Abstract
Cytochrome P450 1A1 (CYP1A1) is a heme-containing mono-oxygenase involved in metabolism of environmental contaminants. Two variants of dog CYP1A1 with a single residue difference were identified and designated Sap1 and Sap2. Compared with Sap1, Sap2 had a Trp50Leu substitution. The biochemical characteristics of the variants were comparatively analyzed using heterologous expression in Escherichia coli. The membrane fraction of E. coli expressing Sap2 exhibited higher CYP holoprotein and heme contents than the Sap1-containing membranes, although the level of total CYP1A1 protein (i.e., apoprotein + holoprotein) was comparable between the groups. As normalized to holo-CYP content, the Sap2-expressing membranes showed lower CYP1A1-specific enzyme activities, such as 7-ethoxyresorufin O-dealkylation (EROD), than the Sap1 group. In single substitution variants of residue 50, proteins with hydrophobic residues having mass similar to Leu exhibited lower EROD activities than those with hydrophobic residues having larger mass than Leu. In addition, variants with polar or charged residues having mass similar to Leu showed activities that were comparable to those of Sap2. Taken together, these findings suggest that the Trp50Leu substitution leads to an enhancement of holo-CYP1A1 formation, but diminishes the enzyme activity because of the small size of Leu compared with Trp.
Keywords: CYP1A1, dog, enzyme activity, heme, holoenzyme
The cytochrome P450s (CYPs) are a superfamily of mono-oxygenases that play important roles in the oxidative metabolism of small hydrophobic chemicals [16]. Each CYP holoprotein is composed of an apoprotein moiety and a heme prosthetic group. The main mammalian CYP families include CYP1, CYP2 and CYP3. The CYP1 family consists of at least three proteins, CYP1A1, CYP1A2 and CYP1B1. CYP1A1 was one of the first CYP isozymes to be characterized and exhibits strong conservation among different mammalian species [10, 16]. Endogenous substrates of CYP1A1 include fatty acids and steroids. CYP1A1 is also involved in bioactivation and detoxification of environmental contaminants, such as polycyclic aromatic hydrocarbons (PAHs). Metabolisms of PAHs by CYP1A1 may result in the formation of diol-epoxides, radical cations and o-quinones [13]. These reactive metabolites produce DNA adducts, leading to DNA mutations, alteration of gene expression profiles and carcinogenesis.
Various human CYP1A1 genotypes have been associated with different human cancers, including prostate, esophageal, lung, cervical, renal cell, and head and neck cancers [1, 3, 8, 9, 11, 14, 21, 22, 27, 31]. Breast cancer is a common malignancy in women, and the mammary gland is also a common site for tumor development in bitches [25]. Carcinomas of the prostate are a common condition in men and occur frequently in neutered dogs [2].
In many mammalian CYP proteins, a proline-rich (PR) region is present following an N-terminal signal anchor and a short hydrophilic linker sequence [29]. One of the motifs in the PR region is the sequence PPGPxxxPxxGx, where the first four residues, PPGP, are highly conserved [5, 6]. The PR region is known to be involved in protein folding and holoenzyme expression in some CYP isozymes [7, 30].
The dog has served as a primary companion animal to humans and has also been widely used in various biomedical research fields, especially for assessing the metabolism, efficacy and safety of drug candidates [12, 20, 23]. However, the number of studies on dog CYP isozymes, especially CYP1A1, is limited compared with those of other laboratory species, such as rodents and rabbits. In this study, we cloned two cDNAs of dog CYP1A1. The two variants exhibited a difference in residue 50, which is predicted to be located within the PR region consensus sequence. Our findings suggest that the two variant apoproteins have different affinity for the prosthetic heme group, and that the variant enzymes have different catalytic activities.
MATERIALS AND METHODS
Chemicals
All chemicals used were of analytical grade or higher and were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.) unless specified.
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committees of Jeju National University (2015-0030). Approximately 3-year-old male and female dogs of the Sapsaree breed have been reared in the animal facilities at the College of Veterinary Medicine, Jeju National University. The breed of the dogs was certified by the National Research Institute of Cultural Heritage (Daejeon, Republic of Korea).
Mononuclear cells
Mononuclear cells were isolated from peripheral blood of dogs using a Lymphoprep Kit (Stemcell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. Isolated cells were washed twice with phosphate-buffered saline (PBS) and suspended in RPMI 1,640 medium containing 2 mg/ml sodium bicarbonate, 10% (v/v) fetal bovine serum and 100 U/ml penicillin. The cells were then treated with 10 nM 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; CIL Inc., Round Rock, TX, U.S.A.) to induce expression of CYP1A1 mRNA at 37°C for 24 hr in a humidified 5% CO2 incubator and used for RNA separation.
cDNA cloning
Total RNA was extracted from TCDD-treated mononuclear cells using TRIzol Reagent (Invitrogen, Carlsbad, CA, U.S.A.) and used to synthesize cDNA using M-MLV reverse transcriptase (Invitrogen) in the presence of random primers, according to the manufacturer’s instructions. Open reading frame CYP1A1 cDNAs were amplified using polymerase chain reactions (PCRs) with primers (Supplementary Table 1) that were designed based on the dog CYP1A1 sequence [26]. The PCR products of about 1.6-kb were subcloned into pTOP Blunt V2 vector (Enzynomics, Daejeon, Republic of Korea) and used for sequencing reactions on a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, U.S.A.).
Genomic DNA
Genomic DNA was extracted from blood samples using a QIAamp blood and tissue kit (Qiagen, Valencia, CA, U.S.A.). The region encompassing codons 50 and 52 was amplified using PCRs with the primers provided in Supplementary Table 1. The PCR products were used for sequencing reactions on a 3730xl DNA analyzer.
Sequence alignment
To compare the amino acid sequences of CYP1A1s from different mammalian species and of different dog CYP isozymes, multiple-sequence alignments were generated using the ESPript 3.0 alignment program [19].
