The disruption caused by GCs has led to significant morphological changes with the appearance of prostatic dysplasia, mediated by changes in combinations of oestrogens and glucocorticoids in the patterns of androgen receptors. A variation was observed in the effects produced by this hormone according to the dose applied, with a higher dose having milder effects and a lower dose being more aggressive. The influence of GCs on nuclear receptors was reversed or attenuated after the rest period; however, this effect was not observed, in the proliferation and apoptosis rates. In addition, morphological changes and dysplasia were present in all groups, demonstrating that the rest period used in the study was not able to reverse all of the damage to the prostate via the exogenous administration of GCs.
Funding source
This work was financed by the State of Sao Paulo Research Foundation – FAPESP (Process Nr. 09⁄16790‐5 and 11/06335‐9) and CNPq – National Research Council (fellowship to SRT – Grant Nr. 301596/2011‐5).
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Acknowledgements
This study is part of the Master thesis of Julia Quilles Antoniassi from the Institute of Biology, UNICAMP, and was supported by grants from the Brazilian Agencies FAPESP – São Paulo Research Foundation (Proc. Nr. 2013/13459‐1) and CNPq – Brazilian National Research and Development Council (Proc. Nr. 301596/2011‐5). The authors wish to thank Mr. Luiz Roberto Falleiros Júnior for his technical assistance, as well as all other researchers at the Microscopy and Microanalysis Laboratory.
References
- Andersen ML, Ribeiro DA, Bergamaschi CT et al (2009) Distinct effects of acute and chronic sleep loss on DNA damage in rats. Prog.Neuropsychopharmacol. Biol. Psychiatry 33, 562–567. [DOI] [PubMed] [Google Scholar]
- Behmer A.O., Tolosa E.M.C. & Neto A.G.F. (1976) Manual de praticas para histologia normal e patologica. São Paulo, Brazil: Edart‐Edusp. [Google Scholar]
- Biddie S.C., Conway‐Campbell B.L. & Lightman S.L. (2011) Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland R., Worker C.A., Noble B.S. et al (1999) Characterization of 11b‐hydroxysteroid dehydrogenase activity and corticosteroid receptor expression in human osteosarcoma cell lines. J. Endocrinol. 161, 455–464. [DOI] [PubMed] [Google Scholar]
- Bruni‐Cardoso A., Vilamaior P.S., Taboga S.R. & Carvalho H.F. (2008) Localized matrix metalloproteinase (MMP)‐2 and MMP‐9 activity in the rat ventral prostate during the first week of postnatal development. Histochem. Cell Biol. 129(6), 805–815. [DOI] [PubMed] [Google Scholar]
- Burgess L.H. & Handa R.J. (1993) Hormonal regulation of androgen receptor mRNA in the brain and anterior pituitary gland of the male rat. Brain Res. 19, 31–38. [DOI] [PubMed] [Google Scholar]
- Burstein K.L., Maiorino J.L. & Caneron D.J. (1995) Androgen and glucocorticoid regulation of androgen receptor cDNA expression. Mol. Cell. Endocrinol. Elsevier Science Ireland.115, 177–186. [DOI] [PubMed] [Google Scholar]
- Busillo J.M. & Cidlowski J.A. (2013) The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 24(3), 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J.R. & Furst D.E. (1991) The efficacy and safety of low‐dose corticosteroids for rheumatoid arthritis. Semin. Arthritis Rheum. 21, 1–11. [DOI] [PubMed] [Google Scholar]
- Calvo N. & Volosin M. (2001) Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety‐like response induced by restraint. Neuroendocrinology 73, 261–271. [DOI] [PubMed] [Google Scholar]
- Chang C., Kokontis J., Chang C.T. & Liao S. (1987) Cloning and sequence analysis of the rat ventral prostate glucocorticoid receptor cDNA. Nucleic Acid Res. 15, 9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang J., Yu G., Liu W. & Pearce D. (1997) Androgen and Glucocorticoid Receptor Heterodimer Formation: a possible mechanism for mutual inhibition of transcriptional activity. J. Biol. Chem. 272, 14087–14092. [DOI] [PubMed] [Google Scholar]
- Cockrem J.F. (2013) Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 181, 45–58. [DOI] [PubMed] [Google Scholar]
