Abstract
Toxoplasma gondii is a zoonotic parasite found in vertebrates worldwide for which felids serve as definitive hosts. Despite low densities of felids in northern Canada, Inuit people in some regions show unexpectedly high levels of exposure, possibly through handling and consumption of Arctic wildlife. Free-ranging caribou (Rangifer tarandus) are widely harvested for food across the Canadian North, show evidence of seroexposure to T. gondii, and are currently declining in numbers throughout the Arctic. We experimentally infected three captive reindeer (conspecific with caribou) with 1000, 5000 or 10,000 oocysts of T. gondii via stomach intubation to assess clinical signs of infection, pathology, and tissue distribution. An unexposed reindeer served as a negative control. Signs of stress, aggression, and depression were noted for the first two weeks following infection. By 4 weeks post infection, all infected reindeer were positive on a modified agglutination test at the highest titer tested (1:200) for antibodies to T. gondii. At 20 weeks post infection, no gross abnormalities were observed on necropsy. Following histopathology and immunohistochemistry, tissue cysts were visualized in the reindeer given the highest and lowest dose of oocysts. Focal pleuritis and alveolitis were associated with respiratory problems in reindeer given the middle dose. DNA of T. gondii was detected following traditional DNA extraction and conventional PCR on 25 mg samples from 17/33 muscles and organs, and by magnetic capture DNA extraction from 100 g samples from all 26 tissues examined. This research demonstrated that reindeer/caribou can serve as intermediate hosts for T. gondii, and that the parasite may be associated with health effects in wildlife. The presence of T. gondii in all tissues tested, many of which are commonly consumed raw, smoked, or dried in northern communities, suggests that caribou may serve as a source of human exposure to T. gondii.
Keywords: Experimental infection, Magnetic capture, Reindeer, Toxoplasma
Graphical abstract
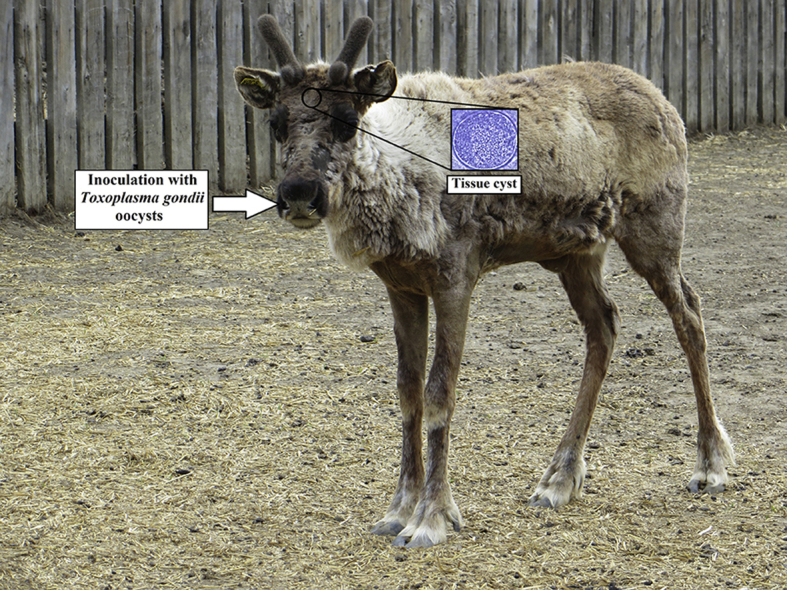
Highlights
-
•
Rangifer sp. are susceptible to Toxoplasma gondii sporulated oocysts.
-
•
Toxoplasma gondii may be associated with detrimental health effect in Rangifer sp.
-
•
Rangifer sp. may serve as a source of human exposure to Toxoplasma gondii.
1. Introduction
The apicomplexan parasite, Toxoplasma gondii, has a worldwide distribution and can be found in virtually all species of warm-blooded animals. Felids, the only known definitive hosts of T. gondii, contribute to environmental contamination by shedding oocysts (Dubey et al., 1970). Exposure to T. gondii is widespread among humans and seroprevalence levels vary widely. While this ubiquitous parasite generally causes no obvious clinical disease in immunocompetent hosts, it can cause clinical syndromes including encephalitis, chorioretinitis and congenital anomalies (Kim and Weiss, 2008). Postnatal infection can occur through the ingestion of oocysts shed by felids in the environment or bradyzoites in animal tissues. Prevention of infection in animal hosts is therefore important to promote food safety, public health, animal welfare, and sustainable livestock production. Many terrestrial animal species harvested for food or fur in the Canadian Arctic have antibodies against T. gondii (Kutz et al., 2000, Elmore et al., 2015). This is enigmatic considering the absence of wild felid definitive hosts above the treeline, and suggests that terrestrial Arctic herbivores are infected when they seasonally migrate further south; for example, Kutz et al. (2001) found a higher seroprevalence levels in mainland caribou compared to High Arctic Island caribou, probably due to their migration below the tree line where free-ranging felids (such as Lynx canadensis) may be shedding oocysts. In other parts of the world, serological evidence for exposure to T. gondii has been found in reindeer from Finland and Norway, which correlate with age and feeding management style (Oksanen et al., 1997).
However, serology provides information on exposure to a pathogen, not whether animals are actively infected or clinical. A single report of toxoplasmosis in experimentally infected reindeer from Fennoscandia described cases of acute enteritis a few days post-intrarumenal inoculation with T. gondii oocysts (Oksanen et al., 1996). Congenital toxoplasmosis has also been documented in a full term stillborn reindeer, leading to fatal toxoplasmosis (Dubey et al., 2002). Other than these two studies, the clinical and pathological effects of T. gondii on Rangifer spp. remain largely unknown.