Expression constructs
Open reading frame cDNA clones for the two dog CYP1A1 variants were modified for expression in Escherichia coli [4, 18]. The second N-terminal residue of CYP1A1, Met, was replaced with Ala, and the nucleotide sequences encoding residues 3–9 were changed to AT-rich sequence (5′- ATGGCTTCTATGTTTAGACTTTCTATT-3′) without substitution of residues. Each modified fragment was inserted into the NdeI and XbaI restriction sites of pCW-NPR, a NADPH-cytochrome P450 reductase-containing bicistronic expression vector. A pCW-NPR-based bacterial construct for human CYP1A1 expression was from Lee et al. [7].
Bacterial expression
E. coli DH5α cells were transformed with the expression constructs and grown overnight at 37°C in Luria–Bertani broth containing 50 µg/ml ampicillin. The overnight culture was inoculated 1:1,000 into Terrific Broth medium containing 50 µg/ml ampicillin and 1 mM thiamine. Cultures were incubated at 37°C with shaking at 200 rpm until they attained an OD600 of 0.5–0.7, then were supplemented with 1 mM isopropyl-β-D-thiogalactopyranoside (Amresco, Solon, OH, U.S.A.) and 0.5 mM δ-aminolevulinic acid (Cayman Chemical, Ann Arbor, MI, U.S.A.) and cultured for 24 hr.
Culture was centrifuged and washed with PBS, and resuspended in 100 mM Tris-acetate buffer, pH 7.6, containing 500 mM sucrose and 0.5 mM ethylenediaminetetraacetic acid. Lysozyme was added to 0.2 mg/ml, and the resulting spheroplasts were sonicated and centrifuged at 10,000 ×g at 4°C for 20 min. Supernatants were then centrifuged, and sedimented membrane fractions were resuspended in 100 mM potassium phosphate buffer, pH 7.6, containing 6 mM magnesium acetate, 20% glycerol (v/v) and 10 mM ME. The membrane preparation was stored at −70°C until use.
CYP and heme contents
Expression of the holo-CYP protein, or holo-CYP content, was determined by reduced CO difference spectra [17]. Sodium dithionite was added to reduce ferric CYPs. Ferrous-CO CYP complexes were generated by passing CO gas through solutions of the ferrous CYPs. The spectra were collected on a SpectraMax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, U.S.A.) at room temperature. Heme content was quantified using a pyridine hemochromogen assay and calculated from the difference in absorption between 557 and 575 nm [24].
Immunoblot analysis
Immunoblot analyses were conducted using a primary anti-CYP1A1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and a secondary horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (GenDepot, Barker, TX, U.S.A.). Blots were developed with a chemiluminescent detection kit (AbFrontier, Seoul, Republic of Korea). Protein concentrations were determined with a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, U.S.A.) using bovine serum albumin as the standard.
Site-directed mutagenesis
Site-directed mutagenesis was conducted to generate various single residue variants of dog and human CYP1A1 using an EZchange site-directed mutagenesis kit (Enzynomics) according to the manufacturer’s instructions. Primers used for mutagenesis are listed in Supplementary Table 1.
7-Ethoxyresorufin O-dealkylation (EROD) and 7-methoxyresorufin O-dealkylation (MROD)
7-Ethoxyresorufin (ER) and 7-methoxyresorufin (MR) are CYP1A1 probe substrates. EROD and MROD activities were assayed by fluorometric detection of resorufin using excitation and emission wavelengths of 544 and 595 nm, respectively. The fluorescence was measured with a Victor3 microplate spectrofluorometer (PerkinElmer, Waltham, MA, U.S.A.). The reaction mixture contained 20 µg of dilauroylphosphatidyl choline, 10 µM ER or MR, and E. coli membrane fractions (containing 50 pmol CYP) in a total volume of 0.5 ml of 50 mM potassium phosphate buffer, pH 7.6. Following a 5-min preincubation at 37°C in a water bath, reactions were initiated by the addition of the NADPH-generation system (final concentrations: 0.25 mM NADP+, 2.5 mM glucose 6-phosphate and 0.25 IU of yeast glucose 6-phosphate dehydrogenase). ER concentrations of 0.5–20 µM were used to determine the KM and Vmax parameters for EROD. Incubations were terminated after 10 min by the addition of 1 ml ice-cold methanol.
Benzo[a]pyrene (BaP) hydroxylation
Determination of BaP hydroxylation activity represents CYP1A1 activity. The reaction mixture contained 20 µg of dilauroylphosphatidyl choline, 3 mM MgCl2, 80 µM BaP (dissolved in acetone) and E. coli membrane fractions (containing 50 pmol CYP) in a total volume of 1 ml of 50 mM potassium phosphate buffer, pH 7.6. Following a 5-min preincubation at 37°C in a water bath, reactions were initiated by the addition of the NADPH-generation system. Incubation was terminated after 10 min by the addition of 1 ml of ice-cold acetone. After addition of acetone, 3.25 ml of hexane were added, and 2 ml of the organic phase was extracted with 4 ml of 1 N NaOH. The fluorescence was measured in the alkaline extract using excitation and emission wavelengths of 396 and 522 nm, respectively. BaP hydroxylation was determined using quinine sulfate in 0.1 N H2SO4 as a standard [15]. In the case of quinine sulfate, the wavelengths were set at 350 nm for excitation and 450 nm for emission. The fluorescence was measured with the Victor3 microplate spectrofluorometer.
Statistics
All data are expressed as the mean ± standard deviation (SD). Differences between sample groups were analyzed using the Student t-test or one-way analysis of variance followed by Tukey’s post hoc test. Statistical analysis was performed with SPSS 19.0 for Windows (SPSS, Chicago, IL, U.S.A.). A P-value of <0.05 was considered significant.
