Abstract
A therapeutic approach to improve treatment outcome of ovarian cancer (OC) in patients is urgently needed. Myxoma virus (MYXV) is a candidate oncolytic virus that infects to eliminate OC cells. We found that in vitro MYXV treatment enhances cisplatin or gemcitabine treatment by allowing lower doses than the corresponding IC50 calculated for primary OC cells. MYXV also affected OC patient ascites-associated CD14+ myeloid cells, one of the most abundant immunological components of the OC tumor environment; without causing cell death, MYXV infection reduces the ability of these cells to secrete cytokines such as IL-10 that are signatures of the immunosuppressive tumor environment. We found that pretreatment with replication-competent but not replication-defective MYXV-sensitized tumor cells to later cisplatin treatments to drastically improve survival in a murine syngeneic OC dissemination model. We thus conclude that infection with replication-competent MYXV before cisplatin treatment markedly enhances the therapeutic benefit of chemotherapy. Treatment with replication-competent MYXV followed by cisplatin potentiated splenocyte activation and IFNγ expression, possibly by T cells, when splenocytes from treated mice were stimulated with tumor cell antigen ex vivo. The impact on immune responses in the tumor environment may thus contribute to the enhanced antitumor activity of combinatorial MYXV-cisplatin treatment.
Keywords: myxoma virus, MYXV, oncolytic, virotherapy, synergism, chemotherapy, M062R-null MYXV, syngeneic, ovarian cancer, immunotherapy
Introduction
Prolonging survival and preventing relapse for ovarian cancer (OC) patients remain challenging, despite the availability of appropriate surgery and highly effective first-line chemotherapy.1 It is estimated that 70% of patients with advanced OC eventually relapse in spite of remission achieved after initial treatment.1, 2 Developing novel treatment approaches is urgently needed.
Immune response and especially immune status within the tumor environment play critical roles in OC progression, overall prognosis, and survival.3, 4 The presence of tumor-infiltrating lymphocytes is associated with prolonged survival.3 Tumor cells, however, cultivate the tumor environment to impair antitumor immunity, an activity that has been associated with poor survival and prognosis;5 this immunosuppressive tumor environment is also a major obstacle for effective treatments of OC, including dendritic cell vaccination.6 Thus, an integrated treatment strategy that not only complements current chemotherapy but also alleviates immune suppression in the tumor environment could benefit the treatment of patients with OC.
Oncolytic virotherapy can be applied as a novel anticancer strategy because it not only can promote cytoreductive activity specifically against tumor cells7, 8 but also stimulates the innate immunity-mediating antitumor bystander effect9 and the adaptive immune response for a prolonged therapeutic effect.10, 11 The mechanism by which a long-lasting treatment benefit is triggered depends on the virus used. In general, oncolytic virotherapy has a direct cytolytic effect on cancer cells, resulting in the release of tumor antigens that leads to a cascade of events ultimately inducing antitumoral adaptive immunity. This outcome is especially valuable in the elimination of micrometastases in distant locations. Moreover, oncolytic virotherapy can facilitate the elimination of the immunosuppressive environment that protects cancer cells from systemic immune surveillance.12 The immunotherapeutic value of oncolytic virotherapy is gradually being recognized.13
The combination of oncolytic virotherapy and cytotoxic chemotherapy agents for improved treatment outcomes has been proposed.14 The synergistic effect observed through this combination treatment may be due to an escalated induction of systemic antitumor immunity.15 Myxoma virus (MYXV) is a candidate oncolytic virus on the path to clinical usage16 and possesses immunogenic properties.17, 18 Although in vitro MYXV oncolytic potential has been evaluated that can effectively infect and kill patient ascites-derived OC cells grown in monolayer19 (Figures 1B and 1D), other than three-dimensional spheroids,20 it has not been investigated in the animal model.
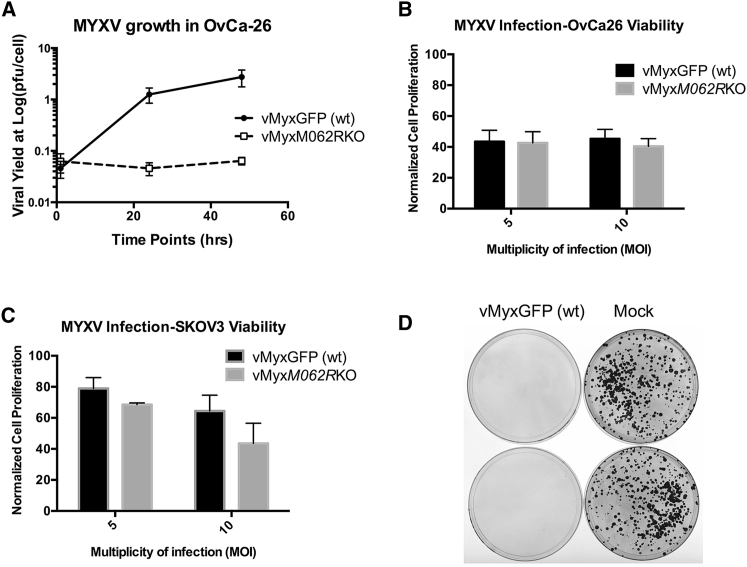
Figure 1.
MYXV Infection in OC Cells and Oncolysis
(A) MYXV multistep growth comparison in primary OC cells. Replication-competent (vMyxGFP [WT]) or -defective (vMyxM062RKO) MYXV was used at an MOI of 0.1 to infect primary OvCa-26 OC cells. Cell lysates were harvested at given time points and viral yields estimated by titering on BSC-40 cells. The mean titer from the titration in triplicate is shown at each time point, and the error bar represents the SD. Shown is a representative of two independent experiments. (B and C) Infection by MYXV led to reduced cell viability. In both primary OC cells, such as OvCa-26 (B) and the OC cell line SKOV3 (C), infection by WT or M062R-null MYXV at an MOI of either 5 or 10 led to reduced cell viability as measured by MTT assay. However, the differences in cell viability between the two virus treatments did not reach statistical significance. The error bar represents SD from the quantification in triplicate, and shown is a representative of two independent experiments. (D) MYXV infection eliminated colony formation by OC tumor cells. SKOV3 cells (5 × 103) were either mock infected or infected with MYXV (MOI = 10) for 1 hr and then seeded in plates. After approximately 3 weeks of culture, cells were fixed and stained with crystal violet to detect colony formation.
The combination of MYXV and gemcitabine tested in a pancreatic tumor model showed a significant treatment benefit.21 Based on the findings in this study, the use of MYXV to treat tumor dissemination within the peritoneal space shows promise. Moreover, in the pancreatic syngeneic model tested in this earlier study, an immunosuppressive tumor environment is present,22, 23 which shares similarity to the immunological property of the OC environment. Because gemcitabine is a second-line chemotherapy option for OC, we considered it as one treatment option in our OC model as a control.
