Abstract
The present study aimed to determine whether circulating serum concentrations of 25-hydroxyvitamin D [25-(OH) D] differed between healthy dogs and dogs with acute pancreatitis (AP). Twenty-two healthy dogs and twenty client-owned dogs with AP were enrolled in the study. Serum concentrations of 25-(OH) D, blood ionized calcium (iCa), and serum C-reactive protein (CRP) were measured. Concentrations of serum 25-(OH) D and blood iCa in dogs with AP were significantly lower than those of healthy dogs, and serum concentrations of CRP in dogs with AP were significantly higher than those of healthy dogs. A significant difference in 25-(OH) D serum concentrations was observed between survivor and non-survivor dogs with AP. After resolution of clinical signs, concentrations of serum 25-(OH) D, blood iCa, and serum CRP did not differ compared to those before treatment. This study shows that dogs with AP exhibit decreased 25-(OH) D levels, which might be associated with calcium imbalances and mortality rate in canine AP.
Keywords: canine, C-reactive protein, hypocalcemia, inflammation, vitamin D
Vitamin D is a secosteroid hormone, which plays a crucial role in calcium homeostasis and bone metabolism [14]. Dogs are unable to synthesize vitamin D in the skin under the influence of ultraviolet B light. Therefore, they rely solely on dietary intake of vitamin D for their vitamin D requirements [20]. After ingestion, 25-hydroxyvitamin D [25-(OH) D] is produced in the liver and is the major circulating form of vitamin D, which is available for further activation by 1α-hydroxylation in the kidney [14]. In addition to regulating calcium homeostasis, vitamin D has immunologic properties, which were recently identified [27]. In humans, vitamin D deficiency is associated with increased risk and/or severity of a variety of diseases including cancer, cardiovascular disease, autoimmune disease, sarcopenia, osteoarthritis, infections, and transplant rejection [13].
Acute pancreatitis (AP) is defined as an acute inflammatory process of the pancreas with variable involvement of other regional tissues or remote organ systems [7, 25]. The inflammatory process is initiated in the pancreas and produces a systemic inflammatory response with massive release of cytokines including tumor necrosis factor (TNF)-α and interleukin (IL)-6 [16, 25, 31]. Consequently, AP may result in a wide range of clinical signs (vomiting, anorexia, weakness, diarrhea and abdominal pain) of differing severity and may cause multisystemic inflammation [25, 32, 35, 38]. The mortality rate among dogs with AP is often high, ranging from 27 to 58%, because of the systemic effects of the disease, and surviving animals usually require intensive treatment and hospitalization [8, 26, 32].
Recently, an imbalance in vitamin D was described and suggested to be associated with disease progression in humans with pancreatitis [5, 34, 39]. Serum levels of 1,25-dihydroxyvitamin D [1,25-(OH)2D] were significantly reduced in women with chronic pancreatitis and were associated with the severity grade of the disease [39]. Levels of 25-(OH) D also decreased over the first two days of admission in patients with AP, and these changes were associated with alterations in C-reactive protein (CRP), a marker of inflammation [5]. To date, however, no studies have investigated the association between vitamin D status and AP in dogs. Therefore, the purpose of the present study was to examine differences in concentrations of serum 25-(OH) D between healthy dogs and AP dogs, and if so, to characterize correlations among serum 25-(OH) D, blood ionized calcium (iCa), and serum CRP.
MATERIALS AND METHODS
Animals and ethical issues
One-hundred and twenty-five dogs with newly diagnosed AP were enrolled in this case-controlled study. Based on their body condition scores (BCS; 9-point scale) [22], forty dogs with normal BCS (BCS 4) were selected because we suspected that serum calcium concentrations might be affected by abdominal fat mass involved in peripancreatic fat necrosis [38]. Among these 40 dogs, five dogs with recurrent bouts of AP and three dogs referred from other hospitals (diagnosed with AP and hospitalized for >3 days) were excluded on the basis of clinical data. Twelve dogs with concurrent diseases, including chronic kidney disease (four dogs), chronic valvular heart disease (three dogs), hyperadrenocorticism (two dogs), diabetes mellitus (one dog), immune-mediated hemolytic anemia (one dog), or protein-losing enteropathy (one dog), were also excluded. Thus, 20 dogs with AP were included in the present study (Table 1). Twenty-two healthy, client-owned dogs with normal BCS (BCS 4) were included as a control. The healthy dogs were recruited from the same veterinary medical center when the dogs presented for health examinations and were enrolled on the basis of a normal medical history and physical examination. The controls were individually matched to each AP dogs by ages, sex and neuter status. Informed consent was obtained from the owners and the University Ethics committee approved all of the animal studies.