Some zoonotic parasitoses are relatively common in people in Arctic communities, including T. gondii in the Inuit in Nunavut and Nunavik, Canada, where the seroprevalence is 2–5 times higher than the North American average of ∼15% (Jones et al., 2008, Messier et al., 2009). In Nunavik, toxoplasmosis was epidemiologically associated with consumption of raw caribou, seal meat and skinning of animals for fur (McDonald et al., 1990). Caribou play a crucial role in culture and survival for northern people; for generations, they were the most abundant cervid in Canada (Festa-Bianchet et al., 2011). They are also supporting many predator populations in northern Canada, including wolves, grizzly bears and Arctic foxes (Mowat and Heard, 2006, Musiani et al., 2007, Samelius et al., 2007). Although Rangifer populations historically fluctuated, the species is vulnerable to climate and habitat changes, possibly mediated through factors driving the population dynamics of predators, alternate prey, and parasites or diseases (Vors and Boyce, 2009). At the population level, declining caribou could negatively affect northern indigenous communities socioeconomically since caribou remains the most important terrestrial subsistence resource for these people (Kendrick et al., 2005). At the individual level, caribou represents a potential health risk for humans (or wildlife carnivores) through foodborne transmission of zoonotic pathogens. It is not known, in infected caribou, which organs or muscles contain T. gondii cysts that can be infective for both humans and animals.
Therefore, the first objective of this study was to determine infectivity and tissue distribution of T. gondii in experimentally infected reindeer. Our second objective was to determine whether clinical and pathological effects occur in experimentally infected reindeer. Additionally, we compared serological testing to detect antibodies to T. gondii, and two molecular techniques to detect T. gondii DNA in tissue: traditional DNA extraction vs magnetic capture. Although increasingly applied for food safety testing in Europe, magnetic capture (MC) DNA extraction methods for T. gondii have not been widely used in wildlife yet, despite the potential for greatly increased sensitivity due to the ability to examine large tissue samples (Opsteegh et al., 2010). The comparison of these techniques in experimentally infected reindeer provides information regarding sensitivity of a conventional PCR targeting the 529bp repeat element (Homan et al., 2000) when used with MC technique instead of traditional DNA extraction.
2. Materials and methods
2.1. T. gondii oocysts
Toxoplasma gondii oocysts of the Type III VEG strain obtained from experimentally infected cats, sporulated in 2% sulfuric acid and stored at 4 °C for approximately a year were donated by the Canadian Food Inspection Agency's Centre for Foodborne and Animal Parasitology, Saskatoon, SK, Canada. No viability assay was performed at the time of the reindeer inoculation due to the lack of resources. A few hours before the inoculation, three doses of 1,000, 5000 and 10,000 oocysts were washed free of acid and suspended in 5 ml of saline. These doses were administered by nasogastric intubation to reindeer 1, 2, and 3 respectively.
2.2. Animals
Four captive male reindeer (Rangifer tarandus) three years of age were relocated from the University of Calgary captive herd in December 2014 and maintained in outdoor animal containment facilities at the Alternative Livestock Facility (ALF), Western College of Veterinary Medicine (WCVM) University of Saskatchewan (U of S) for at least 4 months prior to infection. Following blood sampling at the ALF, all animals were negative for IgG antibodies to T. gondii prior to inoculation based on negative findings on modified agglutination test (MAT) at 1:25 dilution (New Life Diagnostic LLC, Carlsbad, CA, United States). Reindeer were fed a mix of alfalfa/hay, beet pulp, and coccidiostat-free Buck (Deer) Ration (Federated Co-Operatives Limited, Saskatoon, SK, Canada) supplemented with selenium. Salt blocks and water were available ad libitum. At inoculation, the weight of each reindeer was estimated to be approximately 150 kg.
All animal use and procedures followed the Guidelines of the Canadian Council on Animal Care, and were reviewed and approved by the U of S Animal Care Committee (UCACS protocol number 20130107).
2.3. Experimental design
A few hours prior to inoculation, three reindeer were transported to Biocontainment level 2 indoor facilities at the Vaccine and Infectious Disease Organization (VIDO), U of S. They were inoculated via naso-gastric tubes (0.05” inner diameter) with T. gondii oocysts suspended in saline (Table 1). They were kept indoors under observation for 72 h post-inoculation (PI), to allow biocontainment of transitory oocysts passed in feces. They were observed for any signs of anorexia, weakness, injected conjunctiva, respiratory distress, nasal discharge, and unusual behavior. Afterwards, the animals were transported back to the ALF where they were housed in a small pen for the first week for close observation. Ten days PI, they were moved to a large fenced pasture of six acres. They were observed daily for 30–60 min for the first week and every 2–3 days afterwards to assess any clinical signs, behavioral changes, and food intake (full, half, or empty trough). Blood samples were collected at week 1, week 4, and week 20 PI, the time of euthanasia. Reindeer 3 had to be euthanized at 16 wks (vs 20 wks) due to welfare reasons unrelated to the experimental infection. A fourth reindeer kept at the ALF died of unrelated causes one week prior to inoculation (enteritis caused by Clostridium perfringens). Tissues (heart, tongue, liver, spleen, lung, kidney, rumen, brain, and biceps femoris) were harvested at necropsy and used as negative controls.
Table 1.