In this study, we focused on using the combination of MYXV and cisplatin to treat disseminated OC associated with an immunosuppressive tumor microenvironment. Cisplatin, an alkylating agent that causes DNA damage and subsequent apoptosis,24 is a first-line platinum-based chemotherapy agent against OC. Cisplatin has also been observed to impact host immune response by reducing regulatory T cells while enhancing antigen-specific CD8+ T cell activities.25 When cisplatin and oncolytic virotherapy are used together to achieve therapeutic benefit, however, the immunological impact differs, and specific characterization of the mechanism involved is needed.26, 27 Our study marks a first attempt to utilize MYXV and cisplatin together to treat disseminated OC in vivo. We show that combinatorial MYXV/cisplatin produced a significant improvement in overall survival in a mouse model of this deadly cancer.
Results
MYXV Oncolytic Potential against OC Cells
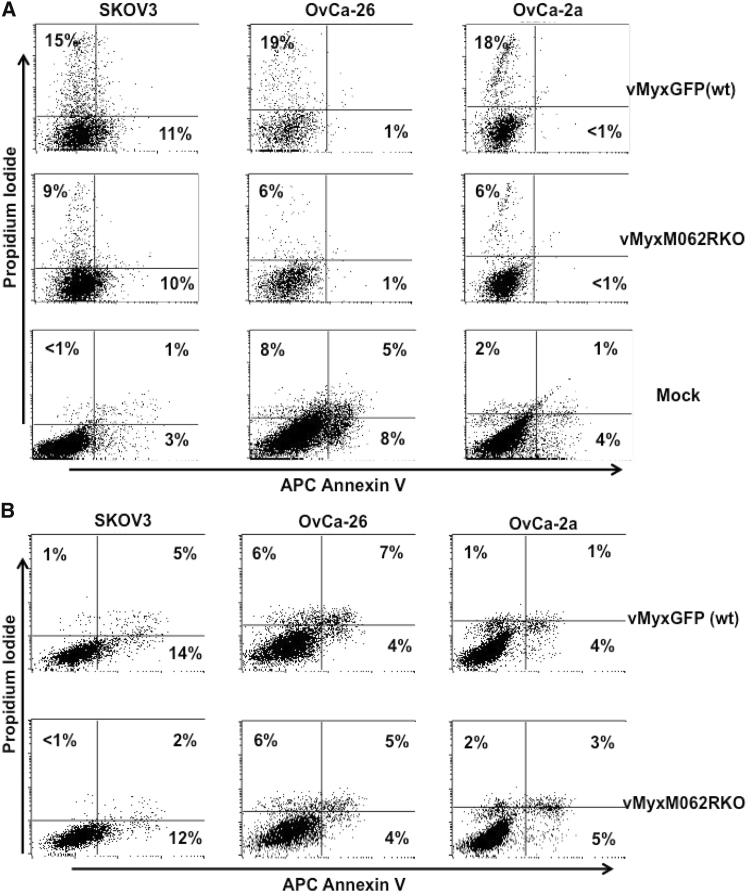
We evaluated the oncolytic potential of MYXV in the established OC cell line SKOV3 and in two primary OC cell lines, OvCa-2a and OvCa-26, that were developed from patient ascites. Wild-type (WT) MYXV (vMyxGFP) could productively infect the cells, while a mutant virus lacking the replication-essential gene M062R (M062R-null MYXV or vMyxM062RKO) caused abortive infection in all OC cell lines tested (Figure 1A). Interestingly, however, infection by either mutant (M062R-null MYXV) or WT MYXV caused comparable reductions in cell viability as measured by MTT assay (Figures 1B and 1C). In patient ascites-derived OC cells, MYXV infection-associated reduction in viability is much more evident than that in the established cell line, SKOV3 (Figures 1B and 1C, respectively). Infection of SKOV3 cells with MYXV led to loss of the ability to form colonies (Figure 1D); comparable effects by MYXV were also seen in the two primary OC cell lines (data not shown). Cell death associated with MYXV infection in the two primary OC cell lines did not show a signature of apoptosis (Figure 2). WT MYXV infection in OvCa-2a and OvCa-26 caused a moderate increase of cell death in infected cells that was not seen in replication-defective infection (GFP+; Figure 2A). On the other hand, MYXV infection of SKOV3 cells caused a slight increase in the number of cells positive for early signs of apoptosis; however, this effect was replication independent (Figure 2).
Figure 2.
Apoptosis Was Not the Main Cause for MYXV Infection-Associated Cell Death in OC Tumor Cells
SKOV3, OvCa-26, and OvCa-2a cells were mock treated, infected with either vMyxGFP (WT) or vMyxM062RKO at an MOI of 5 for 24 hr before being stained with Annexin V and propidium iodide (PI) for flow cytometry. Cells from the infection group were gated first with forward scatter to side scatterplot (FSC/SSC) followed by the GFP-positive population (A) to analyze the Annexin V-PI staining. Next, GFP-negative cells were gated (B) to analyze the Annexin V-PI staining. This is a representative view of three independent experiments. In the quadrants shown, x and y axes indicate fluorescence intensity, while quadrants of each cell line were defined with the use of mock-treated samples, respectively.
MYXV Treatment as a Complement to Chemotherapy Drugs In Vitro
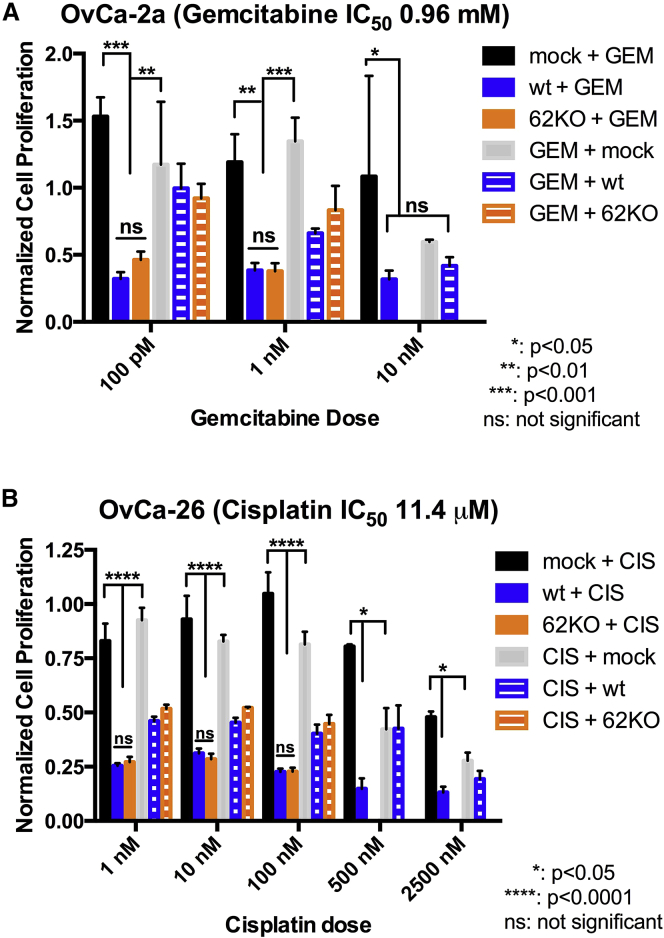
We tested the sensitivity of OvCa-2a and OvCa-26 to cisplatin in comparison to SKOV3 and found OvCa-26 (calculated IC50 = 11.4 μM) to be slightly more resistant than OvCa-2a (calculated IC50 = 3.6 μM) and SKOV3 (8.4 μM). We thus focused on OvCa-26 for further testing on cisplatin response. We also found OvCa-2a (calculated IC50 = 0.96 mM) to be relatively resistant to the gemcitabine treatment compared with SKOV3 (IC50 = 9.32 μM) and OvCa-26 (14.6 μM) and chose to focus on OvCa-2a for further testing related to gemcitabine. At doses comparable to IC50, additive effects of drug and virus were observed in primary OC cells (Figure S1). When we treated primary OC cells with cisplatin or gemcitabine at doses much lower than their calculated IC50, we did not observe significant growth inhibition with drug treatment alone (Figure 3); the reduced cell viability in groups treated with the MYXV treatment following cisplatin or gemcitabine is caused by MYXV infection (Figures 1B and 3B). When we evaluated the effects of combinatorial treatment with MYXV followed by chemotherapy (cisplatin or gemcitabine), consistent and significant further reductions in cell viability could be detected compared with the treatment outcomes of drug first followed by MYXV (Figure 3) or MYXV alone (Figure 1B).
