Abstract
In the present study, bull sperm in the first and second ejaculates were divided into subpopulations based on their motility characteristics using a cluster analysis of data from computer-assisted sperm motility analysis (CASA). Semen samples were collected from 4 Japanese black bulls. Data from 9,228 motile sperm were classified into 4 clusters; 1) very rapid and progressively motile sperm, 2) rapid and circularly motile sperm with widely moving heads, 3) moderately motile sperm with heads moving frequently in a short length, and 4) poorly motile sperm. The percentage of cluster 1 varied between bulls. The first ejaculates had a higher proportion of cluster 2 and lower proportion of cluster 3 than the second ejaculates.
Keywords: bovine, cluster analysis, computer-assisted sperm analysis, motility, spermatozoa
Sperm motility is regarded as the most common factor for predicting male fertility [2]. However, a previous study reported that correlations between the percentages of motile sperm evaluated by conventional methods and fertility fluctuate widely because the standard semen analysis, sperm observations under microscopy performed by practitioners, is a subjective technique and associated with large inter-laboratory variations [18]. Mocé and Graham [25] suggested that inconsistencies are derived from differences in the multifactorial nature of sperm function, and partly by the inaccuracy of in vitro measurements. Computer-assisted sperm motility analysis (CASA) has been developed as a tool for the objective evaluation of sperm motility in humans and animals [10, 16], and provides information on the motility characteristics of individual sperm. Nevertheless, previous studies have mostly reported average values for kinetic parameters without focusing on individual sperm characteristics [10, 12, 14, 23, 35].
The criteria for evaluating fertility in men were defined in the fourth edition of the WHO guidelines [36] as the average value of progressively motile sperm using straight line velocity (VSL: the straight line distance from beginning to end of a sperm track for 1 sec), which has been categorized into 4 grades; A: progressive with VSL ≥25 µm/sec, B: progressive with VSL <25 µm/sec, C: non-progressive, and D: immotile. However, these criteria were omitted from the fifth edition [37] and sperm motility evaluated by microscopic observations was divided into 3 grades: progressively motile, non-progressively motile, and immotile, possibly due to the difficulties technicians have with consistently and reproducibly distinguishing between grades “A” and “B” [5]. This may indicate that sperm velocity is not effective to predict male fertility. Holt et al. [15] showed that a linear regression model containing the amplitude of lateral head displacement (ALH; the mean width of sperm head oscillations) or beat cross frequency (BCF) of sperm heads indicated the significant coefficient of determination with the litter size in boar sperm. These suggest that the moving pattern of sperm heads correlates with fertility, similar to velocity parameters. Therefore, assessing the velocity and head movements of sperm is necessary for precisely evaluating its fertility.
Previous studies reported the potential to divide sperm derived from humans and animals into subpopulations based on the kinetic characteristics of individual sperm using CASA data [1, 7, 16, 24, 29, 32]. A cluster analysis, which is a statistical method for constructing small groups (clusters) using multiple parameters from a large set of data that may contribute to a better understanding of the clumping structure of data, simultaneously using data on sperm head movement and velocity parameters may be more suitable for the prediction of fertility. Previous studies using subpopulations reported that the proportion of the “progressively motile sperm” subpopulation, which had the highest velocity and linearity was related to male fertility [1, 7, 16, 24, 29, 33, 38]. However, a previous study in stallion indicated the relationship between fertility and a subpopulation with low velocity and high linearity [32]. We hypothesized that a cluster analysis of CASA data will become a powerful tool for the evaluation of male fertility if sperm subpopulations other than progressively motile and high velocity sperm are related to pregnancy rates in artificial insemination (AI).
In order to accurately evaluate the relationship between fertility and sperm motility, we need to compare sperm subpopulation structures with semen having consistent results of fertility. However, the pregnancy rate after AI in cattle fluctuates between practitioners [11], season [4], and reproductive management [31]. Semen is generally collected from bulls twice a week at AI centers. On the day of semen collection, the first and second ejaculates are collected within a short interval of time. During repetitive ejaculations, changes have been reported in semen constituents and characteristics [21], such as pH, osmolality, and the concentration of fructose, which affect sperm motility and fertility [17, 34]. Sperm in the first ejaculate have been shown to exhibit similar [9] or higher motility [6, 10] to that in the second ejaculate. However, the second ejaculate showed higher fertility than the first ejaculate when used for AI without freezing [6]. This discrepancy between these findings may be caused by sperm characteristics, which cannot be investigated by conventional methods using light microscopy. Therefore, we herein used the first and second ejaculates as low and high potential fertility sperm models, respectively.
The purpose of this study is to investigate the potential of an analysis of sperm subpopulation structures for the evaluation of sperm motility probably related to fertility by using CASA data on the first and second ejaculates. Furthermore, the sperm subpopulation structures of individual bulls were examined because differences in fertility between bulls were reported [8].