Table 1. Characteristics of dogs with acute pancreatitis (AP) and healthy dogs.
| Dogs with AP (n=20) | Healthy dogs (n=22) | ||
|---|---|---|---|
| Sex | |||
| Female | 2 | 4 | |
| Neutered female | 7 | 7 | |
| Male | 3 | 4 | |
| Neutered male | 8 | 7 | |
| Body weight, kg (mean ± SEM; range) | 6.15 ± 0.64; 2.1–10.8 | 6.62 ± 1.12; 3.8–12.0 | |
| Age, years (mean ± SEM; range) | 9.47 ± 3.18; 3–14 | 9.06 ± 2.66; 2–15 | |
| Breed | |||
| Miniature Schnauzer | 5 | 6 | |
| Miniature Poodle | 2 | 2 | |
| Maltese | 2 | 1 | |
| Shih Tzu | 2 | 2 | |
| Yorkshire Terrier | 2 | 2 | |
| Pomeranian | 2 | 1 | |
| Dachshund | 1 | 0 | |
| Cocker Spaniel | 1 | 0 | |
| Beagle | 1 | 4 | |
| Pekinese | 0 | 1 | |
| Others | 2 | 3 | |
| Serum total calcium (mM/l, mean ± SEM; range) | 2.33 ± 0.11, 1.65–3.55a) | 2.58 ± 0.04, 2.25–2.85 | |
| Serum albumin (g/l, mean ± SEM; range) | 23.55 ± 1.51, 12–38a) | 29.82 ± 0.63, 26–35 | |
a) P<0.05 compared with the healthy group.
Diagnosis of acute pancreatitis
In the AP group, a diagnosis of AP was established only if all the abnormal findings were compatible with acute onset, i.e., increased serum activity of amylase or lipase, a presence of appropriate pancreatic changes on abdominal ultrasonography performed by a board-certified radiologist, a positive result in a SNAP cPL test (IDEXX Laboratories Inc., Westbrook, ME, U.S.A.), and increased Spec cPL concentrations (IDEXX Laboratories Inc.) as described previously [26, 28, 31, 40]. Clinical evidence of AP was confirmed if acute onset vomiting, anorexia, and/or abdominal pain were present during physical examination on admission, or when history was established [26]. Ultrasonographic findings suggestive of pancreatitis involvement included hypo/hyperechoic lesions, or mixed patterns, in a possibly enlarged or irregularly-shaped pancreas [15, 33]. In addition, alterations secondary to pancreatitis, such as hyperechoic mesentery, localized free abdominal fluid, thickened duodenal or gastric walls, spasmodic duodenum, irritated appearance of the adjacent intestine, and a dilated common bile duct, were also considered to be ultrasonographic evidence of AP [15, 33]. The results of SNAP cPL tests were interpreted as abnormal only if the color of the sample spot was more intense than that of the reference spot [43]. In the Spec cPL assays, concentrations >400 µg/l were considered to be consistent with pancreatitis [43].
Dogs in the healthy group (Table 1) were considered to be healthy based on a physical examination, indirect measurement of their systolic blood pressure, examination of fecal specimens to determine the presence of parasites using a floating technique, heartworm antigen testing, complete blood count analysis, serum biochemical analysis including amylase, lipase and Spec cPL, urinalysis, adrenocorticotropic hormone response testing, thyroid function testing, and diagnostic imaging including survey radiography and abdominal ultrasonography.