Figure 3.
MYXV Treatment Complements Chemotherapy in a Replication-Independent Manner against Primary OC Tumor Cells
(A) MYXV complements gemcitabine for improved cytotoxicity. Primary patient-ascites-derived OvCa-2a tumor cells were mock-treated or infected with MYXV (WT or M062R null MYXV) at an MOI of 10; gemcitabine treatment at the given doses followed, or pretreatment with gemcitabine was given before viral infection. The first treatment (virus or chemotherapy) lasted 48 hr, followed by 24 hr of growth in fresh medium; cells were then treated with a second treatment (chemotherapy or virus) for another 48 hr before cell viability was measured. (B) MYXV complements cisplatin-associated cytotoxicity. The primary OvCa-26 OC cell line was used in this study, together with cisplatin. The study was conducted similarly to that with gemcitabine as described above. The error bar represents the SEM, and the mean is calculated from quantification in triplicate. Shown is a representative of two independent experiments.
MYXV Infection in OC Patient Ascites-Associated CD14+ Monocytes
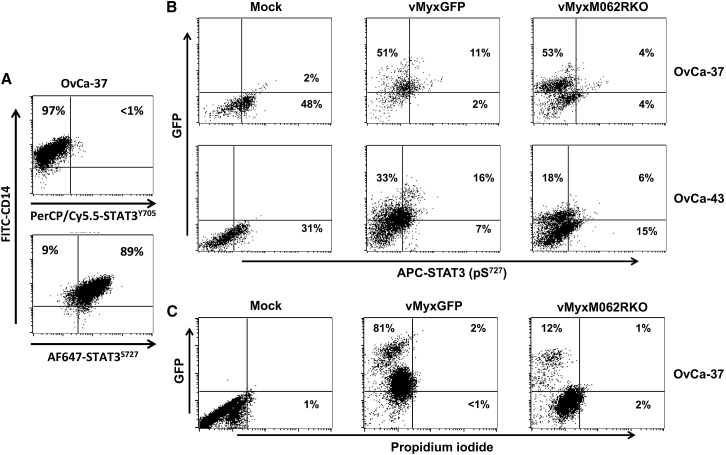
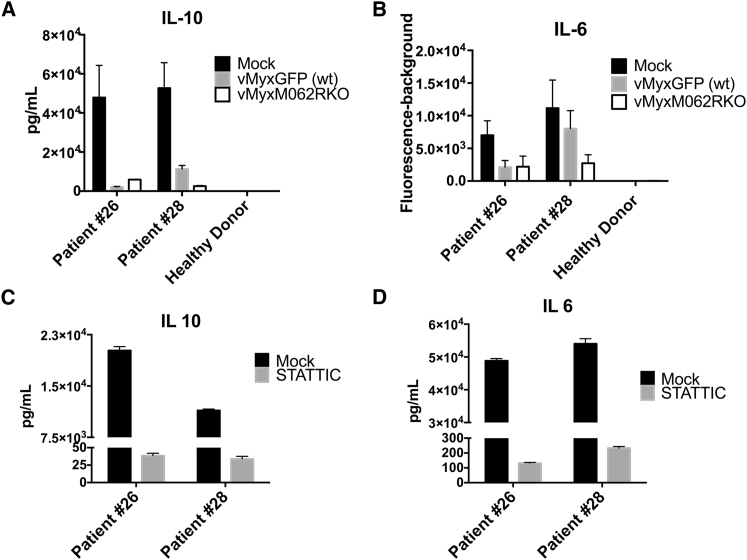
We investigated whether MYXV infection could impact the immunological properties of the OC tumor environment. We chose to examine the interaction between MYXV and OC patient ascites-associated CD14+ cells, as these cells are one of the most abundant and important players in ascites maintaining the immunosuppressive tumor environment.28 Moreover, MYXV prefers to bind and enter human CD14+ myeloid cells rather than other immune cell types29 and can activate the type I interferon (IFN) response by RIG-I in differentiated macrophages through an attachment-based induction.17 However, interestingly, MYXV infection does not cause cell death in either healthy monocytes (data not shown) or OC ascites-associated CD14+ cells (Figure 4C). We observed an unusual pattern of STAT3 phosphorylation in OC patient ascites-associated CD14+ cells with minimal phosphorylation at Y705 but a high level of phosphorylation at S727 (Figure 4A). In OC patient ascites-associated CD14+ monocytes, infection by MYXV led to reduced phosphorylation of STAT3 at serine 727 (pS727) (Figure 4B) and AKT (data not shown). The consequence of MYXV infection was a significant reduction in cytokine secretion, as shown by multiplex array (Figures 5A and 5B), which was comparable to the outcome caused by STAT3 inhibitor (Stattic) treatment (Figures 5C and 5D). Treatment of Stattic did not cause noticeable toxicity, and unchanged levels of IL-8 were detected by multiplex array (data not shown).
Figure 4.