RESULTS
Sequence comparisons
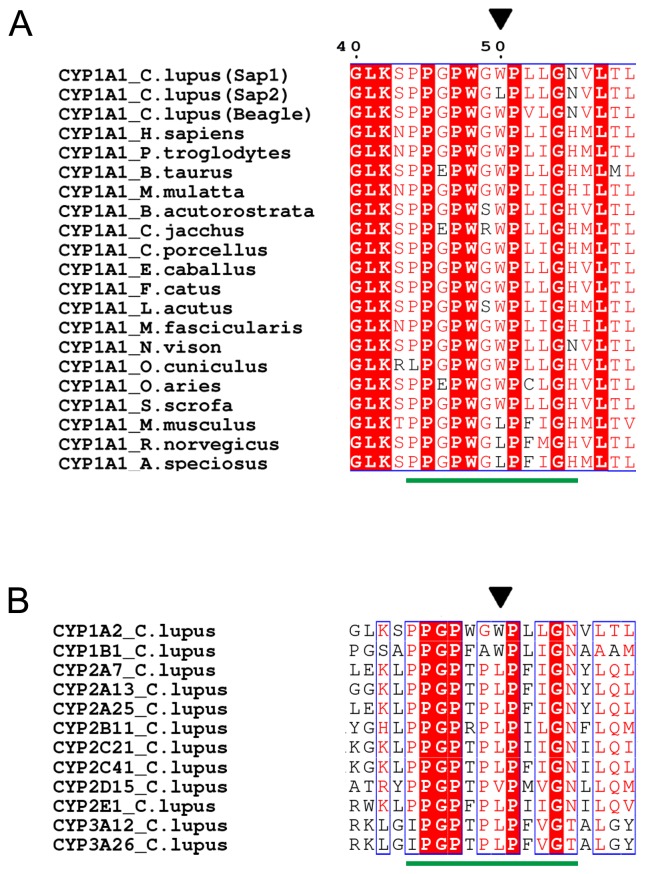
Two full-length CYP1A1 cDNA clones were isolated from TCDD-treated mononuclear cells of two dogs, a female and a male, and were designated Sap1 and Sap2 (Fig. 1A); the female had Sap1 and Sap2 variants, while the male had only Sap2. The Sap1 and Sap2 sequences were deposited in the NCBI under accession numbers KP340900 and KP340901, respectively. Compared with Sap1, Sap2 was found to have a G to T transversion in the second base of codon 50, which leads to a Trp50Leu substitution. Sap1 and Sap2 proteins have a Val52Leu substitution in comparison to CYP1A1 isolated from a Beagle [26]. Sequencing analyses for the region encompassing codons 50 and 52 using genomic DNA samples from the dogs confirmed the two nucleotide polymorphisms.
Fig. 1.
Sequence comparison of segments encompassing the PR region of (A) CYP1A1s from dogs and other mammalian species and of (B) dog CYP isozymes. The species and associated accession numbers for CYP1A1s are: C. lupus (KP340900; Sap1), C. lupus (KP340901; Sap2), C. lupus (P56590; Beagle), H. sapiens (NP_001306146.1), P. troglodytes (XP_001137654.1), B. taurus (XP_005222075.1), M. mulatta (NP_001035328.1), B. acutorostrata (BAE46562.1), C. jacchus (JAB11728.1), C. porcellus (NP_001166411.1), E. caballus (XP_014589581.1), F. catus (NP_001020030.1), L. acutus (AAV34440.1), M. fascicularis (NP_001306411.1), N. vison (ABS87659.1), O. cuniculus (NP_001164543.1), O. aries (NP_001123377.1), S. scrofa (NP_999577.1), M. musculus (NP_001129531.1), R. norvegicus (NP_036672.2) and A. speciosus (BAJ24835.1). The different dog CYP isozymes and associated accession numbers are: CYP1A2 (CDO96707.1), CYP1B1 (ACO52509), CYP2A7 (NP_001041492), CYP2A13 (NP_001032422), CYP2A25 (ABB43281), CYP2B11 (CDO96708), CYP2C21 (NP_001183973), CYP2C41 (NP_001003334), CYP2D15 (NP_001003333), CYP2E1 (NP_001003339), CYP3A12 (NP_001003340) and CYP3A26 (NP_001003338). Conserved residues are in blue boxes; identical residues are shown with a red background and similar residues in red typeface. Residue 50 of dog CYP1A1 and corresponding residues are indicated by a black triangle. A green horizontal bar indicates the PR region consensus sequence.
A sequence comparison among CYP1A1s of dogs and other mammalian species demonstrated that only Trp and Leu occur as the seventh residue of the PR region consensus sequence; aside from in Sap2, Leu was observed only in some rodent species (Fig. 1A). An alignment among other dog CYP isozymes showed that Leu occurs predominantly at this position, and Trp was observed only in two CYP1 family isozymes, CYP1A2 and CYP1B1 (Fig. 1B).