Experimental oral exposure to oocysts of Toxoplasma gondii and serological monitoring of three reindeer (Rangifer tarandus).
| Animal ID | Dose of oocysts | Weeka of necropsy | Post infection serology (MAT) |
||||
|---|---|---|---|---|---|---|---|
| 1:25 | 1:50 | 1:100 | 1:200 | Weeka blood sampled | |||
| Reindeer control | 0 | −1 | – | – | – | – | −1 |
| Reindeer 1 | 1000 | 20 | – | – | – | – | 0 |
| + | + | + | + | 4 | |||
| + | + | + | + | 20 | |||
| Reindeer 2 | 5000 | 20 | – | – | – | – | 0 |
| + | + | + | + | 4 | |||
| + | + | + | + | 20 | |||
| Reindeer 3 | 10,000 | 16 | – | – | – | – | 0 |
| + | + | + | + | 4 | |||
| + | + | + | + | 16 | |||
Relative to inoculation.
2.4. Reindeer anesthesia protocol
Zuclopenthixol acetate (Clopixol® Depot, 200 mg/ml, 1 mg/kg, Lundbeck) was administered via intramuscular hand injection for transport. For manipulations (i.e., de-antlering, blood sampling, oocysts inoculation, and euthanasia), a combination of medetomidine hydrochloride (Domitor®, 40 mg/ml, 0.12 mg/kg) and ketamine hydrochloride (Vetalar®, 100 mg/ml, 2 mg/kg) was administered via darting. Atipamezole hydrochloride (Antisedan®, 5 mg/ml, 0.2 mg/kg, Pfizer) was used to reverse sedation.
2.5. Euthanasia and necropsies
The three inoculated reindeer were sedated and euthanized in the field by intravenous barbiturate overdose of sodium pentobarbital (Euthanyl-Forte®, 540 mg/ml, MTC). They were immediately transported to the WCVM for necropsy. The carcasses were examined for gross abnormalities, and organs and tissues collected (brain, heart, lung, liver, spleen, kidneys, eyes, tongue, diaphragm, and 200 g of 17 different skeletal muscles). Fresh brain squashes (impression smears) were prepared and immediately observed by microscopy at 60× objective lens. Half of each tissue sample was fixed in 10% formalin for histology and immunohistochemistry (IHC). The rest of the samples were frozen at −20 °C for molecular testing.
2.6. Histology and immunohistochemistry
To assess tissue infection status, formalin-fixed tissues from the inoculated reindeer (brain, heart, lung, liver, spleen, kidneys, tongue, diaphragm, triceps, external oblique, gluteus medius, and longissimus) and from the unexposed control reindeer (brain, heart, lung, liver, spleen, kidneys, rumen) were processed into paraffin-embedded tissue blocks, sectioned 5 μm thick, and stained with hematoxylin and eosin (HE). Seven blocks were examined for the control (one organ per block) and 7 blocks for each infected reindeer (2 organs/tissues per block excepted brain and heart with one block each). For IHC, 5 μm sections of the same 28 paraffin-embedded blocks were cut and processed by Prairie Diagnostic Services, SK, Canada as previously described (Cruickshank et al., 1990) with some modifications. Briefly, epitope retrieval was performed using a proteinase K treatment and any binding of the primary antibodies (rabbit anti-Toxoplasma gondii (1:100), ThermoFisher Scientific, Waltham, MA, USA) was detected using an avidin-biotin HRP-labelled detection reagent (Vector Labs, Burlingame, CA, USA) on an automated slide stainer (Autostainer Plus, Dako Canada Inc., Mississauga, ON).
2.7. Serology
Sera of each reindeer were tested for anti-T. gondii IgG using a commercially available modified agglutination test (MAT) (New Life Diagnostic LLC, Carlsbad, CA, United States) (Al-Adhami et al., 2016). The kit was used according to the manufacturer instructions, at four dilutions (1:25; 1:50; 1:100; and 1:200), with 1:25 as a cut-off for seropositivity. The positive and negative controls used were the one included with the test. Questionable results consisting of mild agglutination covering less than half of the test well were considered negatives.
2.8. Traditional DNA extraction
DNA extractions were performed on 25 mg of each of 10 tissues (Table 2) for infected reindeer. Heart, tongue, and biceps femoris of the unexposed control reindeer were also tested. Each sample of 25 mg was frozen at −80C for 30min and thawed in a 97 °C water bath for 15min, repeated twice. Samples were analysed via the DNeasy® Blood and Tissue Kit (Qiagen, Toronto, Canada) according to the manufacturer's instructions. Mice brain infected with T. gondii oocysts was used as a positive control.
Table 2.
Detection of DNA of Toxoplasma gondii in organs and muscles of experimentally exposed reindeer using two DNA extraction methods.
| Traditional DNA extraction (25 mg) | Magnetic capture (100 g) | ||
|---|---|---|---|
| Reindeer control | Heart | – | nt |
| Tongue | – | nt | |
| Biceps femoris | – | – | |
| Reindeer 1 (1000 oocysts) |
Brain | + | + |
| Heart | – | + | |
| Diaphragm | + | + | |
| Lung | + | + | |
| Tongue | – | nt | |
| Biceps femoris | + | + | |
| Gluteus medius | – | + | |
| Longissimus | + | + | |
| External oblique | – | + | |
| Triceps | + | + | |
| Reindeer 2 (5000 oocysts) |
Brain | – | + |
| Heart | – | + | |
| Diaphragm | – | + | |
| Lung | – | + | |
| Tongue | – | nt | |
| Biceps femoris | – | nt | |
| Gluteus medius | – | + | |
| Longissimus | + | + | |
| External oblique | + | + | |
| Triceps | + | + | |
| Reindeer 3 (10,000 oocysts) |
Brain | + | + |
| Heart | – | + | |
| Diaphragm | + | + | |
| Lung | + | + | |
| Tongue | + | nt | |
| Biceps femoris | + | + | |
| Gluteus medius | + | + | |
| Longissimus | – | + | |
| External oblique | + | + | |
| Triceps | + | + |
+: positive –: negative nt: not tested.