MYXV Infection in OC Ascites-Associated CD14+ Monocytes Remodels the Intracellular Signaling Pathway
(A) OC patient ascites-associated CD14+ monocytes/macrophages display non-canonical STAT3 signaling. CD14+ monocytes were enriched from ascites fluid of patient (e.g., OvCa37) and tested for phosphorylation of STAT3 on both Y705 and S727 by flow cytometry. The signature of non-canonical STAT3 signaling is characterized by minimal phosphorylation at the Y705 site of STAT3. (B) MYXV infection in ascites-associated CD14+ monocytes causes inhibition of STAT3 phosphorylation at the Ser727 residue. CD14+ monocytes were enriched from patient-ascites fluid and tested for purity via flow cytometry. Monocytes were immediately mock treated and infected with WT or M062R-null MYXV at an MOI of 10 for 1 h; washing with PBS followed before the cells were cultured for 18 hr. Media was harvested for multiplex array (Figure 5). Cells were fixed and permeablized for intracellular staining as described in the Materials and Methods to examine the level of STAT3 phosphorylation at the Ser727 residue. (C) Infection by MYXV does not cause general change in cell viability in ascites CD14+ cells. Patient CD14+ ascites-associated monocytes were mock treated or infected with WT or M062R-null MYXV for 18 hr before the cells were stained with propidium iodide (PI). Live cells were gated to examine GFP (infection) and the presence of PI staining.
Figure 5.
MYXV Infection in Patient-Ascites-Associated CD14+ Monocytes Inhibits Cytokine Secretion that Is the Signature of Immunosuppressive Tumor Environment
Patient-ascites-associated CD14+ monocytes or CD14+ monocytes from a healthy female donor were mock treated or infected with WT or M062R null MYXV for 18 hr before supernatant was collected for multiplex array. Comparisons in levels of IL-10 (A) and IL-6 (B) are shown for mock treatment and infection in patients and healthy monocytes. To test the effect of STAT3 in cytokine secretion, patient-ascites-associated CD14+ monocytes were treated with 5 μM Stattic, a STAT3 inhibitor, for 48 hr before supernatant was collected for multiplex array. (C and D) The effect of Stattic on the levels of IL-10 (C) and IL-6 (D) in patient monocytes. The error bars represents SD, and the mean is calculated from the quantification in triplicate.
MYXV Treatment in a Syngeneic Murine OC Dissemination Model
We examined murine OC ID8 cells and found them to be sensitive to cisplatin treatment (IC50, 2.0 μM), compared to human SKOV3 cells (IC50, 8.4 μM). We evaluated MYXV as either a single agent or in combination with cisplatin in immunocompetent ID8 disseminated tumor-bearing mice.
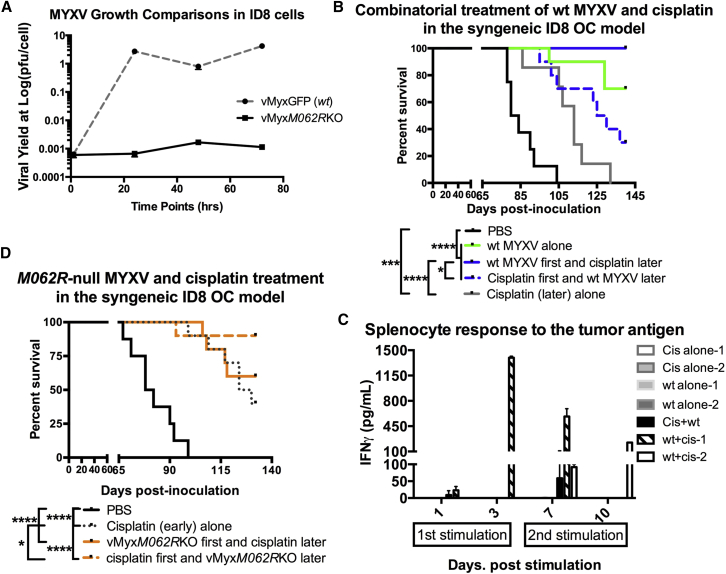
We found that as single-agent treatment given late after tumor cell injection in the syngeneic ID8 OC model, either replication-competent (vMyxGFP or WT MYXV) or -defective (M062R-null MYXV or vMyxM062RKO) MYXV provided similar benefit in prolonging survival. The treatment benefit by MYXV reached statistical significance compared to that of mock treatment, cisplatin, or gemcitabine treatment alone (Figure S2). Gemcitabine treatment alone (50 mg/kg every other day for a total four treatments, which was modified from regimens described previously),21, 30 did not lead to significant therapeutic benefit in this model (Figure S2).
We found that the group of mice treated first with WT MYXV and then later with cisplatin remained healthy 2 months after the median survival time of the mock-treatment group (80 days), while mice in the cisplatin-alone and mock-treatment groups had all succumbed to the disease (Figure 6B). The differences are statistically significant analyzed by log rank (Mantel-Cox) test (p < 0.0001). Interestingly, although it also reached statistical significance in comparison to cisplatin-alone and mock-treatment groups (both p < 0.01), treatment first with cisplatin followed by WT MYXV (30% survival) did not provide the same benefit as MYXV pretreatment followed by cisplatin (100% survival) (Figure 6B) (p = 0.0011). More importantly, when the replication-defective mutant MYXV, vMyxM062RKO, was used for pretreatment before cisplatin (60% survival), we did not observe the same benefit conferred by WT MYXV pretreatment (Figure 6B and solid orange line in Figure 6D; 100% survival). Thus, a replication-competent MYXV is important to sensitize cancer cells to a following cisplatin treatment in order to achieve an optimal therapeutic outcome. Unexpectedly, the regime of M062R-null MYXV treatment after cisplatin (90% survival) provided a better survival outcome than cisplatin alone (40% survival) (log rank Mantel-Cox test; p = 0.0329) or mutant virus followed by cisplatin (Figure 6D) (60% survival; p = 0.1605).
Figure 6.
Combinatorial Treatment of MYXV and Cisplatin for OC Disseminated Tumor in a Syngeneic Murine Model
(A) Multistep growth comparison of viral replication by WT and M062R null MYXV. In the murine OC cell line ID8, similar to most cells tested,47M062R-null MYXV underwent abortive infection. After infection by MYXV, ID8 cell lysates were harvested at given time points. Viral yields are quantified by titration. The error bar represents SEM, and the mean is calculated from the quantification in triplicate. Shown is a representative of two independent experiments. (B) MYXV pretreatment enhanced cisplatin effect in the ID8 OC dissemination model. At 7 days post-tumor-cell-injection, mice were either mock treated or treated with WT MYXV or cisplatin as described in the Materials and Methods. A second treatment occurred 5 days after the first treatment. Statistical analysis was carried out with the log-rank (Mantel-Cox) test (p < 0.0001) and the Gehan-Breslow-Wilcoxon test (p < 0.0001). Median survival (days) is calculated as follows: PBS (n = 8) 82 (days); cisplatin (late) only (n = 7), 113; WT MYXV only (n = 10), undefined; WT MYXV + cisplatin (n = 10), undefined; cisplatin first + WT MYXV later (n = 10), 127.5. *p < 0.05, ***p < 0.001, ****p < 0.0001. (C) Response to tumor antigen in mouse splenocytes from different treatments. Splenocytes harvested from mice treated with WT MYXV followed by cisplatin showed high levels of IFNγ when they were stimulated with ID8 tumor antigen. After 140 days, surviving mice from virus treated alone or combination treatment are euthanized to collect splenocytes; as controls, splenocytes were also harvested from mice with cisplatin alone at approximately 100 days for the test. Freshly harvested splenocytes were immediately stimulated with tumor cell lysate at day 0. Samples of supernatant were collected at days 1 and 3 post-stimulation. These splenocytes continued to be cultured, and at day 6, fresh media were added to include tumor cell lysate. On days 7 and 10, supernatant was collected to test IFNγ by ELISA. The error bar represents SD from the quantification in duplicate. Shown are representative mice of following groups: cisplatin alone (n = 2), WT MYXV alone (n = 2), cisplatin first plus WT MYXV later (n = 1), and WT MYXV first + cisplatin later (n = 2). (D) M062R null MYXV can be used in combination with cisplatin to achieve therapeutic benefit. Similar to (C), M062R-null MYXV was tested in the combinatorial treatment. Statistical significance was determined with the log-rank (Mantel-Cox) test (p < 0.0001), the log-rank test for trend (p < 0.0001), and the Gehan-Breslow-Wilcoxon test (p < 0.0001). Median survival (days) is calculated as following: PBS (n = 8) 80 (days); vMyxM062RKO first + cisplatin later (n = 10), undefined; Cisplatin first + vMyxM062RKO later (n = 10), undefined; cisplatin (early) alone (n = 10), 127. *p < 0.05, ****p < 0.001.