Four Japanese black bulls (A–D, 5–15 years old), which were kept for the production of frozen semen in the AI center (Genetics Hokkaido, Kita-Hiroshima, Japan), were used in the present study. Their frozen-thawed semen were used commercially and indicated acceptable conception rates by AI in the field.
Semen were collected using artificial vaginas, which included a glass tube and the first and second ejaculates within one day were collected separately at 18- to 39-min intervals. Ejaculates were collected in six separate sessions per bull, two sessions per week. Therefore, twelve ejaculates were collected in three weeks. Immediately after collection, the volume of semen was evaluated by visually checking the scale on the tube, while sperm concentrations were assessed using a photometer (SDM 5 12300/0007 DE, Minitube, Tiefenbach, Germany). Sperm motility was also examined under light microscopy, as described previously [30], and subjectively classified into the 5 grades (+++: progressively motile at a high speed, ++: progressively motile at a moderate speed, +: motile at a low speed, ±: motile without progression d -: immotile). The proportions of sperm with +++ and ++ grades were defined as motile sperm. These evaluations were performed independently by two practitioners and the mean value was used as the value for motile sperm. Semen qualities evaluated by practitioners were described in Table 1.
Table 1. Semen quality of Japanese black bulls evaluated by conventional methods.
| Bull | Ejaculation order (replicates) | Semen volume (ml) | Sperm concentration (×106 sperm / ml) | Motile sperma) (%) |
|---|---|---|---|---|
| A | 1st (6) | 7.2 ± 1.8 | 14.5 ± 2.7 | 65.8 ± 2.0 |
| 2nd (6) | 6.4 ± 1.3 | 9.5 ± 1.4 | 65.0 ± 0.0 | |
| Total (12) | 6.7 ± 1.5 | 12.0 ± 3.3 | 65.4 ± 1.4 | |
| B | 1st (6) | 5.0 ± 1.1 | 9.7 ± 3.1 | 70.0 ± 4.5 |
| 2nd (6) | 4.4 ± 0.8 | 8.8 ± 2.0 | 65.8 ± 2.0 | |
| Total (12) | 4.7 ± 1.0 | 9.3 ± 2.5 | 67.9 ± 4.0 | |
| C | 1st (6) | 5.5 ± 0.8 | 11.0 ± 2.7 | 65.8 ± 2.0 |
| 2nd (6) | 5.0 ± 1.1 | 10.9 ± 0.5 | 66.7 ± 2.6 | |
| Total (12) | 5.2 ± 0.9 | 11.0 ± 1.9 | 66.3 ± 2.3 | |
| D | 1st (6) | 4.5 ± 0.5 | 9.6 ± 1.4 | 65.0 ± 0.0 |
| 2nd (6) | 4.0 ± 1.1 | 4.8 ± 2.5 | 65.0 ± 0.0 | |
| Total (12) | 4.2 ± 0.8 | 7.2 ± 3.2 | 65.0 ± 0.0 | |
| Total | 1st (24) | 5.5 ± 1.5 | 11.2 ± 3.1 | 66.7 ± 3.2 |
| 2nd (24) | 4.9 ± 1.4 | 8.5 ± 2.9 | 65.6 ± 1.7 | |
Values are the mean ± standard deviation. a) Progressively and actively motile sperm (+++ and ++) assessed by visual inspections (Okano et al. [30]).
Immediately after semen collection, a small amount of semen was diluted to a concentration of 10–20 × 106 sperm/ml using physiological saline, because phosphate buffered saline significantly inhibited the sperm motility. Immediately after dilution, samples were introduced into a 20-µm-deep chamber (SC20-01-04-B, Leja, GN Nieuw-Vennep, Netherlands) preliminary warmed at 37°C on a hot plate, and sperm motility was evaluated by a CASA system (SMAS, DITECT, Tokyo, Japan) based on the digitalized images obtained using ×10 negative-phase contrast microscope (E200, Nikon, Tokyo, Japan). The percentage of motile sperm was recorded and the following kinetic parameters were analyzed: VSL, curvilinear velocity (VCL: a measure of total distance travelled by a sperm for 1 sec), average path velocity (VAP: the average path velocity of sperm for 1 sec), ALH, and BCF. The number of sperm analyzed per sample was at least 200, including immotile sperm. Linearity (LIN=VSL/VCL; the state of being linear) was calculated automatically using the CASA system. The CASA system recorded 150 frames per second (fps), and sperm having more than 120 frames were used in the analysis.