- Costa M.M., Violato N.M., Taboga S.R., Góes R.M. & Bosqueiro J.R. (2012) Reduction of insulin signalling pathway IRS‐1⁄IRS‐2⁄AKT⁄Mtor and decrease of epithelial cell proliferation in the prostate of glucocorticoid‐treated rats. Int. J. Exp. Path. 93, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz‐Topete D. & Cidlowski J.A. (2015) One hormone two actions: anti‐ and pro‐inflammatory effects of glucocorticoids. NeuroImmunoModulation 22, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. (2004a) Role of stromal‐epithelial interactions in hormonal responses. Arch. Histol. Cytol. 67, 417–434. [DOI] [PubMed] [Google Scholar]
- Cunha G.R., Ricke W., Thomson A. et al (2004b) Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J. Steroid Biochem. Mol. Biol. 92, 221–236. [DOI] [PubMed] [Google Scholar]
- Da Silva D.A.L., Zanatelli M., Shinohara F.Z. et al (2013) Effects of exposure to estradiol and estradiol plus testosterone on the mongolian gerbil (Meriones unguiculatus) female prostate. Microsc. Res. Tech. 1, i–i. [DOI] [PubMed] [Google Scholar]
- Davies P. & Eaton C.L. (1991) Regulation of prostate growth. J. Endocrinol. 131, 5–17. [DOI] [PubMed] [Google Scholar]
- Dinan T.G. (1994) Glucocorticoids and the genesis of depressive illness. A psychobiological model. Br. J. Psychiatry 164, 365–371. [DOI] [PubMed] [Google Scholar]
- Distelhorst C.W. (2002) Recent insights into the mechanism of glucocorticosteroid‐induced apoptosis. Cell Death Differ. 9, 6–19. [DOI] [PubMed] [Google Scholar]
- Faria C.D.C. & Loungui C.A. (2006) Aspectos moleculares da sensibilidade aos glicocorticoides. Arq. Bras. Endocrinol. Metab. 50, 983–995. [DOI] [PubMed] [Google Scholar]
- Feng Z1, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. (1995) Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J. Cell Biol. 131, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M., Deak T., Spencer R.L., Laudenslager M.L., Watkins L.R. & Maier S.F. (1995) A long‐term increase in basal levels of corticosterone and a decrease in corticosteroid‐binding globulin after acute stressor exposure. Endocrinology 136, 5336–5342. [DOI] [PubMed] [Google Scholar]
- Fochi R.A., Santos F.C.A., Goes R.M. & Taboga S.R. (2013) Progesterone as a morphological regulatory factor of the male and female gerbil prostate. Int. J. Exp. Pathol. 94, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier C. (1959) Pituitary ACTH and plasma free corticosteroids following bilateral adrenalectomy in the rat. Proc. Soc. Exp. Biol. Med. 100, 13–16. [DOI] [PubMed] [Google Scholar]
- García‐Flórez M., Oliveira C.A. & Carvalho H.F. (2005) Early effects of estrogen on the rat ventral prostate. Braz. J. Med. Biol. 38(4), 487–497. [DOI] [PubMed] [Google Scholar]
- Glaser R. & Kiecolt‐Glaser J.K. (2005) Stress‐induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251. [DOI] [PubMed] [Google Scholar]
- Gøtzsche P.C. & Johansen H.K. (2009) Alpha1‐antitrypsin deficiency. N. Engl. J. Med. 361, 2101–2102. [DOI] [PubMed] [Google Scholar]
- Herr I., Ucur E., Herzer K. et al (2003) Glucocorticoid co‐treatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 63, 3112–3120. [PubMed] [Google Scholar]
- Hipólide D.C., Suchecki D., de Carvalho P. et al (2006) Paradoxical sleep deprivation and sleep recovery: effects on the hypothalamic‐pituitary‐adrenal axis activity, energy balance and body composition of rats. J. Neuroendocrinol. 18, 231–238. [DOI] [PubMed] [Google Scholar]
- Huttunen E., Romppanen T. & Helminen H.J. (1981) A histoquantitative study on the effects of castration on the rat ventral prostate lobe. J. Anat. 132, 357–370. [PMC free article] [PubMed] [Google Scholar]
- Juruena M.F., Clearea A.J. & Pariantea C.M. (2006) O eixo hipotálamo‐hipófise‐adrenal, a função dos receptores de glicocorticóides e sua importância na depressão. Rev. Bras. Pisquiatr. 26, 189–201. [DOI] [PubMed] [Google Scholar]
- Kato K., Takeda Y., Nakada T. & Sendo F. (1995) Inhibition by dexamethasone of human neutrophil apoptosis in vitro. Nat. Immun. 14, 198–208. [PubMed] [Google Scholar]
- Kofler R. (2000) The molecular basis of glucocorticoid‐induced apoptosis of lymphoblastic leukemia cells. Histochem. Cell Biol. 114, 1–7. [DOI] [PubMed] [Google Scholar]
- Koutsilieris M., Grondin F. & Lehoux J.G. (1992) The expression of mRNA for glucocorticoid receptor gene and functional glucocorticoid receptors detected in PA‐III rat prostate adenocarcinoma cells. Can. Res. 12, 899–904. [PubMed] [Google Scholar]
