Abstract
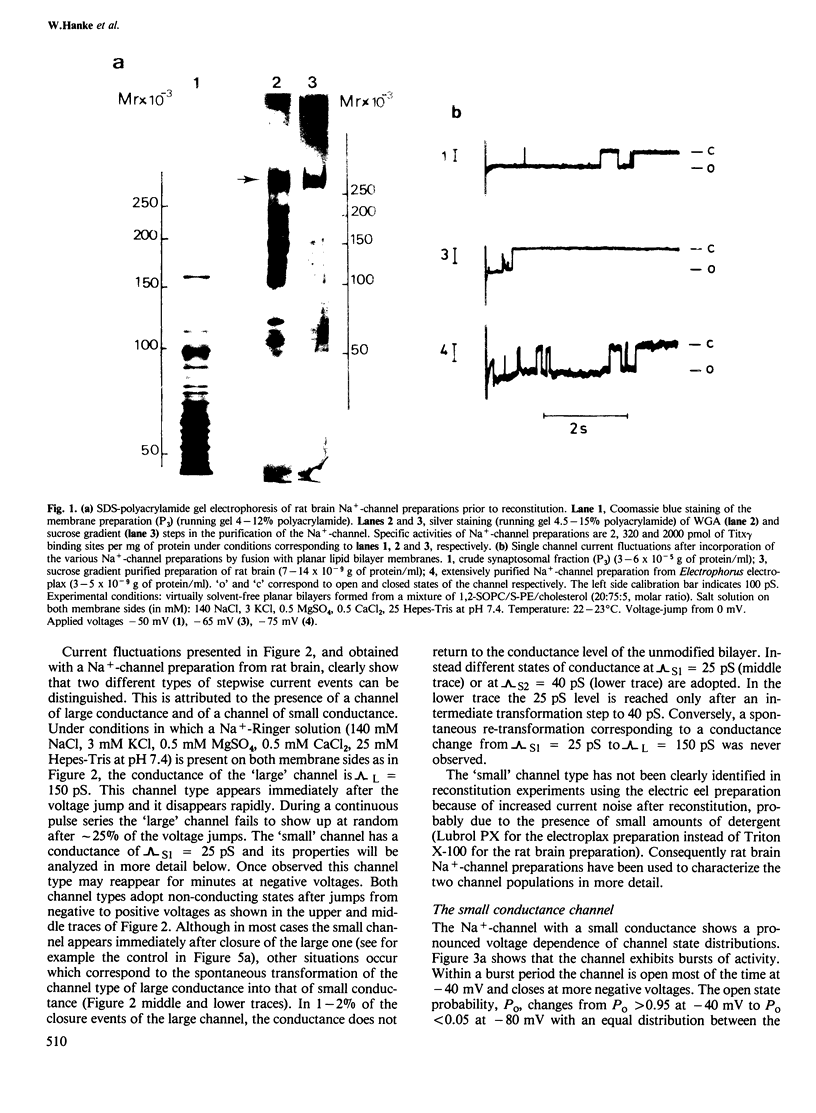
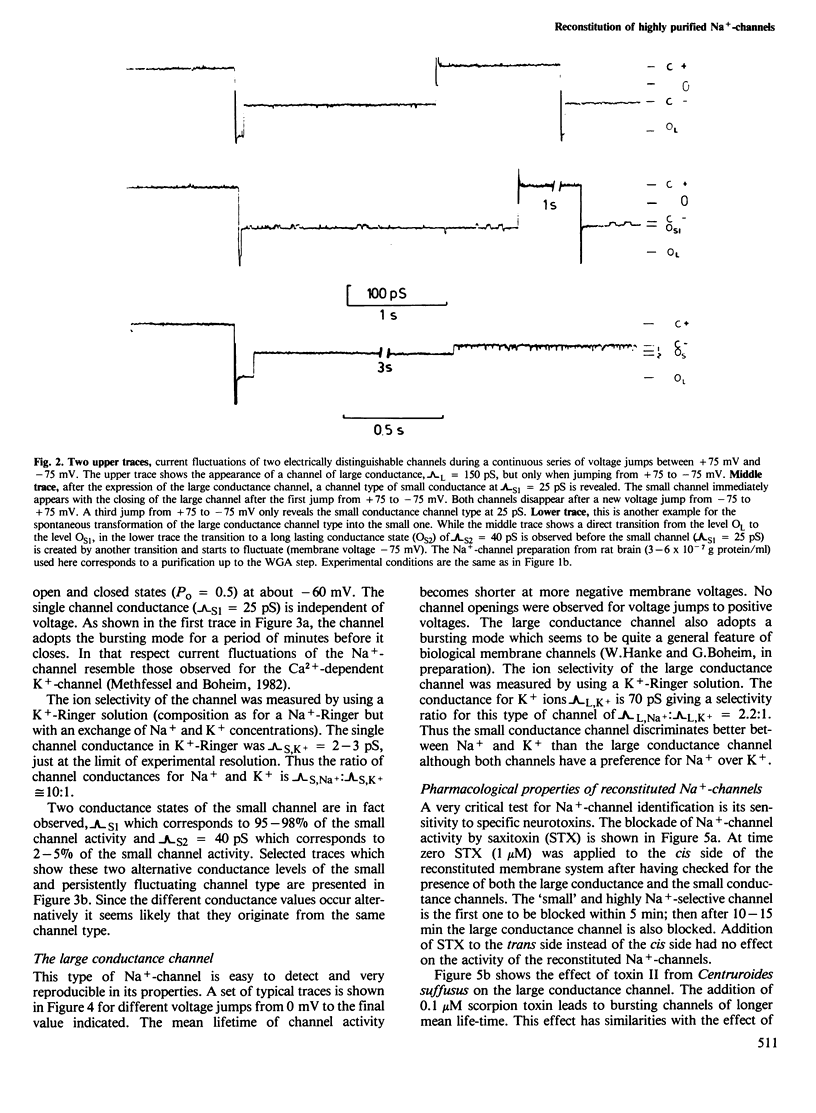
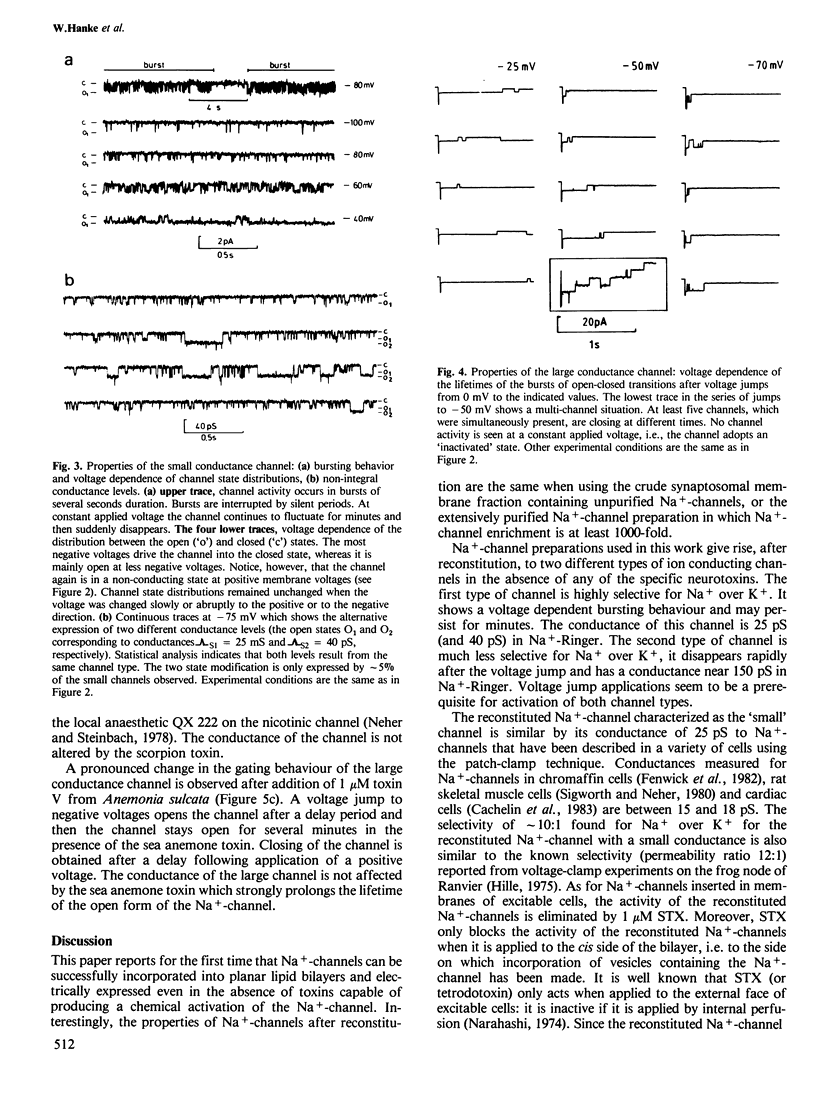
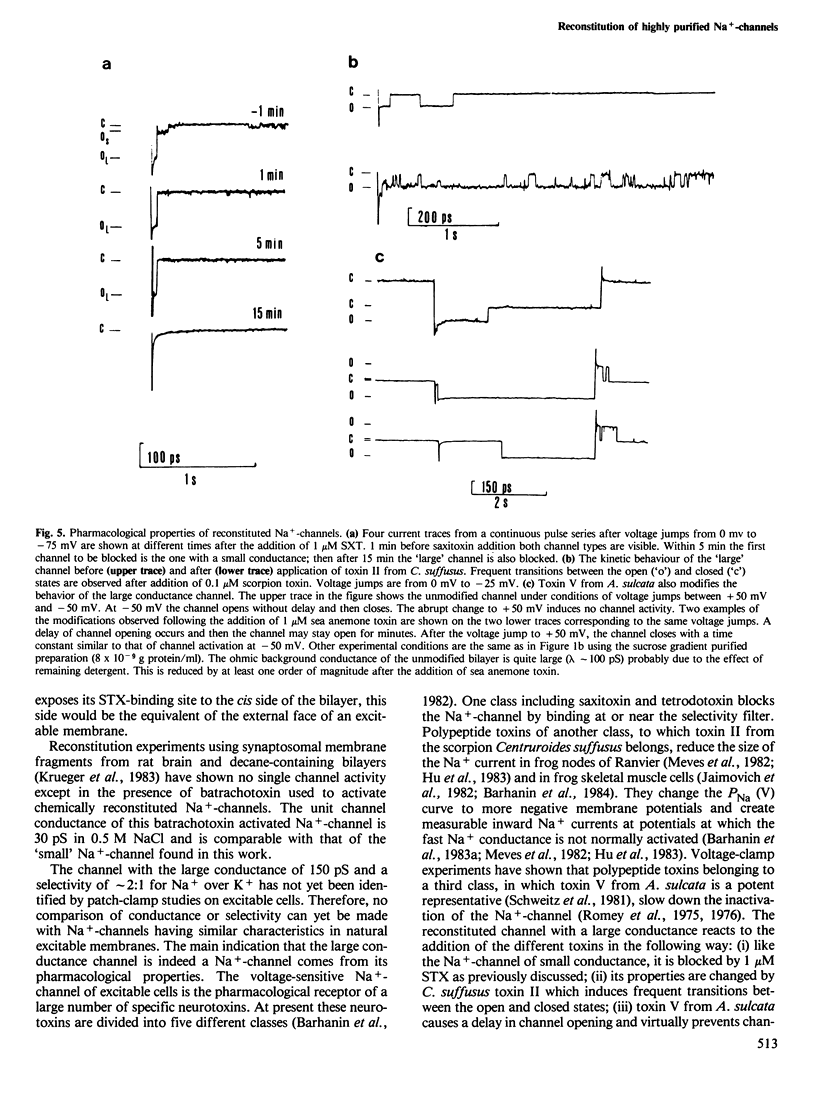
Highly purified Na+-channels isolated from rat brain have been reconstituted into virtually solvent-free planar lipid bilayer membranes. Two different types of electrically excitable channels were detected in the absence of any neurotoxins. The activity of both channels was blocked by saxitoxin. The first channel type is highly selective for Na+ over K+ (approximately 10:1), it shows a bursting behavior, a conductance of 25 pS in Na+-Ringer and undergoes continuous opening and closing events for periods of minutes within a defined range of negative membranes voltages. The second channel type has a conductance of 150 pS and a lower selectivity for Na+ and K+ (2.2:1); only a few opening and closing events are observed with this channel after one voltage jump. The latter type of channel is also found with highly purified Na+-channel from Electrophorus electricus electroplax. A qualitative analysis of the physicochemical and pharmacological properties of the high conductance channel has been carried out. Channel properties are affected not only by saxitoxin but also by a scorpion (Centruroides suffusus suffusus) toxin and a sea anemone (Anemonia sulcata) toxin both known to be selective for the Na+-channel. The spontaneous transformation of the large conductance channel type into the small one has been considered; the two channel types may represent the expression of activity of different conformational states of the same protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barchi R. L., Cohen S. A., Murphy L. E. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J., Giglio J. R., Léopold P., Schmid A., Sampaio S. V., Lazdunski M. Tityus serrulatus venom contains two classes of toxins. Tityus gamma toxin is a new tool with a very high affinity for studying the Na+ channel. J Biol Chem. 1982 Nov 10;257(21):12553–12558. [PubMed] [Google Scholar]
- Barhanin J., Ildefonse M., Rougier O., Sampaio S. V., Giglio J. R., Lazdunski M. Tityus gamma toxin, a high affinity effector of the Na+ channel in muscle, with a selectivity for channels in the surface membrane. Pflugers Arch. 1984 Jan;400(1):22–27. doi: 10.1007/BF00670531. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Pauron D., Lombet A., Norman R. I., Vijverberg H. P., Giglio J. R., Lazdunski M. Electrophysiological characterization, solubilization and purification of the Tityus gamma toxin receptor associated with the gating component of the Na+ channel from rat brain. EMBO J. 1983;2(6):915–920. doi: 10.1002/j.1460-2075.1983.tb01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lombet A., Wheeler K. P., Lazdunski M., Ellory J. C. Molecular size of different neurotoxin receptors on the voltage-sensitive Na+ channel. J Biol Chem. 1983 Jan 25;258(2):700–702. [PubMed] [Google Scholar]
- Boheim G., Hanke W., Barrantes F. J., Eibl H., Sakmann B., Fels G., Maelicke A. Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3586–3590. doi: 10.1073/pnas.78.6.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Fleming B. D., Coolbear K. P., Keough K. M. Gel to liquid-crystalline transition temperatures of water dispersions of two pairs of positional isomers of unsaturated mixed-acid phosphatidylcholines. Biochemistry. 1981 Jun 9;20(12):3633–3636. doi: 10.1021/bi00515a051. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Lazdunski M. Na+ channels with high and low affinity tetrodotoxin binding sites in the mammalian skeletal muscle cell. Difference in functional properties and sequential appearance during rat skeletal myogenesis. J Biol Chem. 1983 Jun 25;258(12):7256–7259. [PubMed] [Google Scholar]