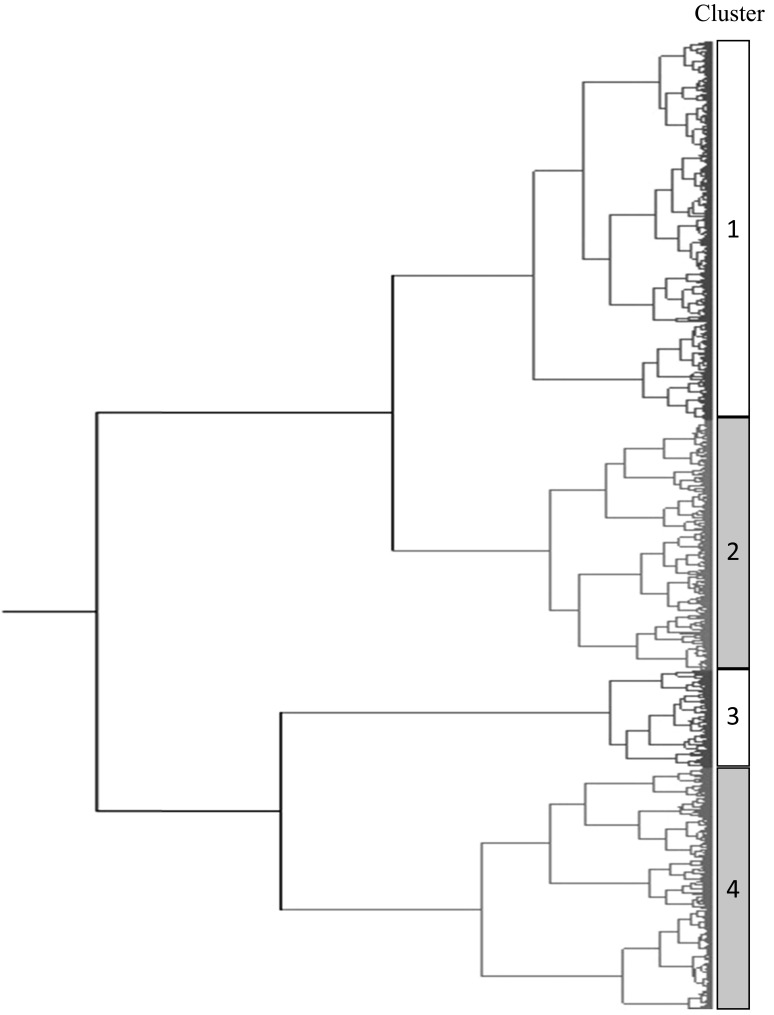
A cluster analysis of sperm motility was performed as described previously [29] with slight modifications. Data from 14,264 sperm in 48 fresh semen samples (2 ejaculates from 4 bulls in 6 replicates) were obtained. In the motility analysis, data from 1,306 immotile sperm and 3,730 motile sperm having less than 120 frames were discarded. Then data from the remaining 9,228 motile sperm were imported into a single data set and analyzed. In the cluster analysis, the 5 independent motility variables: VSL, VCL, VAP, ALH and BCF, were used as parameters after the normalization of data adapted by the following formula: (measurements-average)/standard deviation. The number of clusters was assessed by the shape of the dendrogram according to Ward’s method and determined the cluster number subjectively from the shape of dendrogram (Fig. 1). A multivariate k-means cluster analysis was performed to classify the 9,228 sperm into a reduced number of subpopulations based on their motility variables. Spermatozoa that showed similar motility characteristics were assigned to the same cluster. The k-means clustering model used Euclidean distances, which were computed from the 5 quantitative variables, and the cluster centers were the means of the observations assigned to each cluster. The effects of bulls and the order of ejaculation (ejaculation difference) on sperm motility characteristics assessed by CASA and the percentage of progressively motile sperm evaluated in the visual assessment were analyzed using a two-way ANOVA. Differences in the means of semen quality evaluated by practitioners and motility parameters assessed by CASA between ejaculates and bulls were analyzed by Tukey-Kramer’s HSD test. Differences were considered significant at P<0.05. All analyses were performed using JMP pro 12 (SAS, NC, U.S.A.).
Fig. 1.
Dendrogram described by Ward’s method to determine the number of cluster. The 5 independent motility variables: VSL, VCL, VAP, ALH and BCF, were used as parameters.
There was no interaction in the mean values of kinetic parameters between bulls and the ejaculation difference (Table 2). Therefore, the effects of the two factors were analyzed separately (Table 3). No significant difference was observed in the percentages of motile sperm between bulls and the ejaculation difference. Bull A showed higher VSL than bull D (P<0.05). Bulls A and C had higher VCL, VAP and ALH than bull D (P<0.05). No significant differences were noted in VSL, VCL, or VAP between the first and second ejaculates. However, the first ejaculates had higher ALH and lower BCF than the second ejaculates (P<0.05).