- Labeur M. & Holsboer F. (2010) Molecular mechanisms of glucocorticoid receptor signaling. Medicina 70, 457–462. [PubMed] [Google Scholar]
- Lund T.D., Munson D.J., Haldy M.E., Handa R.J. (2004) Dihydrotestosterone may inhibit hypothalamo‐pituitary‐adrenal activity by acting through estrogen receptor in the male mouse. Neurosci.Lett. 365, 43–47. [DOI] [PubMed] [Google Scholar]
- Marieb E.L. & Hoehn K. (2007) Human Anatomy and Physiology. 9th ed. San Francisco, CA: Pearson: Benjamin Cummings; 1264p. [Google Scholar]
- Marker P.C., Donjacour A.A., Dahiya R. & Cunha G.R. (2003) Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 253, 165–174. [DOI] [PubMed] [Google Scholar]
- McPherson S.J., Ellem S.J. & Risbridger G.P. (2008) Estrogen‐regulated development and differentiation of the prostate. Differentiation 76, 660–670. [DOI] [PubMed] [Google Scholar]
- Messmer U.K., Winkel G., Briner V.A. & Pfeilschifter J. (1999) Glucocorticoids potently block tumor necrosis factor‐a and lipopolysaccharide‐induced apoptotic cell death in bovine glomerular endothelial cells upstream of caspase 3 activation. Br. J. Pharmacol. 127, 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J.L., Chen Y., Hamil K. et al (1996) Androgen and glucocorticoid receptors in the stroma and epithelium of prostatic hyperplasia and carcinoma. Clin. Cancer Res. 2, 889–895. [PubMed] [Google Scholar]
- Nishimura K., Nonomura N., Yasunaga Y. et al (2000) Low doses of oral dexamethasone for hormone‐ refractory prostate carcinoma. Am. Cancer Soc. 89, 2570–2576. [DOI] [PubMed] [Google Scholar]
- Nussinovitch U., de Carvalho J.F., Pereira R.M. & Shoenfeld Y. (2010) Glucocorticoids and the cardiovascular system: state of the art. Curr. Pharm. Des. 16, 3574–3585. [DOI] [PubMed] [Google Scholar]
- Pagliacci M.C., Migliorati G., Smacchia M., Grignani F., Riccardi C. & Nicoletti I. (1993) Cellular stress and glucocorticoid hormones protect L929 mouse fibroblasts from tumor necrosis factor a cytotoxicity. J. Endocrinol. Invest. 16, 591–599. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S. & Cidlowski J.A. (2013) Ligand‐induced repression of the glucocorticoid receptor gene is mediated by an ncor1 repression complex formed by long‐range chromatin interactions with intragenic glucocorticoid response elements. Mol. Cell. Biol. 33, 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie P.S., Bowden J.F., Freeman S.N. et al (1989) Cortisol alters gene expression during involution of the rat ventral prostate. Mol. Endocrinol. 4, 1866–1873. [DOI] [PubMed] [Google Scholar]
- Rhen T. & Cidlowski J.A. (2005) Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723. [DOI] [PubMed] [Google Scholar]
- Ribeiro D.L., Rafacho A., Bosqueiro J.R., Taboga S.R. & Góes R.M. (2008) Cellular changes in the prostatic stroma of glucocorticoid‐treated rats. Cell Tissue Res. 332, 499–508. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Berriguete G.R., Fraile B., Bethencourt F.R. et al (2010) Role of IAPs in prostate cancer progression: immunohistochemical study in normal and pathological (benign hyperplastic, prostatic intraepithelial neoplasia and cancer) human prostate. BMC Cancer 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R.W. & Kantoff P.W. (2007) Hormone‐refractory prostate cancer: choosing the appropriate treatment option. Oncology (Williston Park) 21, 185–193. [PubMed] [Google Scholar]
- Runnebaum I.B. & Bruning A. (2005) Glucocorticoids Inhibit Cell Death in Ovarian Cancer and Up‐regulate Caspase Inhibitor cIAP2. Clin. Cancer Res. 6325–6332. [DOI] [PubMed] [Google Scholar]
- Rutz H.P. & Herr I. (2004) Interference of glucocorticoids with apoptosis signaling and host‐tumor interactions. Cancer Biol.Ther. 3, 715–718. [DOI] [PubMed] [Google Scholar]
- Sasson R., Tajima K. & Amsterdam A. (2001) Glucocorticoids Protect against Apoptosis Induced by Serum Deprivation, Cyclic Adenosine 3*,5*‐ Monophosphate and p53 Activation in Immortalized Human Granulosa Cells: Involvement of Bcl‐2. Endocrinology 142, 802–811. [DOI] [PubMed] [Google Scholar]