CYP and heme contents in CYP1A1s
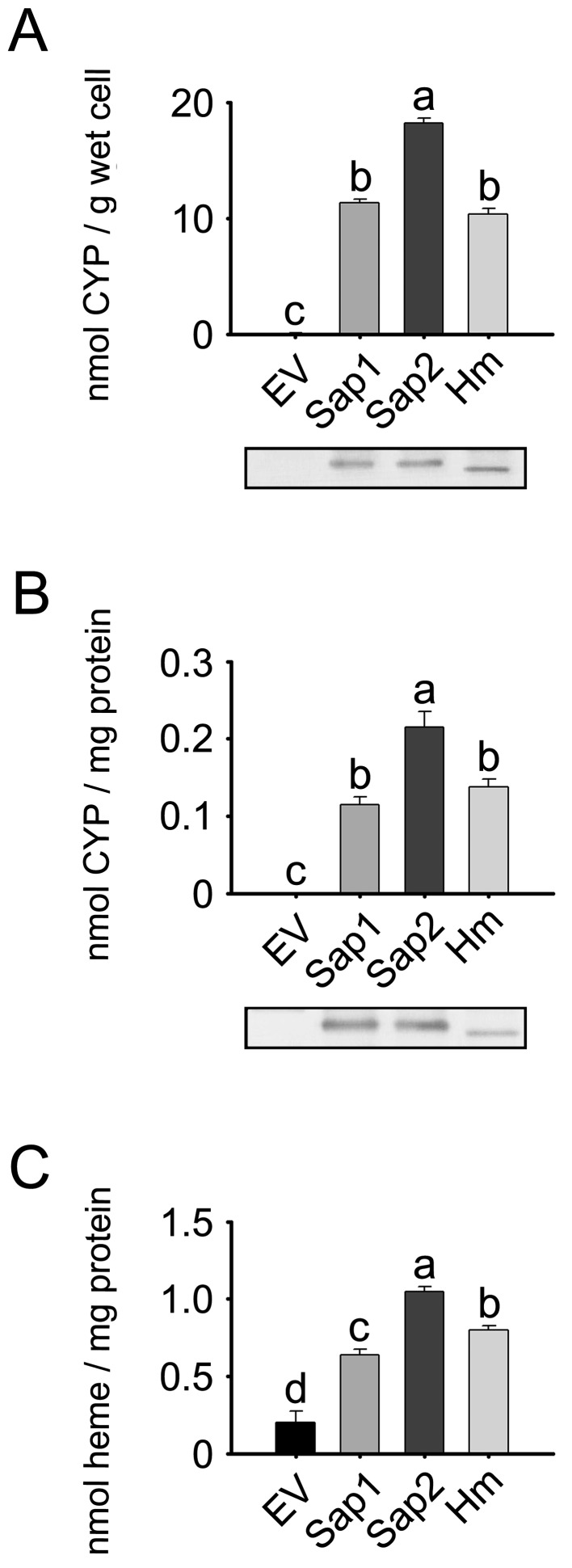
Whole E. coli cells expressing Sap1 and Sap2 variants as well as the cellular membrane fractions were used to produce CO-difference spectra to determine holo-CYP contents (i.e., CYP holoprotein). The cellular holo-CYP content in E. coli expressing Sap1 was about 62.2% of that in Sap2 expressing cells (P<0.05; Fig. 2A). The membrane fraction expressing Sap1 also had lower holo-CYP content, 53.5% of that for Sap2-containing membranes (P<0.05; Fig. 2B). Based upon immunoblots, the total levels of CYP1A1 protein (apoprotein + holoprotein) in the whole cells and membrane fractions of E. coli expressing Sap1 were similar to those in the Sap2 group. CYP1A1 protein was not detected in the whole cells and membrane fractions of E. coli transformed with empty control vector (EV). E. coli cells and their membrane fraction expressing human CYP1A1 were used as positive controls.
Fig. 2.
Expression of the dog CYP1A1 variants, Sap1 and Sap2. E. coli cells were transformed either with pCW-NPR vector including Sap1, Sap2 or human CYP1A1 (Hm) cDNA, or with empty control vector (EV). Total holo-CYP contents were quantified using reduced CO-difference spectra in (A) whole cells and (B) the membrane fractions. Lower insets represent total CYP1A1 protein expression in whole cells and membrane fractions, which were assessed by immunoblotting. (C) Heme contents were determined in the membrane fractions. Each bar represents the mean ± SD of six independent samples. Different lower-case letters indicate significant differences in each panel (P<0.05, Tukey’s post hoc test).
The heme content in the membrane fractions expressing Sap1 was 61.0% of that of the Sap2 group, and was about 3.2-times higher than that in the EV control group (P<0.05; Fig. 2C). The membrane fraction of E. coli transformed with EV had a heme level of 0.20 nmol/mg, which is likely to represent the endogenous heme level in the E. coli membrane fraction.
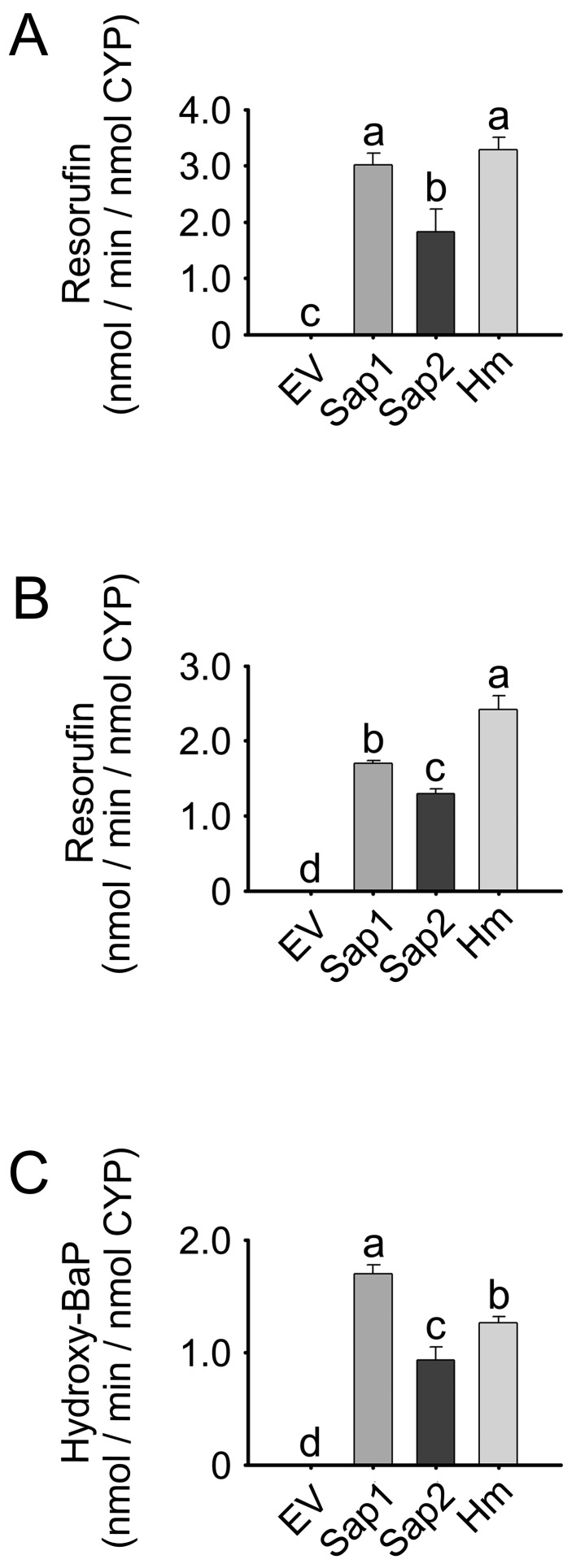
Catalytic activities of CYP1A1 variants
Membrane fractions expressing Sap1 and Sap2 were used for three CYP1A1-specific enzyme assays: EROD, MROD and BaP hydroxylation (Fig. 3). Normalized to holo-CYP content, the EROD, MROD and BaP hydroxylation activities of Sap1-expressing membranes were 1.63-, 1.32- and 1.83-fold higher than those of Sap2 expressing membranes, respectively (P<0.05). The EV control group exhibited very low or negligible catalytic activities in the three assays.