2.9. Magnetic capture DNA extraction
Magnetic capture (MC), as per Opsteegh et al. (2010), was performed on 100 g of each of 9 tissues (Table 2) for infected reindeer. For the uninfected reindeer, only biceps femoris was tested with MC technique as it was the only meat sample having enough grams to be tested. In each run of the protocol, a negative and two spiked beef samples (positive controls) were included. The concentration of the undiluted T. gondii tachyzoite-stock used for spiking was 2.5 × 106/ml. A 10-times dilution series was made in ultrapure water to obtain 2.5 × 105 and 2.5 × 104/ml. For positive controls, 100 μl of these dilutions was added to 100 g of beef samples, resulting in samples spiked with 2500 and 25,000 tachyzoites. Prior to spiking, fluid extracted from the beef muscle tissue was tested by MAT to confirm negativity for antibodies to T. gondii, with negative and positive controls from the test.
2.10. PCR amplification
A conventional PCR using the primers TOX4 and TOX5 described by Homan et al. (2000) was performed on DNA extracted from tissue samples using both the traditional and magnetic DNA extraction techniques. Each PCR run included brain of mice infected with T. gondii oocysts and genomic DNA of T. gondii as positive controls, and PCR negative control for assessing contamination. Controls from the two techniques were tested as well.
Optimization of the PCR assay (hybridization temperature for primer annealing, MgCl2, DNA Taq polymerase and primer concentrations) was performed using T. gondii DNA extracted from experimentally infected reindeer to enhance the sensitivity and specificity of the reaction (Chabbert et al., 2004, Bastien et al., 2008). To minimise the possibility that false-negative were obtained, all negative samples were tested twice using primer sets targeting the internal positive-control, the species-specific reference gene, to assess the quality of DNA extracted.
Following gel observation under ultraviolet light, DNA from suspected-positive bands was purified using a QIAquick PCR Purification Kit (Qiagen, Toronto, Canada) and sent for commercial DNA sequencing (Macrogen, Korea). The resulting sequences were subjected to nucleotide BLAST analysis.
2.11. DNA characterization
2.11.1. GRA6 DNA extraction
For each infected reindeer, 12 ml of crude extract from three positive samples on conventional PCR following the MC technique (brain, heart, and diaphragm) were taken from back-up solution kept frozen at - 20 °C to capture the GRA6 gene following the same MC protocol except that 15 pmol of GRA6-CapF and GRA6-CapR were used rather than 10 pmol (Opsteegh et al., 2010). Approximately 45–50 μL of template DNA were sent within 24 h on dry ice to the National Reference Centre for Parasitology, Research Institute of the McGill University Health Centre, Montreal, QC, Canada for further genetic characterization.
2.11.2. PCR RFLP amplification
The GRA6 gene was amplified according to Zakimi et al. (2006). PCR amplification was performed with 2 μl of DNA template in 50 μl PCR reaction mixture containing, 5× GoTaq Flexi buffer (Promega), 2 mM MgCl2, 50 pmol of each primer, 0.2 mM of each deoxynucleotide triphosphate, and 1.25 U of Taq DNA polymerase. The resulting amplification product was used as a template in the secondary PCR using the internal primers described by Fazaeli et al. (2000), and using 2 μl primary PCR product as the template at an annealing temperature of 60 °C and extension for 2 min with 35 cycles. Five μl of each amplicon was run in a 1.5% agarose gel containing GelRed at 120 V for 40 min with 1 × TE buffer and visualized under Ultraviolet light.
The unpurified PCR product was sequenced at McGill University and Génome Québec Innovation Centre, Montreal, QC, Canada. The Nucleotide sequences were applied to a Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov/blast) to get the 100% similarity with the GRA6 sequences deposited in the GenBank.
RFLP analyses were performed on PCR positive samples to determine the molecular types. GRA6 positive amplicons were incubated with the MseI enzyme according to the manufacturer's instructions (New England BioLabs). The digested PCR products were visualized by electrophoresis on 1.6% agarose gel containing Gel Red.
2.12. Data analysis
All tests performed were read by the same person. The reader was blinded in between assays regarding the infection status of animals.
3. Results
3.1. Serostatus
The serum samples collected before inoculation from the 4 reindeer were all seronegative. The 3 experimentally infected reindeer demonstrated seropositivity at 4 dilutions (1:25; 1:50; 1:100; and 1:200) 4 weeks after inoculation and remained seropositive at time of euthanasia (Table 1).
3.2. Symptoms
Following infection, stress (pacing, not eating) and aggressiveness (pushing away other reindeer) were noted for reindeer 1 and 3 for about 2 weeks. Reindeer 2 self-isolated from the others, and frequently exhibited panting, pacing, and dragging his feet. Behavior returned to normal for all reindeer in the following weeks.
3.3. Necropsy, histopathology and immunohistochemistry
Post-mortem examination of infected reindeer did not show any gross abnormalities. A single T. gondii tissue cyst was observed on squash mount of the brain of reindeer 2 at necropsy (Fig. 1). Toxoplasma gondii cysts were visualized by histopathology and immunohistochemistry on tongue, diaphragm, external oblique, and triceps of reindeer 3 (Fig. 2), and on external oblique and gluteus medius in reindeer 1. Focal to coalescing chronic fibrinous eosinophilic histiocytic pleuritis, with mild focal eosinophilic alveolitis were observed in reindeer 2. No histological changes were detected in the unexposed control reindeer on heart, liver, spleen, lung, kidney, rumen, and brain.