Table 2. Effects of the ejaculation order and bulls on mean values of kinetic parameters evaluated by CASA; probability values analyzed by a two-way ANOVA.
| Parameters | Factors affecting each kinetic parameter | ||
|---|---|---|---|
| Ejaculation order | Bull | Interaction | |
| % of motile sperm | 0.9376 | 0.2112 | 0.8546 |
| VSL | 0.1597 | 0.0074 | 0.5579 |
| VCL | 0.0856 | 0.0011 | 0.5704 |
| VAP | 0.1655 | 0.0037 | 0.4151 |
| ALH | 0.0113 | 0.0077 | 0.2770 |
| BCF | 0.0145 | 0.7670 | 0.9238 |
| LIN | 0.4462 | 0.5092 | 0.4701 |
Table 3. Mean values of kinetic parameters of sperm in each bull and the order of ejaculation.
| Bull | Ejaculation order | Replicates | % of motile sperm | VSL (µm/sec) |
VCL (µm/sec) |
VAP (µm/sec) |
ALH (µm) |
BCF (Hz) |
LIN (%) |
|---|---|---|---|---|---|---|---|---|---|
| A | 1st | 6 | 91.8 ± 3.6 | 117.7 ± 16.9 | 310.9 ± 27.9 | 147.0 ± 17.6 | 5.1 ± 0.2 | 15.6 ± 2.1 | 35.6 ± 3.4 |
| 2nd | 6 | 92.1 ± 3.7 | 104.1 ± 22.9 | 261.7 ± 58.3 | 125.4 ± 27.5 | 4.0 ± 1.0 | 17.4 ± 1.6 | 36.0 ± 2.5 | |
| Total | 12 | 92.0 ± 3.5 | 110.9 ± 21.2a) | 286.3 ± 51.9a) | 136.2 ± 25.5a) | 4.5 ± 0.9a) | 16.5 ± 2.1 | 35.8 ± 3.0 | |
| B | 1st | 6 | 90.9 ± 5.9 | 91.7 ± 23.8 | 250.1 ± 63.6 | 117.5 ± 29.7 | 4.2 ± 1.1 | 15.7 ± 2.2 | 34.2 ± 2.1 |
| 2nd | 6 | 88.1 ± 9.9 | 95.5 ± 20.3 | 243.4 ± 42.9 | 120.2 ± 22.4 | 3.9 ± 0.7 | 16.9 ± 2.6 | 34.6 ± 5.0 | |
| Total | 12 | 89.5 ± 7.9 | 93.6 ± 22.2a,b) | 246.8 ± 54.3a,b) | 118.8 ± 26.3a,b) | 4.0 ± 0.9a,b) | 16.3 ± 2.5 | 34.4 ± 3.8 | |
| C | 1st | 6 | 91.4 ± 5.2 | 107.0 ± 11.3 | 286.5 ± 28.5 | 134.2 ± 12.2 | 4.7 ± 0.5 | 16.0 ± 1.2 | 33.9 ± 1.4 |
| 2nd | 6 | 92.0 ± 6.4 | 103.9 ± 9.7 | 282.8 ± 36.5 | 135.0 ± 14.3 | 4.3 ± 0.8 | 18.0 ± 1.5 | 33.6 ± 1.2 | |
| Total | 12 | 91.7 ± 5.5 | 105.4 ± 10.6a,b) | 284.6 ± 32.8a) | 134.6 ± 13.3a) | 4.5 ± 0.7a) | 17.0 ± 1.7 | 33.8 ± 1.3 | |
| D | 1st | 6 | 86.6 ± 6.7 | 93.9 ± 2.1 | 232.4 ± 18.8 | 114.3 ± 4.5 | 3.8 ± 0.2 | 16.6 ± 1.4 | 35.9 ± 2.1 |
| 2nd | 6 | 87.8 ± 5.4 | 75.0 ± 21.0 | 197.5 ± 42.6 | 96.6 ± 19.5 | 3.2 ± 0.8 | 17.5 ± 1.6 | 32.5 ± 4.5 | |
| Total | 12 | 87.2 ± 5.9 | 84.5 ± 17.6b) | 214.9 ± 37.2b) | 105.4 ± 16.7b) | 3.5 ± 0.6b) | 17.0 ± 1.6 | 34.2 ± 3.9 | |
| Average | 1st | 24 | 90.0 ± 6.4 | 101.0 ± 16.8 | 281.3 ± 46.7 | 131.2 ± 21.4 | 4.7 ± 0.8* | 15.4 ± 2.2 | 33.2 ± 3.7 |
| 2nd | 24 | 90.5 ± 6.6 | 95.2 ± 20.2 | 255.7 ± 52.3 | 121.4 ± 22.6 | 4.1 ± 0.9 | 17.0 ± 2.8* | 33.5 ± 4.0 | |
Values are the mean ± standard deviation. a,b) Superscripts indicate significant differences between bulls (P<0.05). *The asterisk indicates a significant difference between ejaculation orders (P<0.05).
As shown in Fig. 1, 9,228 motile sperm derived from 48 ejaculates were categorized into 4 clusters. Kinetic parameters of sperm in each cluster were described in Table 4. Cluster 1 showed the highest velocities and highest LIN as well as the second highest ALH and BCF. Cluster 2 showed the second highest velocities and highest ALH, but lower BCF and LIN than clusters 1 and 3. Although all 3 velocities of cluster 3 were lower than those of clusters 1 and 2, cluster 3 had the highest BCF, lowest ALH, and second highest LIN. Cluster 4 had the lowest values in all parameters.