Fig. 3.
Enzyme activities of the dog CYP1A1 variants Sap1 and Sap2. (A) EROD, (B) MROD and (C) BaP hydroxylation activities in the membrane fractions of E. coli expressing Sap1, Sap2 or Hm protein, as well as EV controls. Each bar represents the mean ± SD of six independent samples. Different lower-case letters indicate significant differences in each panel (P<0.05, Tukey’s post hoc test).
The Vmax value of EROD for the Sap1-expressing membrane was higher by 89.0% than that for the Sap2-expressing membrane (P<0.05; Table 1), whereas the KM value for the Sap1 group was 36.7% lower than that for the Sap2 group (P<0.05). Thus, the catalytic efficiency of Sap1, as assessed by Vmax/KM, was 3.02-fold higher than that of Sap2 (P<0.05), suggesting that the Trp50Leu substitution produces a decrease in EROD activity.
Table 1. Kinetic parameters of EROD for membrane fractions from E. coli expressing Sap1 and Sap2.
|
Vmax (nmol/min/nmol CYP) |
KM (µM) |
Catalytic efficiency (Vmax/KM) |
|
|---|---|---|---|
| Sap1 | 3.63 ± 0.26a) | 1.71 ± 0.19a) | 2.14 ± 0.22a) |
| Sap2 | 1.93 ± 0.33 | 2.69 ± 0.25 | 0.71 ± 0.066 |
The parameters were determined by using Lineweaver-Burk plots and represent the means ± SD of six independent samples. “a” indicates a significant difference (Student’s t-test, P<0.05).
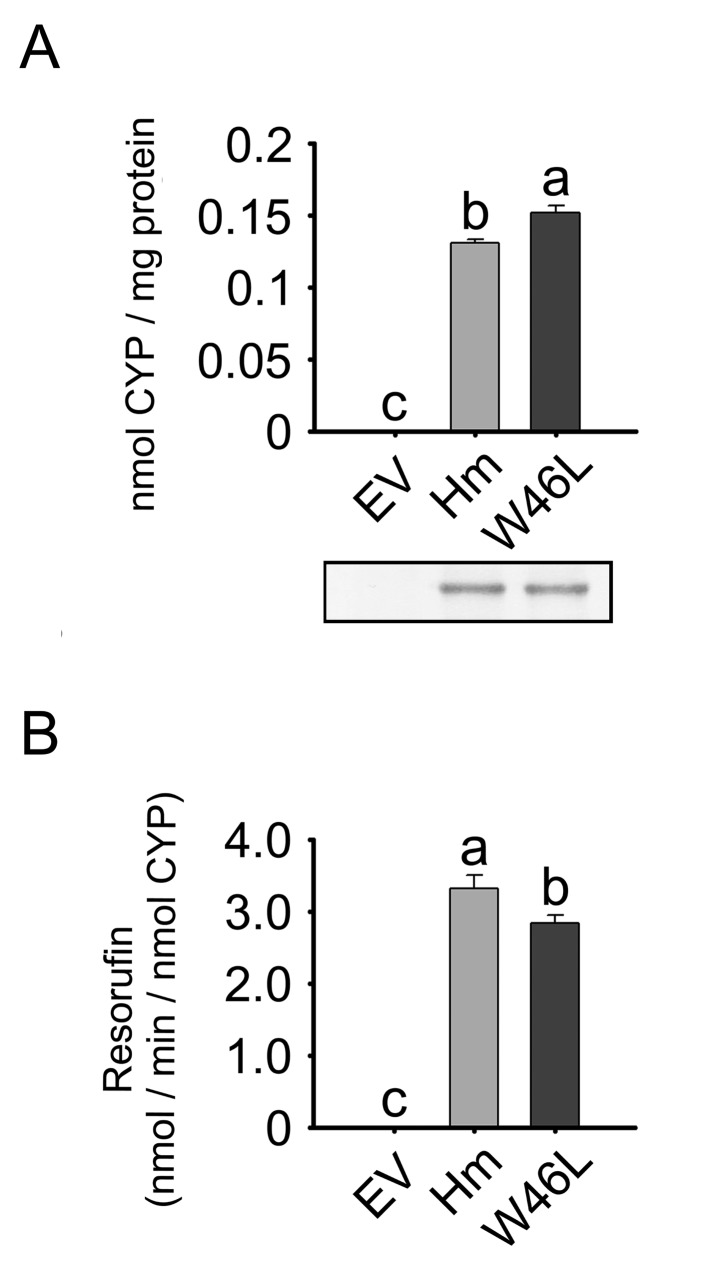
Trp46Leu substitution in human CYP1A1 (Hm)
Based upon sequence comparison (Fig. 1A), Trp46 of Hm was predicted to correspond to residue 50 of dog CYP1A1. By using residue substitution, we studied the structural and functional importance of residue 46 in a Trp46Leu variant of Hm (Fig. 4). The holo-CYP content (holoprotein) in membrane fractions of E. coli expressing wild-type Hm was about 86.2% of that for the Trp46Leu variant (P<0.05). Immunoblots demonstrated that the total level of CYP1A1 protein (apoprotein + holoprotein) in the membrane fractions expressing wild-type Hm was similar to that in the Trp46Leu group. The EROD activities of wild-type Hm-expressing membranes were 1.17-fold higher than those of the Trp46Leu group (P<0.05; Fig. 4B). In the EV controls, holo-CYP content and EROD activity were not detected.