Fig. 1.
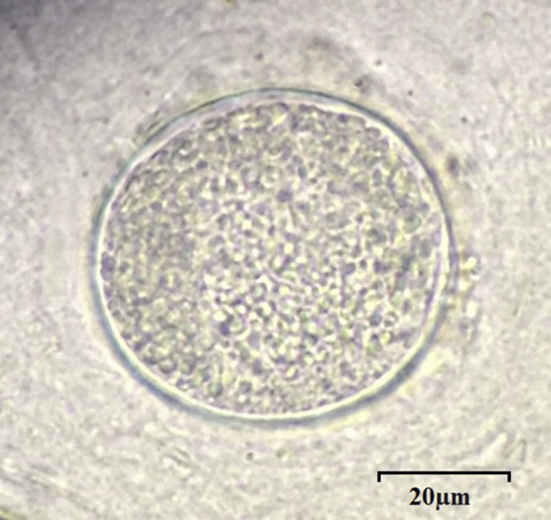
Brain squash picture of T. gondii tissue cyst of reindeer 2 brain visualized on compound microscopy (60×).
Fig. 2.
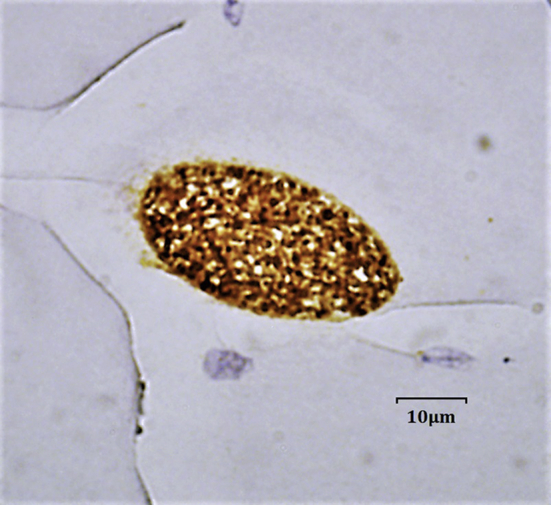
Histological section of reindeer 3 diaphragm containing a T. gondii cyst visualized at 100× with oil immersion after immunohistochemical stain.
3.4. Toxoplasma gondii detection in tissues and organs
Following traditional (kit based) DNA extraction, T. gondii was detected by PCR in 3–8 tissues from each infected reindeer (Table 2); heart was negative for all 3 reindeer and triceps was the only tissue that tested positive in all three reindeer. Following MC DNA extraction, DNA of T. gondii was detected in all tissue samples tested (Table 2). Sequence analysis indicated 99% identity with T. gondii Type III VEG strain. For those tissues tested using both techniques (n = 26), the proportion of positive tissues among all tissues tested by traditional DNA extraction was 61.5% (16/26), and 100% (26/26) for MC. For traditional DNA extraction, positive controls of infected mice brain gave positive results, as well as the spiked beef sample for MC. Genotyping of MC positive samples (brain, heart, diaphragm) of each of the exposed reindeer were identified as T. gondii Type III VEG strain, the same strain given at inoculation.
No evidence of T. gondii was detected after serological and molecular testing of tissues from the unexposed control reindeer. The lack of personnel, time, material, and changes in the protocol after the euthanasia of the unexposed control reindeer explained the difference in number and quantity of samples collected and tested compared to the exposed ones.
4. Discussion
This study reports the first experimental infection in reindeer inoculated orally with oocysts of T. gondii Type III via nasogastric tube, and successfully established subclinical infections. Oral exposure reflects the suspected natural route of infection: i.e. by consuming oocysts in contaminated feed or water. Minor behavioral changes observed for a few days following infection may reflect changes in housing and stress due to handling. Our observations demonstrated that reindeer are suitable intermediate hosts for T. gondii and susceptible to sporulated oocysts. The only other experimental infection reported in reindeer resulted in a marked acute rumenitis associated with intrarumenal inoculation of 5000 and 50,000 oocysts of T. gondii in 2 yearling reindeer (Oksanen et al., 1996) with the type II ME-49 strain (Dubey et al., 1995). With the strain used in our work (VEG III strain), subclinical infections were previously successfully achieved with other animal species (Gajadhar et al., 2004, Forbes et al., 2012). Forbes et al. (2012) detected infection 7–14 days after inoculating pigs with doses as low as 10 to 100 oocysts, although lethal effects have been reported in BALB/c mice at doses ranging from 10 to 1000 oocysts (Dubey et al., 2012). It is thus possible that the differences in pathology observed between our study and the previous reindeer experimental study were due to differences in dose of oocysts (1000–10,000 vs 5000 and 50,000), strain virulence of T. gondii (VEG vs ME-49), method of inoculation (intubation vs intrarumenal), and age of the host (adult vs yearling).
Even though reindeer in our study seemed to tolerate infection well, the behavioral and respiratory changes that we observed could have important implications in wild populations of caribou, which are subject to chase predation by wolves. In intermediate hosts, it is long recognized that T. gondii-infected rodents have an increase risk of predation by exhibiting higher activity and a decreasing in predator vigilance behavioral traits (Hay et al., 1983). Toxoplasma gondii localization in the central nervous system of intermediate hosts may facilitate both escape from host immunity as well as manipulation of host behavior. Increase risk of predation in sea otters by sharks has also been described following infection with the parasite (Miller et al., 2004). Subtle behavioral changes associated with toxoplasmosis could predispose wild caribou to predation or act synergistically with stress from other sources. Toxoplasmosis may also have an impact on fetuses of acutely infected dams. With caribou populations already in decline (Vors and Boyce, 2009), T. gondii could affect calving success and health.