We collected splenocytes from surviving mice from the following groups 100 days after tumor cell injection: cisplatin alone (early treatment group as in Figure 6B, collected after 100 days), WT MYXV alone (Figure 6B) (collected after 140 days), cisplatin plus WT MYXV (Figure 6B) (collected after 140 days), and WT MYXV plus cisplatin (Figure 6B) (collected after 140 days). After stimulation of spleen cells with ID8 tumor antigen, we examined IFNγ secretion in the supernatant as a measurement of immune cell activation. Splenocytes from the group treated with WT MYXV plus cisplatin consistently showed a strong IFNγ response (Figure 6C).
Discussion
The oncolytic potential of MYXV is largely due to the intracellular environment of tumor cells that permits a productive MYXV infection, including highly phosphorylated AKT31 and loss of synergistic effects of the tumor necrosis factor (TNF) and IFN responses, in the transformed cells.32, 33 The mechanism of oncolysis by MYXV, however, varies by the type of cancer.34, 35 We found that apoptosis was not the major driver of oncolysis by MYXV in the OC cells tested, including primary OC cells derived from patient ascites. Although replication competence can moderately increase cell death, such as in WT MYXV-infected primary OC cells, the overall inhibitory effect to OC cell growth seemed to be replication independent. Further investigation on the mechanism is ongoing. More importantly, pretreatment with MYXV sensitized OC cells to much lower doses of chemotherapy agents than those given when the agents are used alone. Further investigation into the mechanism of this sensitization process is needed. It is encouraging to examine whether this treatment approach may be an alternative strategy to target chemoresistance in many OC cells, especially recurrent tumor arising after first-line chemotherapy treatment.
Within human OC ascites, CD14+ monocytes/macrophages are one of the most abundant immune cell populations.5, 28 These myeloid cells have an M2 immunosuppressive phenotype and have been linked to resistance to platinum-based chemotherapy agents.36 The ability of CD14+ myeloid cells to produce IL-10 has a suppressive effect on T cells present in the OC tumor environment in the peritoneal cavity.37, 38 MYXV preferentially binds and enters human CD14+ cells rather than other immune cells to initiate early gene expression without resulting in a productive infection.29 We found that MYXV did not affect the general viability of healthy human CD14+ cells. However, MYXV infection in OC ascites-associated CD14+ cells led to an inhibitory effect on multiple signaling pathways associated with cytokine secretion patterns that contribute to the immunosuppressive tumor environment. Thus, MYXV can be a potential immunotherapeutic tool for targeting CD14+ monocytes in the tumor environment. In the murine ID8 model of OC, we found the presence of CD11b+ cell population but few F4/80+ cells (mature macrophages) in the ascites of mice injected with this clone of ID8 cells (data not shown). It is not an optimal system to investigate the MYXV therapeutic effect against the equivalent of CD14+ cell type in human OC ascites. A recently developed model using p53 null ID8 cells with high levels of macrophages infiltration in the ascites39 can be a system to extend the investigation.
An initial characterization of OC patient ascites-associated CD14+ cells showed active AKT signaling and a non-canonical state of STAT3 signaling (STAT3 pY705low/none pS727high). Targeting STAT3 signaling to reverse chemoresistance in OC has been suggested.40 However, the roles of non-canonical STAT3 signaling in OC disease progression and maintenance of immunological properties of OC are not yet characterized. Phosphorylation at serine 727 of STAT3 permits a maximal transcription activity in principle.41 We utilized a specific inhibitor of STAT3, Stattic, to prevent STAT3 homodimerization and DNA binding42 and observed suppressive effect in cytokine secretion of tumor-associated CD14+ macrophages (Figure 5, control). Our results showed that MYXV infection could suppress STAT3 (STAT3 pY705low/none pS727high) and ATK signaling; this effect could be further enhanced when engineered M062R-null MYXV was used.
Cisplatin treatment can also affect the tumor environment, including induction of a tumor-specific CD8+ T cell response.43 We found that pretreatment with replication-competent MYXV followed by cisplatin greatly improved survival, compared with cisplatin alone. Interestingly, treating mice first with cisplatin followed by replicating MYXV did not achieve the same treatment benefit. Even with replicating MYXV, the viral infection was eliminated within 7 days in immunocompetent mice.21 Thus, it is possible that transient MYXV infection remodels the tumor environment, sensitizing tumor cells to a later cisplatin intervention. It seems that the initial phase of viral replication is crucial to a favorable treatment outcome when the combinatorial and sequential WT MYXV-cisplatin regimen is used.
However, intriguingly, use of the replication-defective MYXV, M062R-null MYXV, after cisplatin treatment in this OC dissemination model led to 90% survival in mice and was much more effective than the use of replicating MYXV after cisplatin treatment (60% survival). Cisplatin inhibits DNA replication of MYXV (data not shown); therefore, cisplatin and MYXV cannot be applied at the same time, as was explored with reovirus virotherapy.26 The lack of statistically significant differences in disease progression between groups treated with cisplatin alone and cisplatin followed by WT MYXV suggests that even 5 days after cessation of cisplatin treatment, the effect of inhibiting a subsequent productive MYXV infection in the tumor environment persists. Accordingly, we speculate that the favorable outcome of cisplatin plus later M062R null MYXV treatment may be unique to this mutant-virus. In human cells, M062R-null MYXV infection activates the anti-neoplastic SAMD9 pathway.44 It is not known whether infection with M062R-null MYXV in this murine model stimulates a similar pathway that can specifically enhance the outcome of preceding cisplatin treatment.
In human OC ascites-associated CD14+ cells, M062R-null MYXV effectively suppresses STAT3 phosphorylation (Figure 4B) and AKT (data not shown) and reduces phosphorylated CREB (data not shown), all important signaling molecules in the maintenance of the M2 state of tumor-associated myeloid cells.