Table 4. Kinetic parameters of sperm in each cluster.
| Cluster | No. of sperm | VSL (µm/sec) |
VCL (µm/sec) |
VAP (µm/sec) |
ALH (µm) |
BCF (Hz) |
LIN (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3,594 | 162.8 ± 25.0 | 350.9 ± 48.8 | 180.5 ± 21.3 | 5.1 ± 1.0 | 20.0 ± 3.4 | 46.9 ± 7.2 |
| 2 | 2,529 | 98.8 ± 38.0 | 333.6 ± 69.7 | 145.0 ± 30.4 | 6.0 ± 1.2 | 12.7 ± 4.1 | 30.4 ± 11.6 |
| 3 | 1,248 | 49.3 ± 30.4 | 153.9 ± 54.4 | 88.2 ± 29.9 | 2.0 ± 1.1 | 27.0 ± 5.7 | 33.3 ± 17.8 |
| 4 | 1,857 | 12.9 ± 15.8 | 75.9 ± 56.5 | 22.4 ± 21.2 | 1.9 ± 1.5 | 8.7 ± 4.1 | 16.5 ± 12.1 |
Values are the mean ± standard deviation.
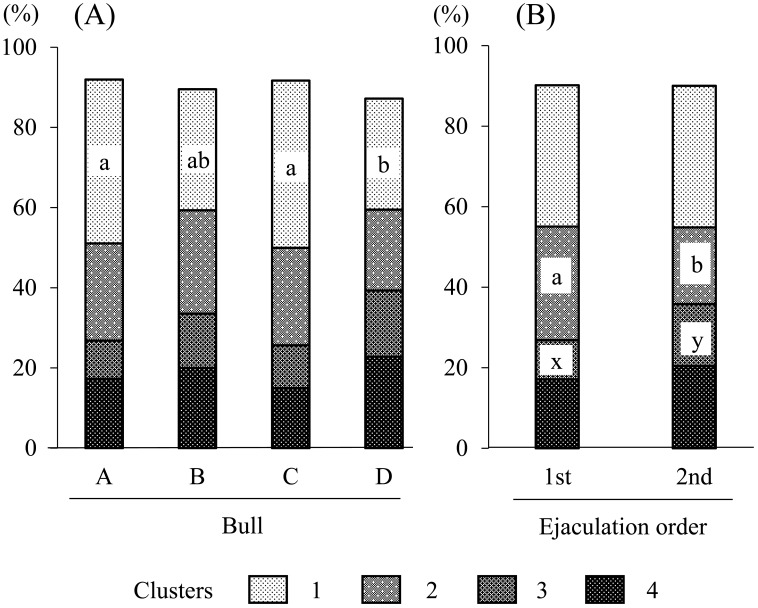
As shown in Table 5, there was no interaction in sperm subpopulation structures between bulls and the order of ejaculation; however, the percentage of cluster 1 was affected by the bull factor, and the percentages of clusters 2 and 3 were affected by the ejaculation difference. Therefore, the effects of the two factors were analyzed individually (Fig. 2). Bulls A and C had a higher percentage of cluster 1 than bull D (P<0.05, Fig. 2A). No significant differences were observed in the percentages of clusters 1 and 4 between the first and second ejaculates (Fig. 2B). However, the first ejaculates included a higher percentage of cluster 2 (28.8%) than the second ejaculates (19.0%, P<0.05), and the second ejaculates had a higher percentage of cluster 3 (15.4%) than the first ejaculates (9.7%, P<0.05). The total percentages of clusters 1, 2 and 3 varied between 64.6 and 76.7%, and the values obtained were similar to the percentages of progressively motile sperm evaluated by practitioners (Table 1).
Table 5. Effects of the ejaculation order and bulls on the proportiona) of each sperm cluster within ejaculates; probability values analyzed by a two-way ANOVA.
| Cluster | Factors affecting the proportion of each cluster | ||
|---|---|---|---|
| Ejaculation order | Bull | Interaction | |
| 1 | 0.9838 | 0.0058 | 0.5214 |
| 2 | 0.0069 | 0.6431 | 0.5094 |
| 3 | 0.0117 | 0.1125 | 0.6819 |
| 4 | 0.2420 | 0.2332 | 0.3141 |
a) The proportions are shown in Fig. 2.
Fig. 2.
Effects of bulls (A) and ejaculation difference (B) on sperm subpopulation structures in semen. a, b and x, y Letters indicate significant differences in the same cluster between groups (P<0.05; see Table 5).