Fig. 4.
Expression of wild-type Hm and the Trp46Leu variant. E. coli cells were transformed either with pCW-NPR vector including wild-type Hm or Trp46Leu cDNA, or with EV. Total holo-CYP contents (A) and EROD activities (B) were analyzed in the membrane fractions. The lower inset in (A) represents total CYP1A1 protein expression in the membrane fractions, which was assessed by immunoblotting. Each bar represents the mean ± SD of six independent samples. Different lower-case letters indicate significant differences in each panel (P<0.05, Tukey’s post hoc test).
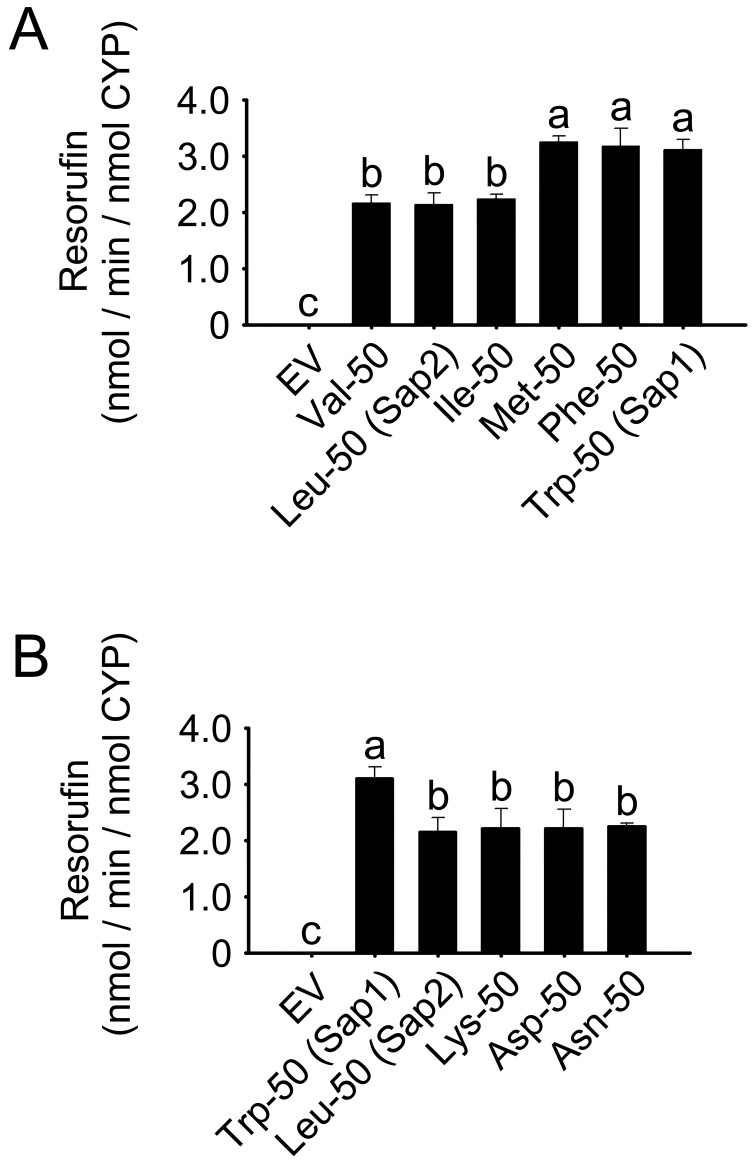
Substitutions of residue 50 with various residues
To study the structural characteristics responsible for the catalytic differences between Sap1 and Sap2 variants, we replaced residue 50 of dog (Sapsaree) CYP1A1 with hydrophobic residues of different mass (Fig. 5A). Membrane fractions of E. coli expressing Sap2 with Leu50 and Ile and Val variants exhibited EROD activities at least 32.0% lower than those for enzymes where residue 50 was larger (P<0.05). No significant differences in EROD activities were observed among Sap1 (Trp50) and Met50, and Phe50 variants, or among Sap2 (Leu50), and Val50 and Ile50 variants. In light of these findings, residue 50 was also substituted with polar or charged residues having mass similar to Leu: Lys, Asp and Asn (Fig. 5B). Membrane fractions of E. coli expressing variants with these polar or charged residues in position 50 had at least 27.6% lower EROD activities than Sap1. However, no significant difference was observed among Sap2 and the three variants with polar or charged groups.
Fig. 5.
EROD activities in membrane fractions of E. coli expressing Sap1, Sap2 and single residue-50 variants of dog CYP1A1, as well as EV controls. (A) Hydrophobic residue variants; (B) polar or charged residue variants. Each bar represents the mean ± SD of six independent samples. Different lower-case letters indicate significant differences in each panel (P<0.05, Tukey’s post hoc test).
DISCUSSION
Two novel cDNAs of dog CYP1A1 with a single base polymorphism were designated Sap1 and Sap2 (Fig. 1). Compared with Sap1, Sap2 had a Trp50Leu substitution. The two hydrophobic residues, Leu and Trp, are the most frequent to occur at the corresponding residue position in different dog CYP isozymes. In CYP1A1s of various mammalian species, Trp is observed more frequently at this position than Leu. Here, the Trp-harboring variants of dog and human CYP1A1s exhibited higher CYP1A1-specific catalytic activities than the Leu-containing forms (Figs. 3 and 4, Table 1). It is suggested that Trp, the largest natural amino acid residue in proteins, is a preferred residue at this position of CYP1A1s, with respect to the occurrence frequency, as well as to the activities of the variants harboring it.