Development of novel treatment approaches for OC patients is urgently needed. We showed that an oncolytic virotherapy candidate, MYXV, could be integrated into and complement an existing chemotherapy regimen to improve the treatment benefit in an immunocompetent preclinical model. We are investigating the mechanism of MYXV immunotherapeutic potential in the OC tumor environment, especially the effect on OC ascites-associated CD14+ cells. To the best of our knowledge, this is the first study that investigates the benefit of combining MYXV with cisplatin in the treatment of OC in a syngeneic model in vivo.
Materials and Methods
Human Subjects
Ovarian cancer patients were recruited from patients attending the Women’s Oncology clinic in the Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences (UAMS), under an IRB-approved protocol. Ovarian tumor ascites samples were recovered at the time of surgery.
Characterization of Patient Samples
(1) Clinical characteristics of patient tumor samples are as follows:
OvCa-2a, clear cell carcinoma
OvCa-26, metastatic adenocarcinoma that is moderately differentiated but lacks clear-cut high-grade serous, clear cell, or endometrioid differentiation
OvCa-2a and OvCa-26, both newly established ovarian cancer cell lines. OvCa-2a is EpCAMhi CD133hi A-cadherinneg and it has a stem-like phenotype. OvCa-26 is EpCAMhi, CD133hi E-cadherinlo and also has elements of a stem-like phenotype. Both cell lines express ALDH1. TP53 mutation status is unknown.
(2) Clinical characterization of patient tumor type with which tumor-associated macrophages were purified from ascites are as follows:
OvCa-28, mucinous cystadenocarcinoma
OvCa-37, high-grade serous carcinoma
OvCa-43, high-grade serous carcinoma.
Cell Lines and Viruses
SKOV3,45 OvCa-26 (primary human OC cells), OvCa-2a (primary human OC cells), and ID8 (courtesy of Katherine Roby, PhD, University of Kansas Medical Center) cells46 were cultured in RPMI1640 (Mediatech, Corning, NY) supplemented with 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 5 × 10−5 M 2-mercaptoethanol (Thermo Fisher Scientific, Waltham, MA), and 100 μg/mL of penicillin/streptomycin (Invitrogen). MYXV viruses, vMyxGFP-WT and M062R-null MYXV (vMyxM062RKO), have been described previously.47 Both viruses were engineered to express GFP driven by a viral synthetic promoter from which GFP is synthesized throughout the course of infection. Viruses are amplified on BSC-40 cells and purified through 36% sucrose gradient as previously described.21, 47, 48 BSC-40 cells were cultured in Dulbecco’s minimal essential medium (Lonza, Basel, Switzerland, and Invitrogen) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 2 mM glutamine (Corning, Corning, NY), and 100 μg/mL of penicillin/streptomycin (Invitrogen).
Reagents and Antibodies
Chemotherapy drugs cisplatin (Sigma-Aldrich, St. Louis, MO) and gemcitabine (Sigma-Aldrich) were diluted to appropriate concentrations in growth medium for treatment in vitro and were diluted in PBS for animal treatment. Stattic (Selleckchem, Houston, TX) was dissolved in DMSO at 10 mM, and patient ascites-associated monocytes were treated at a concentration of 5 μM before cytokine secretion was tested. The antibodies STAT3 pY705, STAT3 pS727, total STAT3, AKT pS473, and pCREB, as well as the Annexin V Apoptosis Detection Kit APC, are from Affymetrix eBioscience (San Diego, CA).
Colony-Formation Assay and MTT Assay
Colony-formation assay was conducted as previously described.21 Briefly, cancer cells were treated with MYXV at an MOI of 50 before they were diluted for seeding in a 10-cm dish. Depending on the cell lines used, after 2–6 weeks of growth, cells were fixed and stained with crystal violet for imaging. Cell viability was measured by MTT assay (Promega, Madison, WI) according to the manufacturer’s protocol.
Combinatorial Treatment Test In Vitro
To calculate IC50 dose for each cell line, we adopted a method similar to what was previously reported.21 Briefly, cells were treated with chemotherapy drug at serial diluted doses for 48–72 hr before cell proliferation was measured with MTT assay.
Primary patient ascites-derived tumor cells (OvCa-2a and OvCa-26) were mock-treated or infected with MYXV (replicating WT or defective M062R-null MYXV) at an MOI of 10 for 48 hr before they were cultured for 24 hr in fresh medium without any treatment; cells were then treated with a second treatment (chemotherapy or virus) for another 48 hr before cell viability was measured with MTT assay (Promega).
Human Healthy CD14+ Monocytes and OC Patient Tumor-Associated CD14+ Monocytes
Human CD14+ monocytes are from healthy female donors (Lonza). OC tumor-associated CD14+ monocytes were purified from patient ascites as described previously.38 Briefly, primary ovarian tumor ascites CD14+ cells were separated magnetically using commercially available columns and anti-CD14 conjugated microbeads (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. The purity of recovered ascites CD14+ cells was typically 95%–98%.
Flow Cytometry, ELISA, and Multiplex Array
After appropriate treatments, cells were fixed and permeablized with fixation/permeablilization concentrate (Affymetrix eBioscience) and stained according to the manufacturer’s instructions with antibodies recognizing intracellular signaling phosphoproteins. The mouse IFNγ ELISA and ProcartaPlex human inflammation panel (20 plex) (Affymetrix eBioscience) were performed according to manufacturer’s instructions. A customized multiplex array (Millipore, Billerica, MA) was used to characterize Stattic-treated patient-ascites monocytes. To examine IFNγ secretion in splenocytes to tumor antigen stimulation, mouse splenocytes were harvested and treated with ACK lysing buffer (Thermo Fisher Scientific) at 1:1 volume ratio for 5 min at room temperature before cells were pelleted. Approximately 10 million cells per mouse were either mock treated or stimulated with ID8 cell lysate (technical replicates in duplicate). At 1 day and 3 days post-stimulation, a sample of supernatant was taken per well and stored at −80°C for ELISA (eBioscience). These splenocytes continued to be cultured, and at day 6, media were replaced to contain ID8 tumor lysate for the second round of stimulation. At days 7 and 10, samples of supernatant were again taken per well for ELISA. To prepare ID8 tumor cell lysate as a crude tumor antigen preparation, we resuspended one million ID8 cells in 1 mL of medium for 3rounds of freeze-thaw cycle followed by sonication; 125 μL of tumor cell lysate was used to stimulate 10 million splenocytes.