In the present study, motile sperm were divided into 4 clusters. Cluster 1 sperm had highly progressive motility, which has been speculated to reflect very fertile sperm [29]. It has been reported that males with high fertility had higher proportion of rapid and linear sperm subpopulation and lower proportion of rapid and nonlinear sperm subpopulation than males with low fertility in red deer [33] and ram [38]. Therefore, bulls A and C may have relatively high fertility. Cluster 2 sperm showed the highest ALH in all clusters and LIN had the second lowest value due to higher VCL and lower VSL. Hyperactivation is a movement pattern observed in sperm at fertilization [13]. Hyperactivated sperm have been shown to have increased VCL and ALH along with decreased LIN [20, 26]. These findings may indicate that cluster 2 includes hyperactivated sperm. Although VSL, VCL, and VAP in cluster 3 were lower than in clusters 1 and 2, cluster 3 had the highest BCF, second highest LIN, and lowest ALH in all groups. We previously reported that bovine sperm penetrated oocytes between 4 and 8 hr after the initiation of in vitro fertilization [22]. Furthermore, sperm with high BCF, low ALH, and high LIN have been suggested to maintain higher activity without hyperactivation 6 hr after being incubated [19]. These findings indicate that sperm with high BCF and low ALH maintain their fecundity and have the ability to penetrate oocytes. Collectively, the results of the present study and previous our findings may indicate that sperm in cluster 3 has higher longevity in the female genital tract than sperm in cluster 2. Cluster 4 may be regarded as poorly motile sperm due to the lowest values in all parameters, and did not correlate with fertilization.
The numbers and characteristics of clusters in the present study were consistent with previous findings [27,28,29]; however, cluster 1 in previous findings [27,28,29] moved more linearly (LIN: 69.8–70.9%) than that in the present study (46.9%). In addition, LIN in cluster 3 was also higher in previous studies at 65.1–79.9% [27,28,29] than that in the present study (33.3%). On the other hand, BCF in the present study (8.7–27.0 Hz) was more than 2 or 3-fold higher than that (2.4–9.5 Hz) in previous studies [27,28,29]. These discrepancies may be derived from differences in the capture rate of frames by the different CASA systems. Since previous studies used a capture rate of 25 fps [27,28,29], maximum BCF was limited theoretically. The present results showed 27.0 ± 5.7 Hz for the highest BCF in cluster 3 because of employing 150 fps. This result indicates that a higher capture rate is necessary for correctly evaluating sperm motility. A previous study also indicated that a lower frame rate resulted in lower VCL because of the loss of detailed trajectory [3], leading to higher LIN. Lower LIN in the present study may be due to the higher frame rate used.
In visual inspections, no differences were detected in sperm motility between the first and second ejaculates in all bulls or between bulls. The percentages of progressively motile sperm evaluated by practitioners were 65.0–70.0%, which appears to reflect the total of clusters 1, 2 and 3. This result indicates that the sperm motility analysis by CASA has the ability to describe sperm motility characteristics in more detail. However, we suggest that sperm motility evaluations based on the average values of CASA data do not have the ability to correctly predict sperm potential fertility. For example, bulls A and C had higher VSL, VCL, and VAP, which indicated higher fertility [27], but higher ALH and lower LIN, which may correlate with shorter longevity [19, 22]. On the other hand, a cluster analysis may detect higher percentages of cluster 1, including higher velocity sperm in bulls A and C than in bull D. Although bull D showed the lowest average value for ALH, based on the cluster analysis, it may be speculated that bulls A and C do not have shorter longevity than bull D. In further study, we should investigate the longevity of sperm in each cluster. Furthermore, in comparisons of the first and second ejaculates, we only found differences in the percentages of clusters 2 and 3, and not in the percentage of cluster 1. If a difference exists in fertility between the first and second ejaculates, as described in a previous study [6], a cluster analysis has the potential to evaluate semen quality.
In conclusion, sperm in fresh semen derived from Japanese black bulls was categorized into 4 clusters by kinetic parameters analyzed using the CASA system. Clusters 1, 2, and 3 sperm may be evaluated as progressively motile sperm by visual assessments, in which differences between them cannot be detected, whereas a cluster analysis has the ability to identify differences and similarities. It means that cluster analysis can distinguish the characteristics of the first and second ejaculates collected at short interval. In further study, we should confirm the relationship between semen fertility and sperm subpopulation analyzed by cluster analysis, and investigate what statistical method is appropriate to the prediction of semen fertility.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to M. Nagano (Nos. 25450441 and 16K08043).