- Scarano W.R., Sousa D.E., Campos S.G.P., Corradi L.S., Vilamaior P.S.L. & Taboga S.R. (2008) Estrogen supplementation following castration promotes stromal remodeling and histopathological alterations in the gerbil ventral prostate. Int. J. Exp. Pathol. 89, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara F.Z., Silva D.A.L., Zanatelli M. et al (2013) Progesterone restores the female prostate activity in ovariectomized gerbil and may act as competitor of testosterone in intraprostatic environment. Life Sci. 92, 1–14. [DOI] [PubMed] [Google Scholar]
- Simanainen U., Lampinen A., Henneicke H. et al (2011) Long‐Term Corticosterone Treatment Induced Lobe‐Specific Pathology in Mouse Prostate. Prostate 71, 289–297. [DOI] [PubMed] [Google Scholar]
- Smith R.G., Syms A.J. & Norris J.S. (1984) Differential effects of androgens and glucocorticoids on the regulation of androgen receptor concentration and cell growth. J. Steroid Biochem. Mol. Biol. 20, 277–281. [DOI] [PubMed] [Google Scholar]
- Stöhr1 T., Almeida O.F.X., Landgraf R., Shippenbergb T.S., Holsboera F., Spanagela R. (1999) Stress‐ and corticosteroid‐induced modulation of the locomotor response to morphine in rats. Behav. Brain Res. 103, 85–93. [DOI] [PubMed] [Google Scholar]
- Thomas S., Waterman P., Chen S. et al (2011) Development of Secreted Protein and Acidic and Rich in Cysteine (SPARC) Targeted Nanoparticles for the Prognostic Molecular Imaging of Metastatic Prostate Cancer. J. Nanomed. Nanotechnol. 2, 2157–7439‐2‐112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms B.G., Petersen S.L. & VomSaal F.S. (1999) Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses. J. Urol. 161, 1694–1701. [PubMed] [Google Scholar]
- Tornello S., Orti E., De Nicola A.F., Rainbow T.C. & McEwen B.S. (1982) Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology 35, 411–417. [DOI] [PubMed] [Google Scholar]
- Turner B.B. (1992) Sex differences in the binding of type I and type II corticosteroid receptors in rat hippocampus. Brain Res. 581, 229–236. [DOI] [PubMed] [Google Scholar]
- Venâncio D.P., Andersen M.L., Vilamaior P.S.L. et al (2012) Sleep Deprivation Alters Rat Ventral Prostate Morphology, Leading to Glandular Atrophy: A Microscopic Study Contrasted with the Hormonal Assays. J. Biomed. Biotechnol. (10.1155/2012/285938) 2012, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilamaior P.S.L., Felisbino S.R., Taboga S.R. & Carvalho H.F. (2000) Collagen fiber reorganization in the rat ventral prostate following androgen deprivation: a possible role for the smooth muscle cells. Prostate 45, 253–258. [DOI] [PubMed] [Google Scholar]
- Walters M.R. & Clark J.H. (1979) Relationship between the quantity of progesterone receptor and the antagonism of estrogen‐induced uterotropic response. Endocrinology 105, 382–386. [DOI] [PubMed] [Google Scholar]
- Weibel E.R. (1978) Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 12, 131–155. [PubMed] [Google Scholar]
- Wen L.P., Madani K., Fahrni J.A., Duncan S.R. & Rosen G.D. (1997) Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN‐g and Fas. Am. J. Physiol. 273, L921–L929. [DOI] [PubMed] [Google Scholar]
- Witorsch R.J. (2016) Effects of elevated glucocorticoids on reproduction and development: relevance to endocrine disruptor screening. Crit. Rev. Toxicol. 46, 420–436. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fukuda K., Miura N., Suzuki R., Kido T. & Komatsu Y. (1998) Inhibition by dexamethasone of transforming growth factor beta‐1 induced apoptosis in rat hepatoma cells: a possible association with Bcl‐xL induction. Hepatology 27, 959–966. [DOI] [PubMed] [Google Scholar]
- Yemelyanov A., Czwornog J., Chebotaev D. et al (2007) Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene 26, 1885–1896. [DOI] [PubMed] [Google Scholar]
- Zanatelli M., Silva D.A.L., Shinohara F.Z. et al (2013) Actions of oestradiol and progesterone on the prostate in female gerbils: reversal of the histological effects of castration. Reprod. Fertil. Dev. 1, i–ii. [DOI] [PubMed] [Google Scholar]
- Zhao B., Choi J.P., Jaehne M. et al (2014) Glucocorticoid receptor in prostate epithelia is not required for corticosteroid‐induced epithelial hyperproliferation in the mouse prostate. Prostate 74, 1068–1078. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Brown H.N., Zhang Y., Stevens R.G. & Zheng T. (2005) Period 3 structural variation: a circadian biomarker associated with breast cancer in young women. Cancer Epidemiol. Biomarkers Prev. 14(1), 268–270. [PubMed] [Google Scholar]