Histological evidence of chronic eosinophilic inflammation in the lungs of reindeer 2 might explain observations of panting and depression in this animal. Lung inflammation following T. gondii infection has been described in the literature (Dubey, 2010). No histopathological changes were associated with T. gondii seen on IHC. We noted more tissue cysts in the reindeer given the highest dose of oocysts, consistent with earlier observations with the VEG strain of T. gondii (Dubey et al., 1996). Low sensitivity of detection of tissue cysts on histopathology and IHC was expected, as only a small amount of tissue can be fixed on one slide and as few as one tissue cyst can be present in an entire organ.
We detected T. gondii DNA in all tissues examined from infected reindeer using MC as compared to only 62% of tissues from which DNA was extracted using a conventional kit. This likely reflects the larger amount of tissue (100 g) examined in the MC technique, as compared to 25 mg of tissue. We suggest the use of MC technique when sufficient amounts of tissue are available, and as a useful screening tool in exposure assessment in wildlife and other animals intended for human consumption. In a previous study, this technique has shown an equal sensitivity compared with the mouse bioassay, which is often considered to be the gold standard for detection of T. gondii in meat samples (Garcia et al., 2006). Thus, the MC DNA extraction technique in combination with PCR can be a valuable alternative to mouse bioassay, which is time consuming and ethically challenging.
We found a wide distribution of the parasites in multiple organs and muscles, even in the reindeer infected with the lowest dose. This suggests that the doses we used were high relative to natural infections, where only a few organs and tissues might harbour cysts. Experimentally infected animals have less variation in infectious dose, time after infection, and host and parasite genetics than with naturally infected animals (Opsteegh et al., 2010). Therefore, the presence of T. gondii in multiple tissues following experimental infection might not reflect the distribution of the parasite in naturally infected caribou in the wild. In our study, we used a terrestrial Type III VEG strain administered orally to establish subclinical infection and avoid acute toxoplasmosis.
Caribou migrating south might be exposed to different strains, of varying virulence, and experience different clinical signs, but there is no well documented report of natural clinical toxoplasmosis in Rangifer, which could reflect relatively low levels of environmental contamination with T. gondii oocysts throughout their migration range. However, a high prevalence of antibodies (37%) has been detected in mainland caribou that migrate into boreal regions where lynx may be present (Kutz et al., 2001), suggesting relatively high levels of exposure. Since cats infected for the first time can shed 100's of millions potentially infectious oocysts during peak patency, environmental contamination can be high in specific areas. Levels of contamination can vary greatly along the urban-rural-wild gradient (Gilot-Fromont et al., 2012). One study from the Morro Bay area in California estimated the annual burden in the environment between 94 and 4671 oocysts/m2 (Dabritz et al., 2007). Another study in France estimated rate of soil contamination by T. gondii oocysts between 31 and 3600 oocysts/m2 per year (Afonso et al., 2010).
In this experimental infection study, we detected the parasites in multiple muscles and organs commonly consumed by subsistence harvesters in northern communities. Caribou is consumed in every Inuit community across Canada, with preferences depending on the region. As a nutrient dense food, practically all parts of caribou are traditionally consumed including meat, milk, organs, blood, and bone marrow (Meis Mason et al., 2007). Inuit in Canada's north traditionally relied on “country food” (hunting caribou, seal, fish, wild berries etc.). However, modernization and the high cost of hunting are changing Inuit eating habits, with store-bought foods being increasingly popular in Inuit communities (Sheikh et al., 2011). Nevertheless, traditional foods remain frequently consumed, highly preferred, and an integral part of the social and cultural life (Meis Mason et al., 2007). In these regions, meat and organs are often eaten raw, dried, or smoked (Messier et al., 2009). Consumption of muscles (legs, shoulder, ribs, rump, front end, breastbone, backbone), fat, tongue, head, stomach content, heart, kidney, and liver has been demonstrated (Condon et al., 1995, Wein et al., 1996). Therefore, further work is needed to determine the tissue infection status and tissue cysts viability in caribou harvested for human consumption.
Finally, since it was a small study with only three exposed animals, more data would be needed to validate the results. Future work on the public health significance could include characterizing tissue distribution of T. gondii in naturally infected caribou as well as determining the effects of traditional methods of food preparation on viability of tissue cysts for a holistic understanding of the epidemiology and clinical significance of toxoplasmosis in free-ranging caribou. From a wildlife health and conservation viewpoint, further work is needed to determine the effects of changing climate and habitat on the transmission of T. gondii in the Canadian North.
Conflict of interest
None.
Acknowledgements
We thank the University of Calgary for providing reindeer, staff at the Alternative Livestock Facility for housing the animals, the Canadian Food Inspection Agency's Centre for Food-borne and Animal Parasitology for providing T. gondii oocysts, and the Vaccine and Infectious Disease Organization for helping with inoculation. We thank B. Blackmore, S. Walker, B. Ambros, T. Shury for help with the experimental design; and C. Fernando, B. Wagner, A. Iqbal, and A. Gupta for laboratory assistance. This study was supported by the Natural Science and Engineering Research Council (424278-2012-RGPNS and 386666-2012-RGPIN), Western College of Veterinary Medicine Wildlife Health Fund and Interprovincial Graduate Student Fund, the University of Saskatchewan, and the Canadian Foundation for Innovation Leaders Opportunity Fund (23105) for the Zoonotic Parasite Research Unit.