Murine Model of OC Dissemination and Treatments
The animal studies were approved by the IACUC at the University of Arkansas for Medical Sciences (UAMS). Cisplatin was administered at 3 mg/kg every 3 days as described previously.49 In a regimen modified from those described previously,21, 30 gemcitabine was administered at 50 mg/kg every other day for a total four treatments. Virotherapy was carried out every other day for a total of four or five intraperitoneal (i.p.) injections as described previously.21
To test the therapeutic effect of combined virus and chemotherapy treatment, treatment started 7 days after i.p. injection of a dose of 6 × 106 tumor cells/mouse. Injecting fewer cells of this ID8 clone (e.g., 1 × 106 or 3 × 106 cells) failed to provide the same disease progression and survival outcome as shown in this study (e.g., Figure S2). For combinatorial treatment of virus followed by cisplatin, four i.p. injections of 1 × 108 plaque-forming units (PFUs)/mouse every other day were carried out, and cisplatin treatment was begun 5 days after the last injection of virus as described above. For combination treatment of cisplatin first followed by virus, cisplatin treatment was carried out as described above with a 5-day interval before the virus treatment (five injections every other day). As a control, cisplatin treatment was tested at either 7 days (early) or 16 days (late) post-injection of tumor cells. To test single-agent virotherapy, cisplatin, or gemcitabine treatment, treatments were initiated at 16 days post-tumor-injection.
Author Contributions
Investigation: B.N., J. Liem, M.C., J. Liu; Methodology, Funding Acquisition, and Writing – Review and Editing: M.C., J. Liu; Conceptualization, Supervision, Visualization, and Writing – Original Draft: J. Liu.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by NIH K22-A99184 and P20GM103625, the Cookie Laughlin Award from Rivkin Center for Ovarian Cancer, and a start-up fund by the UAMS Department of Microbiology and Immunology to J. Liu. This work was supported in part by P50 CA-136393 (Mayo Clinic SPORE in Ovarian Cancer) to M.C. This work was also supported by Microgrants for Collaborative Exploratory Research (MiCER) from the Department of Microbiology and Immunology at UAMS to both J. Liu and M.C. The Flow Cytometry Core service is supported in part by the NIH Clinical and Translational Science Award-funded Translational Research Institute at UAMS (UL1TR000039) and by the NIH COBRE Center for Microbial Pathogenesis and Host Inflammatory Responses (P20GM103625). We thank Katherine Roby, PhD, of the University of Kansas Medical Center, for providing ID8 cells for this study. We also thank Y. Li, S. Blair, and S. Chancellor for technical assistance.
Footnotes
Supplemental Information includes two figures and can be found with this article online at http://dx.doi.org/10.1016/j.omto.2017.08.002.
Supplemental Information
References
- 1.Bukowski R.M., Ozols R.F., Markman M. The management of recurrent ovarian cancer. Semin. Oncol. 2007;34(Suppl 2):S1–S15. doi: 10.1053/j.seminoncol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Ozols R.F. Challenges for chemotherapy in ovarian cancer. Ann. Oncol. 2006;17(Suppl 5):v181–v187. doi: 10.1093/annonc/mdj978. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Conejo-Garcia J.R., Katsaros D., Gimotty P.A., Massobrio M., Regnani G., Makrigiannakis A., Gray H., Schlienger K., Liebman M.N. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 4.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Cannon M.J., Ghosh D., Gujja S. Signaling circuits and regulation of immune suppression by ovarian tumor-associated macrophages. Vaccines (Basel) 2015;3:448–466. doi: 10.3390/vaccines3020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyne H.E., Cannon M.J. Dendritic cell vaccination, immune regulation, and clinical outcomes in ovarian cancer. Front. Immunol. 2013;4:382. doi: 10.3389/fimmu.2013.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuhara H., Ino Y., Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arulanandam R., Batenchuk C., Varette O., Zakaria C., Garcia V., Forbes N.E., Davis C., Krishnan R., Karmacharya R., Cox J. Microtubule disruption synergizes with oncolytic virotherapy by inhibiting interferon translation and potentiating bystander killing. Nat. Commun. 2015;6:6410. doi: 10.1038/ncomms7410. [DOI] [PubMed] [Google Scholar]
- 10.Bilsland A.E., Spiliopoulou P., Evans T.R. Virotherapy: cancer gene therapy at last? [version 1; referees: 2 approved] F1000Res. 2016;5:2105. doi: 10.12688/f1000research.8211.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woller N., Gürlevik E., Ureche C.I., Schumacher A., Kühnel F. Oncolytic viruses as anticancer vaccines. Front. Oncol. 2014;4:188. doi: 10.3389/fonc.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne S.H., Liang W., Sampath P., Schmidt T., Sikorski R., Beilhack A., Contag C.H. Targeting localized immune suppression within the tumor through repeat cycles of immune cell-oncolytic virus combination therapy. Mol. Ther. 2010;18:1698–1705. doi: 10.1038/mt.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller B.A., Bell J.C. Oncolytic viruses-immunotherapeutics on the rise. J. Mol. Med. (Berl.) 2016;94:979–991. doi: 10.1007/s00109-016-1453-9. [DOI] [PubMed] [Google Scholar]
- 14.Wennier S.T., Liu J., McFadden G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr. Pharm. Biotechnol. 2012;13:1817–1833. doi: 10.2174/138920112800958850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson G.R., Relph K., Harrington K., Melcher A., Pandha H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: recent advances. Oncolytic Virother. 2016;5:1–13. doi: 10.2147/OV.S66083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan W.M., Rahman M.M., McFadden G. Oncolytic myxoma virus: the path to clinic. Vaccine. 2013;31:4252–4258. doi: 10.1016/j.vaccine.2013.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F., Gao X., Barrett J.W., Shao Q., Bartee E., Mohamed M.R., Rahman M., Werden S., Irvine T., Cao J. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 2008;4:e1000099. doi: 10.1371/journal.ppat.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F., Barrett J.W., Ma Y., Dekaban G.A., McFadden G. Induction of alpha/beta interferon by myxoma virus is selectively abrogated when primary mouse embryo fibroblasts become immortalized. J. Virol. 2009;83:5928–5932. doi: 10.1128/JVI.02587-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa R.J., Komar M., Tong J.G., Sivapragasam M., Rahman M.M., McFadden G., Dimattia G.E., Shepherd T.G. Myxoma virus-mediated oncolysis of ascites-derived human ovarian cancer cells and spheroids is impacted by differential AKT activity. Gynecol. Oncol. 2012;125:441–450. doi: 10.1016/j.ygyno.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong J.G., Ramos Valdes Y., Barrett J.W., Bell J.C., Stojdl D., McFadden G., McCart J.A., DiMattia G.E., Shepherd T.G. Evidence for differential viral oncolytic efficacy in an in vitro model of epithelial ovarian cancer metastasis. Mol. Ther. Oncolytics. 2015;2:15013. doi: 10.1038/mto.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennier S.T., Liu J., Li S., Rahman M.M., Mona M., McFadden G. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol. Ther. 2012;20:759–768. doi: 10.1038/mt.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moo-Young T.A., Larson J.W., Belt B.A., Tan M.C., Hawkins W.G., Eberlein T.J., Goedegebuure P.S., Linehan D.C. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J. Immunother. 2009;32:12–21. doi: 10.1097/CJI.0b013e318189f13c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan M.C., Goedegebuure P.S., Belt B.A., Flaherty B., Sankpal N., Gillanders W.E., Eberlein T.J., Hsieh C.S., Linehan D.C. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J. Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng C.W., Hung C.F., Alvarez R.D., Trimble C., Huh W.K., Kim D., Chuang C.M., Lin C.T., Tsai Y.C., He L. Pretreatment with cisplatin enhances E7-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. Clin. Cancer Res. 2008;14:3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandha H.S., Heinemann L., Simpson G.R., Melcher A., Prestwich R., Errington F., Coffey M., Harrington K.J., Morgan R. Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma. Clin. Cancer Res. 2009;15:6158–6166. doi: 10.1158/1078-0432.CCR-09-0796. [DOI] [PubMed] [Google Scholar]
- 27.Moehler M., Sieben M., Roth S., Springsguth F., Leuchs B., Zeidler M., Dinsart C., Rommelaere J., Galle P.R. Activation of the human immune system by chemotherapeutic or targeted agents combined with the oncolytic parvovirus H-1. BMC Cancer. 2011;11:464. doi: 10.1186/1471-2407-11-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke C.M., Kryczek I., Zou W. Antigen-presenting cell (APC) subsets in ovarian cancer. Int. Rev. Immunol. 2011;30:120–126. doi: 10.3109/08830185.2011.567362. [DOI] [PubMed] [Google Scholar]
- 29.Chan W.M., Bartee E.C., Moreb J.S., Dower K., Connor J.H., McFadden G. Myxoma and vaccinia viruses bind differentially to human leukocytes. J. Virol. 2013;87:4445–4460. doi: 10.1128/JVI.03488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gujar S.A., Clements D., Dielschneider R., Helson E., Marcato P., Lee P.W. Gemcitabine enhances the efficacy of reovirus-based oncotherapy through anti-tumour immunological mechanisms. Br. J. Cancer. 2014;110:83–93. doi: 10.1038/bjc.2013.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G., Barrett J.W., Stanford M., Werden S.J., Johnston J.B., Gao X., Sun M., Cheng J.Q., McFadden G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc. Natl. Acad. Sci. USA. 2006;103:4640–4645. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartee E., McFadden G. Human cancer cells have specifically lost the ability to induce the synergistic state caused by tumor necrosis factor plus interferon-beta. Cytokine. 2009;47:199–205. doi: 10.1016/j.cyto.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartee E., Mohamed M.R., Lopez M.C., Baker H.V., McFadden G. The addition of tumor necrosis factor plus beta interferon induces a novel synergistic antiviral state against poxviruses in primary human fibroblasts. J. Virol. 2009;83:498–511. doi: 10.1128/JVI.01376-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunlap K.M., Bartee M.Y., Bartee E. Myxoma virus attenuates expression of activating transcription factor 4 (ATF4) which has implications for the treatment of proteasome inhibitor-resistant multiple myeloma. Oncolytic Virother. 2015;4:1–11. doi: 10.2147/OV.S72372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartee M.Y., Dunlap K.M., Bartee E. Myxoma virus induces ligand independent extrinsic apoptosis in human myeloma cells. Clin. Lymphoma Myeloma Leuk. 2016;16:203–212. doi: 10.1016/j.clml.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkgraaf E.M., Heusinkveld M., Tummers B., Vogelpoel L.T., Goedemans R., Jha V., Nortier J.W., Welters M.J., Kroep J.R., van der Burg S.H. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 37.Loercher A.E., Nash M.A., Kavanagh J.J., Platsoucas C.D., Freedman R.S. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian carcinoma that inhibits cytokine protein expression and proliferation of autologous T cells. J. Immunol. 1999;163:6251–6260. [PubMed] [Google Scholar]
- 38.Goyne H.E., Stone P.J., Burnett A.F., Cannon M.J. Ovarian tumor ascites CD14+ cells suppress dendritic cell-activated CD4+ T-cell responses through IL-10 secretion and indoleamine 2,3-dioxygenase. J. Immunother. 2014;37:163–169. doi: 10.1097/CJI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton J., Blagih J., Ennis D., Leung E., Dowson S., Farquharson M., Tookman L.A., Orange C., Athineos D., Mason S. CRISPR/Cas9-mediated Trp53 and Brca2 knockout to generate improved murine models of ovarian high-grade serous carcinoma. Cancer Res. 2016;76:6118–6129. doi: 10.1158/0008-5472.CAN-16-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han Z., Hong Z., Gao Q., Chen C., Hao Z., Ji T., Hu W., Yan Y., Feng J., Liao S. A potent oncolytic adenovirus selectively blocks the STAT3 signaling pathway and potentiates cisplatin antitumor activity in ovarian cancer. Hum. Gene Ther. 2012;23:32–45. doi: 10.1089/hum.2011.101. [DOI] [PubMed] [Google Scholar]
- 41.Nair R.R., Tolentino J.H., Hazlehurst L.A. Role of STAT3 in transformation and drug resistance in CML. Front. Oncol. 2012;2:30. doi: 10.3389/fonc.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schust J., Sperl B., Hollis A., Mayer T.U., Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 43.Beyranvand Nejad E., van der Sluis T.C., van Duikeren S., Yagita H., Janssen G.M., van Veelen P.A., Melief C.J., van der Burg S.H., Arens R. Tumor eradication by cisplatin is sustained by CD80/86-mediated costimulation of CD8+ T cells. Cancer Res. 2016;76:6017–6029. doi: 10.1158/0008-5472.CAN-16-0881. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., McFadden G. SAMD9 is an innate antiviral host factor with stress response properties that can be antagonized by poxviruses. J. Virol. 2015;89:1925–1931. doi: 10.1128/JVI.02262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirandola L., Yu Y., Cannon M.J., Jenkins M.R., Rahman R.L., Nguyen D.D., Grizzi F., Cobos E., Figueroa J.A., Chiriva-Internati M. Galectin-3 inhibition suppresses drug resistance, motility, invasion and angiogenic potential in ovarian cancer. Gynecol. Oncol. 2014;135:573–579. doi: 10.1016/j.ygyno.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Roby K.F., Taylor C.C., Sweetwood J.P., Cheng Y., Pace J.L., Tawfik O., Persons D.L., Smith P.G., Terranova P.F. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]
- 47.Liu J., Wennier S., Zhang L., McFadden G. M062 is a host range factor essential for myxoma virus pathogenesis and functions as an antagonist of host SAMD9 in human cells. J. Virol. 2011;85:3270–3282. doi: 10.1128/JVI.02243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Wennier S., Moussatche N., Reinhard M., Condit R., McFadden G. Myxoma virus M064 is a novel member of the poxvirus C7L superfamily of host range factors that controls the kinetics of myxomatosis in European rabbits. J. Virol. 2012;86:5371–5375. doi: 10.1128/JVI.06933-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang C.L., Hsu Y.T., Wu C.C., Lai Y.Z., Wang C., Yang Y.C., Wu T.C., Hung C.F. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res. 2013;73:119–127. doi: 10.1158/0008-5472.CAN-12-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.