REFERENCES
- 1.Abaigar T., Holt W. V., Harrison R. A. P., del Barrio G.1999. Sperm subpopulations in boar (Sus scrofa) and gazelle (Gazella dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments. Biol. Reprod. 60: 32–41. doi: 10.1095/biolreprod60.1.32 [DOI] [PubMed] [Google Scholar]
- 2.Amelar R. D., Dubin L., Schoenfeld C.1980. Sperm motility. Fertil. Steril. 34: 197–215. doi: 10.1016/S0015-0282(16)44949-6 [DOI] [PubMed] [Google Scholar]
- 3.Castellini C., Dal Bosco A., Ruggeri S., Collodel G.2011. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil. Steril. 96: 24–27. doi: 10.1016/j.fertnstert.2011.04.096 [DOI] [PubMed] [Google Scholar]
- 4.Cavestany D., el-Wishy A. B., Foote R. H.1985. Effect of season and high environmental temperature on fertility of Holstein cattle. J. Dairy Sci. 68: 1471–1478. doi: 10.3168/jds.S0022-0302(85)80985-1 [DOI] [PubMed] [Google Scholar]
- 5.Cooper T. G., Yeung C. H.2006. Computer-aided evaluation of assessment of “grade a” spermatozoa by experienced technicians. Fertil. Steril. 85: 220–224. doi: 10.1016/j.fertnstert.2005.07.1286 [DOI] [PubMed] [Google Scholar]
- 6.Davis H. P., Williams N. K.1939. Evaluating bovine semen. I. Influence of the number of ejaculates upon various physical and chemical characteristics and the relationship between those factors. J. Anim. Sci. 1: 232–242. [Google Scholar]
- 7.Davis R. O., Drobnis E. Z., Overstreet J. W.1995. Application of multivariate cluster, discriminate function, and stepwise regression analyses to variable selection and predictive modeling of sperm cryosurvival. Fertil. Steril. 63: 1051–1057. doi: 10.1016/S0015-0282(16)57547-5 [DOI] [PubMed] [Google Scholar]
- 8.Dejarnette J. M.2005. The effect of semen quality on reproductive efficiency. Vet. Clin. North Am. Food Anim. Pract. 21: 409–418. doi: 10.1016/j.cvfa.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 9.Everett R. W., Bean B.1982. Environmental influences on semen output. J. Dairy Sci. 65: 1303–1310. doi: 10.3168/jds.S0022-0302(82)82344-8 [DOI] [PubMed] [Google Scholar]
- 10.Farrell P. B., Presicce G. A., Brockett C. C., Foote R. H.1998. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology 49: 871–879. doi: 10.1016/S0093-691X(98)00036-3 [DOI] [PubMed] [Google Scholar]
- 11.García-Ispierto I., López-Gatius F., Santolaria P., Yániz J. L., Nogareda C., López-Béjar M.2007. Factors affecting the fertility of high producing dairy herds in northeastern Spain. Theriogenology 67: 632–638. doi: 10.1016/j.theriogenology.2006.09.038 [DOI] [PubMed] [Google Scholar]
- 12.Hirano Y., Shibahara H., Obara H., Suzuki T., Takamizawa S., Yamaguchi C., Tsunoda H., Sato I.2001. Relationships between sperm motility characteristics assessed by the computer-aided sperm analysis (CASA) and fertilization rates in vitro. J. Assist. Reprod. Genet. 18: 213–218. doi: 10.1023/A:1009420432234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho H. C., Suarez S. S.2001. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction 122: 519–526. doi: 10.1530/rep.0.1220519 [DOI] [PubMed] [Google Scholar]
- 14.Hoflack G., Opsomer G., Rijsselaere T., Van Soom A., Maes D., de Kruif A., Duchateau L.2007. Comparison of computer-assisted sperm motility analysis parameters in semen from Belgian blue and Holstein-Friesian bulls. Reprod. Domest. Anim. 42: 153–161. doi: 10.1111/j.1439-0531.2006.00745.x [DOI] [PubMed] [Google Scholar]
- 15.Holt C., Holt W. V., Moore H. D., Reed H. C., Curnock R. M.1997. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J. Androl. 18: 312–323. [PubMed] [Google Scholar]
- 16.Holt W.1995. Can we predict fertility rates? Making sense of sperm motility. Reprod. Domest. Anim. 31: 17–24. doi: 10.1111/j.1439-0531.1995.tb00001.x [DOI] [Google Scholar]
- 17.Hopwood M. L., Rutherford E. R., Gassner F. X.1956. The concept of fructose utilization by bull sperm and its relation to fertility. J. Dairy Sci. 39: 51–59. doi: 10.3168/jds.S0022-0302(56)94704-X [DOI] [Google Scholar]
- 18.Jørgensen N., Auger J., Giwercman A., Irvine D. S., Jensen T. K., Jouannet P., Keiding N., Le Bon C., MacDonald E., Pekuri A. M., Scheike T., Simonsen M., Suominen J., Skakkeboek N. E.1997. Semen analysis performed by different laboratory teams: an intervariation study. Int. J. Androl. 20: 201–208. doi: 10.1046/j.1365-2605.1997.00052.x [DOI] [PubMed] [Google Scholar]