References
- Afonso E., Thulliez P., Gilot-Fromont E. Local meteorological conditions, dynamics of seroconversion to Toxoplasma gondii in cats (Felis catus) and oocyst burden in a rural environment. Epidemiol. Infect. 2010;138:1105–1113. doi: 10.1017/S0950268809991270. [DOI] [PubMed] [Google Scholar]
- Al-Adhami B.H., Simard M., Hernández-Ortiz A., Boireau C., Gajadhar A.A. Development and evaluation of a modified agglutination test for diagnosis of Toxoplasma infection using tachyzoites cultivated in cell culture. Food Waterborne Parasitol. 2016;2:15–21. [Google Scholar]
- Bastien P., Procop G.W., Reischl U. Quantitative real-time PCR is not more sensitive than “conventional” PCR. J. Clin. Microbiol. 2008;46:1897–1900. doi: 10.1128/JCM.02258-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert E., Lachaud L., Crobu L., Bastien P. Comparison of two widely used PCR primer systems for detection of Toxoplasma in amniotic fluid, blood, and tissues. J. Clin. Microbiol. 2004;42:1719–1722. doi: 10.1128/JCM.42.4.1719-1722.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon R.G., Collings P., Wenzel G. The best part of life: subsistence hunting, ethnicity, and economic adaptation among young adult Inuit males. Arctic. 1995;48:31–46. [Google Scholar]
- Cruickshank J.J., Haines D.M., Palmer N.C., St Aubin D.J. Cysts of a Toxoplasma-like organism in an Atlantic bottlenose dolphin. Can. Vet. J. 1990;31:213–215. [PMC free article] [PubMed] [Google Scholar]
- Dabritz H.A., Miller M.A., Atwill E.R., Gardner I.A., Leutenegger C.M., Melli A.C., Conrad P.A. Detection of Toxoplasma gondii-like oocysts in cat feces and estimates of the environmental oocyst burden. J. Am. Vet. Med. Assoc. 2007;231:1676–1684. doi: 10.2460/javma.231.11.1676. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. second ed. CRC Press; 2010. Toxoplasmosis of Animals and Humans; p. 313. [Google Scholar]
- Dubey J.P., Ferreira L.R., Martins J., McLeod R. Oral oocyst-induced mouse model of toxoplasmosis: effect of infection with Toxoplasma gondii strains of different genotypes, dose, and mouse strains (transgenic, out-bred, in-bred) on pathogenesis and mortality. Parasitology. 2012;139:1–13. doi: 10.1017/S0031182011001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey J.P., Goodwin M.A., Ruff M.D., Shen S.K., Kwok O.C., Wizlkins G.L., Thulliez P. Experimental toxoplasmosis in chukar partridges (Alectoris graeca) Avian. Pathol. 1995;24:95–107. doi: 10.1080/03079459508419051. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Lewis B., Beam K., Abbitt B. Transplacental toxoplasmosis in a reindeer (Rangifer tarandus) fetus. Vet. Parasitol. 2002;110:131–135. doi: 10.1016/s0304-4017(02)00320-5. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Lunney J.K., Shen S.K., Kwok O.C., Ashford D.A., Thulliez P. Infectivity of low numbers of Toxoplasma gondii oocysts to pigs. J. Parasitol. 1996;82:438–443. [PubMed] [Google Scholar]
- Dubey J.P., Miller N.L., Frenkel J.K. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 1970;132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S.A., Samelius G., Fernando C., Alisauskas R.T., Jenkins E.J. Evidence for Toxoplasma gondii in migratory vs. nonmigratory herbivores in a terrestrial arctic ecosystem. Can. J. Zool. 2015;93:671–675. [Google Scholar]
- Fazaeli A., Carter P.E., Darde M.L., Pennington T.H. Molecular typing of Toxoplasma gondii strains by GRA6 gene sequence analysis. Int. J. Parasitol. 2000;30:637–642. doi: 10.1016/s0020-7519(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Festa-Bianchet M., Ray J.C., Boutin S., Côté S.D., Gunn A. Conservation of caribou (Rangifer tarandus) in Canada: an uncertain future. Can. J. Zool. 2011;89:419–434. [Google Scholar]
- Forbes L.B., Parker S.E., Gajadhar A.A. Performance of commercial ELISA and agglutination test kits for the detection of anti-Toxoplasma gondii antibodies in serum and muscle fluid of swine infected with 100, 300, 500 or 1000 oocysts. Vet. Parasitol. 2012;190:362–367. doi: 10.1016/j.vetpar.2012.06.040. [DOI] [PubMed] [Google Scholar]
- Gajadhar A.A., Measures L., Forbes L.B., Kapel C., Dubey J.P. Experimental Toxoplasma gondii infection in grey seals (Halichoerus grypus) J. Parasitol. 2004;90:255–259. doi: 10.1645/GE-144R. [DOI] [PubMed] [Google Scholar]
- Garcia J.L., Gennari S.M., Machado R.Z., Navarro I.T. Toxoplasma gondii: detection by mouse bioassay, histopathology, and polymerase chain reaction in tissues from experimentally infected pigs. Exp. Parasitol. 2006;113:267–271. doi: 10.1016/j.exppara.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Gilot-Fromont E., Lélu M., Dardé M.-L., Richomme C., Aubert D., Afonso E., Mercier A., Gotteland C., Villena I. The life cycle of Toxoplasma gondii in the natural environment. In: Djurkovic-Djakovic O., editor. Toxoplasmosis – Recent Advances. InTech; Rijeka: 2012. pp. 1–36. [Google Scholar]