- Goldin S. M., Rhoden V., Hess E. J. Molecular characterization, reconstitution, and "transport-specific fractionation" of the saxitoxin binding protein/Na+ gate of mammalian brain. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6884–6888. doi: 10.1073/pnas.77.11.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Horn R., Patlak J., Stevens C. F. Sodium channels need not open before they inactivate. Nature. 1981 Jun 4;291(5814):426–427. doi: 10.1038/291426a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Meves H., Rubly N., Watt D. D. A quantitative study of the action of Centruroides sculpturatus toxins III and IV on the Na currents of the node of Ranvier. Pflugers Arch. 1983 Apr;397(2):90–99. doi: 10.1007/BF00582045. [DOI] [PubMed] [Google Scholar]
- Jaimovich E., Ildefonse M., Barhanin J., Rougier O., Lazdunski M. Centruroides toxin, a selective blocker of surface Na+ channels in skeletal muscle: voltage-clamp analysis and biochemical characterization of the receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3896–3900. doi: 10.1073/pnas.79.12.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Worley J. F., 3rd, French R. J. Single sodium channels from rat brain incorporated into planar lipid bilayer membranes. Nature. 1983 May 12;303(5913):172–175. doi: 10.1038/303172a0. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Boheim G. The gating of single calcium-dependent potassium channels is described by an activation/blockade mechanism. Biophys Struct Mech. 1982;9(1):35–60. doi: 10.1007/BF00536014. [DOI] [PubMed] [Google Scholar]