- 19.Kang S. S., Koyama K., Huang W., Yang Y., Yanagawa Y., Takahashi Y., Nagano M.2015. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro. J. Reprod. Dev. 61: 99–105. doi: 10.1262/jrd.2014-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kathiravan P., Kalatharan J., Karthikeya G., Rengarajan K., Kadirvel G.2011. Objective sperm motion analysis to assess dairy bull fertility using computer-aided system--a review. Reprod. Domest. Anim. 46: 165–172. doi: 10.1111/j.1439-0531.2010.01603.x [DOI] [PubMed] [Google Scholar]
- 21.Kirton K. T., Hafs H. D., Hunter A. G.1964. Levels of some normal constituents of bull semen during repetitive ejaculation. J. Reprod. Fertil. 8: 157–164. doi: 10.1530/jrf.0.0080157 [DOI] [PubMed] [Google Scholar]
- 22.Koyama K., Kang S. S., Huang W., Yanagawa Y., Takahashi Y., Nagano M.2014. Estimation of the optimal timing of fertilization for embryo development of in vitro-matured bovine oocytes based on the times of nuclear maturation and sperm penetration. J. Vet. Med. Sci. 76: 653–659. doi: 10.1292/jvms.13-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen L., Scheike T., Jensen T. K., Bonde J. P., Ernst E., Hjollund N. H., Zhou Y., Skakkebaek N. E., Giwercman A., The Danish First Pregnancy Planner Study Team 2000. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. Hum. Reprod. 15: 1562–1567. doi: 10.1093/humrep/15.7.1562 [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Pastor F., Tizado E. J., Garde J. J., Anel L., de Paz P.2011. Statistical Series: Opportunities and challenges of sperm motility subpopulation analysis. Theriogenology 75: 783–795. doi: 10.1016/j.theriogenology.2010.11.034 [DOI] [PubMed] [Google Scholar]
- 25.Mocé E., Graham J. K.2008. In vitro evaluation of sperm quality. Anim. Reprod. Sci. 105: 104–118. doi: 10.1016/j.anireprosci.2007.11.016 [DOI] [PubMed] [Google Scholar]
- 26.Mortimer S. T.2000. CASA--practical aspects. J. Androl. 21: 515–524. [PubMed] [Google Scholar]
- 27.Muiño R., Tamargo C., Hidalgo C. O., Peña A. I.2008. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein bulls: effects of cryopreservation and between-bull variation. Anim. Reprod. Sci. 109: 27–39. doi: 10.1016/j.anireprosci.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 28.Muiño R., Peña A. I., Rodríguez A., Tamargo C., Hidalgo C. O.2009. Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls. Theriogenology 72: 860–868. doi: 10.1016/j.theriogenology.2009.06.009 [DOI] [PubMed] [Google Scholar]
- 29.Muiño R., Peña A. I., Quintela L. A., Becerra J., Herradón P., Gonzalez F. H.2015. Management of the storage of cryopreserved sperm on dairy cattle farms. J. Anim. Sci. Biotechnol. 31: 85–100. [Google Scholar]
- 30.Okano T., Murase T., Asano M., Tsubota T.2004. Effects of final dilution rate, sperm concentration and times for cooling and glycerol equilibration on post-thaw characteristics of canine spermatozoa. J. Vet. Med. Sci. 66: 1359–1364. doi: 10.1292/jvms.66.1359 [DOI] [PubMed] [Google Scholar]
- 31.Pursley J. R., Kosorok M. R., Wiltbank M. C.1997. Reproductive management of lactating dairy cows using synchronization of ovulation. J. Dairy Sci. 80: 301–306. doi: 10.3168/jds.S0022-0302(97)75938-1 [DOI] [PubMed] [Google Scholar]
- 32.Quintero-Moreno A., Miró J., Teresa Rigau A., Rodríguez-Gil J. E.2003. Identification of sperm subpopulations with specific motility characteristics in stallion ejaculates. Theriogenology 59: 1973–1990. doi: 10.1016/S0093-691X(02)01297-9 [DOI] [PubMed] [Google Scholar]
- 33.Ramón M., Soler A. J., Ortiz J. A., García-Alvarez O., Maroto-Morales A., Roldan E. R. S., Garde J. J.2013. Sperm population structure and male fertility: an intraspecific study of sperm design and velocity in red deer. Biol. Reprod. 89: 110. doi: 10.1095/biolreprod.113.112110 [DOI] [PubMed] [Google Scholar]
- 34.Turner T. T., Howards S. S.1978. Factors involved in the initiation of sperm motility. Biol. Reprod. 18: 571–578. doi: 10.1095/biolreprod18.4.571 [DOI] [PubMed] [Google Scholar]
- 35.Verstegen J., Iguer-Ouada M., Onclin K.2002. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 57: 149–179. doi: 10.1016/S0093-691X(01)00664-1 [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. 1999. WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction, 4th ed., Cambridge University Press, Cambridge. [Google Scholar]
- 37.World Health Organization. 2010. WHO Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucus Interaction, 5th ed., Cambridge University Press, Cambridge. [Google Scholar]
- 38.Yániz J. L., Palacín I., Vicente-Fiel S., Sánchez-Nadal J. A., Santolaria P.2015. Sperm population structure in high and low field fertility rams. Anim. Reprod. Sci. 156: 128–134. doi: 10.1016/j.anireprosci.2015.03.012 [DOI] [PubMed] [Google Scholar]