The similar levels of total CYP1A1 proteins in the Sap1 and Sap2 expressing membranes (Fig. 2) suggest that the expression of the apoprotein and its incorporation into E. coli membranes were not affected by the Trp50Leu substitution. Considering the higher level of the CYP holoprotein in the Sap2 expressing membrane (Fig. 2), it is plausible that the Sap2 variant has a structural characteristic that is readily accessed by heme. However, the heme-carrying pool of the Sap2 variant is likely to have decreased enzymatic activity, when compared with that of the Sap1 variant (Fig. 3 and Table 1).
After subtracting these EV heme levels from those observed for the Sap1 and Sap2 groups, the heme level of the Sap1 membrane fraction was 51.8% of the Sap2 group ((0.64–0.20)/(1.05–0.20)=0.518), which was comparable with the difference in holo-CYP content between the Sap1 and Sap2 groups (Fig. 2). These findings suggest that the Trp50Leu substitution leads to an enhancement of holo-CYP1A1 formation by increasing the heme content of the protein without influencing total levels of the CYP1A1 protein (apoprotein + holoprotein). Based on these observations, we hypothesized that the substitution promotes heme incorporation.
Like the Trp50Leu substitution in dog CYP1A1, a corresponding substitution in human CYP1A1 (Trp46Leu) increased the holo CYP-protein level of E. coli membranes expressing the enzyme (Figs. 3 and 4), suggesting an elevated affinity of the apoproteins for the heme group. Trp46 is within the PR region (Fig. 1). In a three-dimensional structure of human CYP1A1 (Supplementary Fig. 1), Trp46 appears to hydrogen bond with Arg77 that belongs to β-sheet 1 [28]. Residues in β-sheet 1 have indirect interactions with the heme group via residues of substrate recognition site 5. In addition, a Trp46Gly substitution in human CYP1A1 diminishes both CYP and heme contents in E. coli membranes expressing the enzyme [7]. These findings indicate an important structural role of residue 46 in holoenzyme formation by human CYP1A1. It is plausible that the corresponding residue of dog CYP1A1, residue 50, has an equivalent function within that protein.
Sap2 and variants with hydrophobic residues with similar mass to Leu in position 50 exhibited catalytic activities lower than those of variants with other hydrophobic residues with mass larger than Leu (Fig. 5). Substitutions of Leu with polar or charged residues having similar mass to Leu did not affect the catalytic activity, suggesting polarity independence. It is thus possible that the difference in enzymatic activities between Sap1 and Sap2 is due to the size difference between Trp and Leu at residue position 50.
In the present study, two single-residue variants of dog CYP1A1 were identified and compared with respect to CYP holoprotein formation and catalytic activities. Future studies are warranted to expand our understanding of the influence of the polymorphism on gene regulation, as well as on any resulting susceptibility to environmentally-associated diseases, such as cancers.
CONFLICT OF INTEREST. The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was supported in part by Research Institute for Veterinary Sciences, Seoul National University.
REFERENCES
- 1.Ding G., Xu W., Liu H., Zhang M., Huang Q., Liao Z.2013. CYP1A1 MspI polymorphism is associated with prostate cancer susceptibility: evidence from a meta-analysis. Mol. Biol. Rep. 40: 3483–3491. doi: 10.1007/s11033-012-2423-0 [DOI] [PubMed] [Google Scholar]
- 2.Dobson J. M.2013. Breed-predispositions to cancer in pedigree dogs. ISRN Vet. Sci. 2013: 941275. doi: 10.1155/2013/941275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong F. F., Lu S. S., Hu C. Y., Qian Z. Z., Feng F., Wu Y. L., Yang H. Y., Sun Y. H.2014. Cytochrome P450 1A1 (CYP1A1) polymorphism and susceptibility to esophageal cancer: an updated meta-analysis of 27 studies. Tumour Biol. 35: 10351–10361. doi: 10.1007/s13277-014-2341-y [DOI] [PubMed] [Google Scholar]
- 4.Guengerich F. P., Martin M. V.2006. Purification of cytochromes P450: products of bacterial recombinant expression systems. Methods Mol. Biol. 320: 31–37. [DOI] [PubMed] [Google Scholar]
- 5.Kemper B.2004. Structural basis for the role in protein folding of conserved proline-rich regions in cytochromes P450. Toxicol. Appl. Pharmacol. 199: 305–315. doi: 10.1016/j.taap.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 6.Kusano K., Sakaguchi M., Kagawa N., Waterman M. R., Omura T.2001. Microsomal p450s use specific proline-rich sequences for efficient folding, but not for maintenance of the folded structure. J. Biochem. 129: 259–269. doi: 10.1093/oxfordjournals.jbchem.a002853 [DOI] [PubMed] [Google Scholar]
- 7.Lee S. H., Yu H. J., Lee S., Ryu D. Y.2015. Characterization of the Gly45Asp variant of human cytochrome P450 1A1 using recombinant expression. Toxicol. Lett. 239: 81–89. doi: 10.1016/j.toxlet.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 8.Li W., Song L. Q., Tan J.2014. Combined effects of CYP1A1 MspI and GSTM1 genetic polymorphisms on risk of lung cancer: an updated meta-analysis. Tumour Biol. 35: 9281–9290. doi: 10.1007/s13277-014-2212-6 [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Wu G., Xue F., Li Y., Shi J., Han J., Zhang X., Na Y., Zhang H., Tang X., Pu H., Yuan Q., Zhang L., Yang M.2013. Functional CYP1A1 genetic variants, alone and in combination with smoking, contribute to development of head and neck cancers. Eur. J. Cancer 49: 2143–2151. doi: 10.1016/j.ejca.2013.01.028 [DOI] [PubMed] [Google Scholar]