- Meves H., Rubly N., Watt D. D. Effect of toxins isolated from the venom of the scorpion Centruroides sculpturatus on the Na currents of the node of Ranvier. Pflugers Arch. 1982 Mar;393(1):56–62. doi: 10.1007/BF00582392. [DOI] [PubMed] [Google Scholar]
- Miller C. Voltage-gated cation conductance channel from fragmented sarcoplasmic reticulum: steady-state electrical properties. J Membr Biol. 1978 Apr 20;40(1):1–23. doi: 10.1007/BF01909736. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Fritz L. C., Raftery M. A., Brockes J. P. Isolation and characterization of a monoclonal antibody against the saxitoxin-binding component from the electric organ of the eel Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1673–1677. doi: 10.1073/pnas.79.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman R. I., Schmid A., Lombet A., Barhanin J., Lazdunski M. Purification of binding protein for Tityus gamma toxin identified with the gating component of the voltage-sensitive Na+ channel. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4164–4168. doi: 10.1073/pnas.80.13.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. F., Kazazoglou T., Lombet A., Chicheportiche R., Jaimovich E., Romey G., Lazdunski M. The Na+ channel in mammalian cardiac cells. Two kinds of tetrodotoxin receptors in rat heart membranes. J Biol Chem. 1983 Jul 25;258(14):8799–8805. [PubMed] [Google Scholar]
- Renaud J. F., Scanu A. M., Kazazoglou T., Lombet A., Romey G., Lazdunski M. Normal serum and lipoprotein-deficient serum give different expressions of excitability, corresponding to different stages of differentiation, in chicken cardiac cells in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7768–7772. doi: 10.1073/pnas.79.24.