- Hay J., Hutchison W.M., Aitken P.P., Graham D.I. The effect of congenital and adult-acquired Toxoplasma infections on activity and responsiveness to novel stimulation in mice. Ann. Trop. Med. Parasitol. 1983;77:483–495. doi: 10.1080/00034983.1983.11811741. [DOI] [PubMed] [Google Scholar]
- Homan W.L., Vercammen M., De Braekeleer J., Verschueren H. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 2000;30:69–75. doi: 10.1016/s0020-7519(99)00170-8. [DOI] [PubMed] [Google Scholar]
- Jones J.L., Kruszon-Moran D., Won K., Wilson M., Schantz P.M. Toxoplasma gondii and Toxocara spp. co-infection. Am. J. Trop. Med. Hyg. 2008;78:35–39. [PubMed] [Google Scholar]
- Kendrick A., Lyver P.O.B., Dene first nation, L.K'E Denesoline (Chipewyan) knowledge of barren-ground caribou (Rangifer tarandus groenlandicus) movements. Arctic. 2005;58:175–191. [Google Scholar]
- Kim K., Weiss L.M. Toxoplasma: the next 100 years. Microbes Infect. 2008;10:978–984. doi: 10.1016/j.micinf.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz S.J., Elkin B., Gunn A., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in muskox (Ovibos moschatus) sera from northern Canada. J. Parasitol. 2000;86:879–882. doi: 10.1645/0022-3395(2000)086[0879:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Elkin B.T., Panayi D., Dubey J.P. Prevalence of Toxoplasma gondii antibodies in barren-ground caribou (Rangifer tarandus groenlandicus) from the Canadian Arctic. J. Parasitol. 2001;87:439–442. doi: 10.1645/0022-3395(2001)087[0439:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McDonald J.C., Gyorkos T.W., Alberton B., MacLean J.D., Richer G., Juranek D. An outbreak of toxoplasmosis in pregnant women in northern Quebec. J. Infect. Dis. 1990;161:769–774. doi: 10.1093/infdis/161.4.769. [DOI] [PubMed] [Google Scholar]
- Meis Mason A., Dana L.-P., Anderson R. The Inuit commercial caribou harvest and related agri-food industries in Nunavut. Int. J. Entrepreneursh. Small Bus. 2007;4:785–806. [Google Scholar]
- Messier V., Levesque B., Proulx J.F., Rochette L., Libman M.D., Ward B.J., Serhir B., Couillard M., Ogden N.H., Dewailly E., Hubert B., Dery S., Barthe C., Murphy D., Dixon B. Seroprevalence of Toxoplasma gondii among Nunavik Inuit (Canada) Zoonoses Public Health. 2009;56:188–197. doi: 10.1111/j.1863-2378.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- Miller M.A., Grigg M.E., Kreuder C., James E.R., Melli A.C., Crosbie P.R., Jessup D.A., Boothroyd J.C., Brownstein D., Conrad P.A. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Mowat G., Heard D.C. Major components of grizzly bear diet across North America. Can. J. Zool. 2006;84:473. [Google Scholar]
- Musiani M., Leonard J.A., Cluff H.D., Gates C.C., Mariani S., Paquet P.C., Vila C., Wayne R.K. Differentiation of tundra/taiga and boreal coniferous forest wolves: genetics, coat colour and association with migratory caribou. Mol. Ecol. 2007;16:4149–4170. doi: 10.1111/j.1365-294X.2007.03458.x. [DOI] [PubMed] [Google Scholar]
- Oksanen A., Åsbakk K., Nieminen M., Norberg H., Näreaho A. Antibodies against Toxoplasma gondii in Fennoscandian reindeer - association with the degree of domestication. Parasitol. Int. 1997;46:255–261. [Google Scholar]
- Oksanen A., Gustafsson K., Lunden A., Dubey J.P., Thulliez P., Uggla A. Experimental Toxoplasma gondii infection leading to fatal enteritis in reindeer (Rangifer tarandus) J. Parasitol. 1996;82:843–845. [PubMed] [Google Scholar]
- Opsteegh M., Langelaar M., Sprong H., den Hartog L., De Craeye S., Bokken G., Ajzenberg D., Kijlstra A., van der Giessen J. Direct detection and genotyping of Toxoplasma gondii in meat samples using magnetic capture and PCR. Int. J. Food. Microbiol. 2010;139:193–201. doi: 10.1016/j.ijfoodmicro.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Samelius G., Alisauskas R.T., Hobson K.A., Larivière S. Prolonging the arctic pulse: long-term exploitation of cached eggs by arctic foxes when lemmings are scarce. J. Anim. Ecol. 2007;76:873–880. doi: 10.1111/j.1365-2656.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Sheikh N., Egeland G.M., Johnson-Down L., Kuhnlein H.V. Changing dietary patterns and body mass index over time in Canadian Inuit communities. Int. J. Circumpolar Health. 2011;70:511–519. doi: 10.3402/ijch.v70i5.17863. [DOI] [PubMed] [Google Scholar]
- Vors L.S., Boyce M.S. Global declines of caribou and reindeer. Glob. Chang. Biol. 2009;15:2626–2633. [Google Scholar]
- Wein E.E., Freeman M.M., Makus J.C. Use of and preference for traditional foods among the Belcher Island Inuit. Arctic. 1996;49:256–264. [Google Scholar]
- Zakimi S., Kyan H., Oshiro M., Sugimoto C., Xuenan X., Fujisaki K. Genetic characterization of GRA6 genes from Toxoplasma gondii from pigs in Okinawa, Japan. J. Vet. Med. Sci. 2006;68:1105–1107. doi: 10.1292/jvms.68.1105. [DOI] [PubMed] [Google Scholar]