- 10.Martignoni M., Groothuis G. M., de Kanter R.2006. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2: 875–894. doi: 10.1517/17425255.2.6.875 [DOI] [PubMed] [Google Scholar]
- 11.Meng F. D., Ma P., Sui C. G., Tian X., Jiang Y. H.2015. Association between cytochrome P450 1A1 (CYP1A1) gene polymorphisms and the risk of renal cell carcinoma: a meta-analysis. Sci. Rep. 5: 8108. doi: 10.1038/srep08108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mise M., Hashizume T., Komuro S.2008. Characterization of substrate specificity of dog CYP1A2 using CYP1A2-deficient and wild-type dog liver microsomes. Drug Metab. Dispos. 36: 1903–1908. doi: 10.1124/dmd.108.022301 [DOI] [PubMed] [Google Scholar]
- 13.Moorthy B., Chu C., Carlin D. J.2015. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol. Sci. 145: 5–15. doi: 10.1093/toxsci/kfv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata M., Shiraishi T., Fukutome K., Watanabe M., Nagao M., Kubota Y., Ito H., Kawamura J., Yatani R.1998. Cytochrome P4501A1 and glutathione S-transferase M1 genotypes as risk factors for prostate cancer in Japan. Jpn. J. Clin. Oncol. 28: 657–660. doi: 10.1093/jjco/28.11.657 [DOI] [PubMed] [Google Scholar]
- 15.Nebert D. W., Gelboin H. V.1968. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J. Biol. Chem. 243: 6242–6249. [PubMed] [Google Scholar]
- 16.Nelson D. R., Koymans L., Kamataki T., Stegeman J. J., Feyereisen R., Waxman D. J., Waterman M. R., Gotoh O., Coon M. J., Estabrook R. W., Gunsalus I. C., Nebert D. W.1996. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6: 1–42. doi: 10.1097/00008571-199602000-00002 [DOI] [PubMed] [Google Scholar]
- 17.Omura T., Sato R.1964. The carbon monoxide-binding pigment of liver microsomes. II. solubilization, purification, and properties. J. Biol. Chem. 239: 2379–2385. [PubMed] [Google Scholar]
- 18.Parikh A., Gillam E. M., Guengerich F. P.1997. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat. Biotechnol. 15: 784–788. doi: 10.1038/nbt0897-784 [DOI] [PubMed] [Google Scholar]
- 19.Robert X., Gouet P.2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42: W320–324. doi: 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherr M. C., Lourenço G. J., Albuquerque D. M., Lima C. S.2011. Polymorphism of cytochrome P450 A2 (CYP1A2) in pure and mixed breed dogs. J. Vet. Pharmacol. Ther. 34: 184–186. doi: 10.1111/j.1365-2885.2010.01243.x [DOI] [PubMed] [Google Scholar]
- 21.Sergentanis T. N., Economopoulos K. P., Choussein S., Vlahos N. F.2012. Cytochrome P450 1A1 (CYP1A1) gene polymorphisms and cervical cancer risk: a meta-analysis. Mol. Biol. Rep. 39: 6647–6654. doi: 10.1007/s11033-012-1470-x [DOI] [PubMed] [Google Scholar]
- 22.Shaik A. P., Jamil K., Das P.2009. CYP1A1 polymorphisms and risk of prostate cancer: a meta-analysis. Urol. J. 6: 78–86. [PubMed] [Google Scholar]
- 23.Shou M., Norcross R., Sandig G., Lu P., Li Y., Lin Y., Mei Q., Rodrigues A. D., Rushmore T. H.2003. Substrate specificity and kinetic properties of seven heterologously expressed dog cytochromes p450. Drug Metab. Dispos. 31: 1161–1169. doi: 10.1124/dmd.31.9.1161 [DOI] [PubMed] [Google Scholar]
- 24.Sinclair P. R., Gorman N., Jacobs J. M.2001. Measurement of heme concentration. Curr. Protoc. Toxicol. 8: 8.3.1–8.3.7. [DOI] [PubMed] [Google Scholar]
- 25.Sleeckx N., de Rooster H., Veldhuis Kroeze E. J., Van Ginneken C., Van Brantegem L.2011. Canine mammary tumours, an overview. Reprod. Domest. Anim. 46: 1112–1131. doi: 10.1111/j.1439-0531.2011.01816.x [DOI] [PubMed] [Google Scholar]
- 26.Uchida T., Komori M., Kitada M., Kamataki T.1990. Isolation of cDNAs coding for three different forms of liver microsomal cytochrome P-450 from polychlorinated biphenyl-treated beagle dogs. Mol. Pharmacol. 38: 644–651. [PubMed] [Google Scholar]
- 27.Vijayalakshmi K., Vettriselvi V., Krishnan M., Shroff S., Jayanth V. R., Paul S. F.2005. Cytochrome p4501A1 gene variants as susceptibility marker for prostate cancer. Cancer Biomark. 1: 251–258. doi: 10.3233/CBM-2005-14-508 [DOI] [PubMed] [Google Scholar]
- 28.Walsh A. A., Szklarz G. D., Scott E. E.2013. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J. Biol. Chem. 288: 12932–12943. doi: 10.1074/jbc.M113.452953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams P. A., Cosme J., Sridhar V., Johnson E. F., McRee D. E.2000. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol. Cell 5: 121–131. doi: 10.1016/S1097-2765(00)80408-6 [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki S., Sato K., Suhara K., Sakaguchi M., Mihara K., Omura T.1993. Importance of the proline-rich region following signal-anchor sequence in the formation of correct conformation of microsomal cytochrome P-450s. J. Biochem. 114: 652–657. doi: 10.1093/oxfordjournals.jbchem.a124232 [DOI] [PubMed] [Google Scholar]
- 31.Zhou S. F., Liu J. P., Chowbay B.2009. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 41: 89–295. doi: 10.1080/03602530902843483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.