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Abita J. P., Schweitz H., Wunderer G., Lazdunski Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Chicheportiche R., Lazdunski M., Rochat H., Miranda F., Lissitzky S. Scorpion neurotoxin - a presynaptic toxin which affects both Na+ and K+ channels in axons. Biochem Biophys Res Commun. 1975 May 5;64(1):115–121. doi: 10.1016/0006-291x(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitz H., Vincent J. P., Barhanin J., Frelin C., Linden G., Hugues M., Lazdunski M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry. 1981 Sep 1;20(18):5245–5252. doi: 10.1021/bi00521a023. [DOI] [PubMed] [Google Scholar]
- Sherman S. J., Lawrence J. C., Messner D. J., Jacoby K., Catterall W. A. Tetrodotoxin-sensitive sodium channels in rat muscle cells developing in vitro. J Biol Chem. 1983 Feb 25;258(4):2488–2495. [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Talvenheimo J. A., Tamkun M. M., Catterall W. A. Reconstitution of neurotoxin-stimulated sodium transport by the voltage-sensitive sodium channel purified from rat brain. J Biol Chem. 1982 Oct 25;257(20):11868–11871. [PubMed] [Google Scholar]
- Villegas R., Villegas G. M., Condrescu-Guidi M., Suárez-Mata Z. Characterization of the nerve membrane sodium channel incorporated into soybean liposomes: a sodium channel active particle. Ann N Y Acad Sci. 1980;358:183–203. doi: 10.1111/j.1749-6632.1980.tb15396.x. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Barhanin J., Lazdunski M. Specific binding of toxin II from Centruroides suffusus suffusus to the sodium channel in electroplaque membranes. Biochemistry. 1982 Oct 26;21(22):5628–5634. doi: 10.1021/bi00265a037. [DOI] [PubMed] [Google Scholar]