Treatment and grouping
Treatment was carried out as recommended in the literature at the time of the study [26, 31, 38]. The mainstay of the treatment was intravenous fluid therapy. The prescribed medication comprised analgesics, antiemetics, broad-spectrum antibiotics, H2 receptor blockers, fresh plasma transfusion, and low-molecular weight heparin, and nil per os (NPO) was maintained until vomiting stopped. Briefly, intravenous fluid therapy was initiated promptly upon hospitalization and dehydration was corrected using crystalloid fluid. Based on the assumption that abdominal pain could be present in all dogs with AP, butorphanol tartrate (Butophan, Myungmoon Pharm. Co., Seoul, Korea) was administered (0.2 mg/kg IV q 6 hr). Maropitant citrate (Cerenia, Pfizer, Pocé-sur-Cisse, France) was used as an antiemetic, which blocks centrally- and peripherally-mediated emesis (1 mg/kg SC q 24 hr). Bacterial complications are rare in dogs with AP, but the dogs were treated with broad-spectrum antibiotics if pyrexia, left shift neutropenia, or documented infection were present. Famotidine (Gaster Inj, Dong-A ST, Seoul, Korea), an H2 receptor blocker, was also administered (0.5 mg/kg IV q 12 hr). Dogs were maintained NPO if vomiting continued despite antiemetic therapy. If dogs had remained NPO and vomiting stopped, water was reintroduced slowly, which was followed by small amounts of a low-fat diet on the next day.
Dogs with AP were categorized into “survivor” or “non-survivor” groups. The survivor group included dogs that exhibited obvious improvements or even recovery at the end of hospitalization. Evidences of the treatment in the survivor group included resolving symptoms related with AP and negative result of SNAP cPL test. The non-survivor group comprised dogs that died during hospitalization.
Assays
Blood samples were collected from dogs with AP and control groups upon admission after fasting for ≥12 hr. Blood samples were also obtained from dogs with AP following their treatment at the time clinical signs resolved, again after fasting for ≥12 hr. Blood was collected from the jugular or peripheral vein. After blood collection, heparinized blood samples were analyzed immediately for iCa measurements with a cage-side i-STAT analyzer (Abaxis Inc., Union City, CA, U.S.A.). Serum was separated from clotted whole blood samples in blood collection tubes (red top tubes) by centrifugation at 1,200 g for 10 min using a refrigerated centrifuge within 1 hr of blood collection, and sera were immediately stored at −80°C.
Circulating concentrations of 25-hydroxyvitamin D [25 (OH) D] were measured at a commercial laboratory (NEODIN, Seoul, Korea) using electrochemiluminescence immunoassay (Roche Diagnostics Limited, Basel, Switzerland) in a Cobas 8000 automated analyzer (Roche Diagnostics Limited) [1]. The lower detection limit of the 25 (OH) D assay was 7.5 nM/l, and both inter-assay and intra-assay variations were <15%. Serum CRP was measured using a Randox immunoterbidimetric assay (Randox Laboratories Ltd., Co., Antrim, U.K.) on a Hitachi 912 analyzer (Roche Diagnostics Limited). The intra-and inter-assay variabilities were <5% and <10%, respectively, and the lower detection limit of the assay was 5 mg/l. Serum biochemical tests were performed on the remaining aliquots of serum. All laboratory tests were performed according to the manufacturers’ instructions by trained laboratory staff.
Statistical analyses
All statistical analyses were performed using Prism 6.0 software (Graphpad software Inc., La Jolla, CA, U.S.A.). A normality test (D’Agostino-Pearson omnibus test) was performed to determine whether data were normally distributed. Unpaired t tests were used to compare the differences between the healthy and AP groups based on normally distributed data. Kruskal-Wallis tests with post hoc test using Mann-Whitney U tests were conducted to compare between healthy dogs, survivor, and non-survivor AP groups based on a lack of normality. Wilcoxon matched-pairs signed rank tests were used to compare data for the AP group before and after treatment, based on a lack of normality. Correlations were examined with the Pearson correlation coefficient based on normally distributed data. Data were expressed as mean (deviation) or median (ranges) based on the results of the normality test. P-values for two-tailed tests and 95% confidence intervals (CI) for differences between means or medians are provided. P<0.05 was considered statistically significant.
RESULTS
Circulating concentrations of 25-(OH) D, iCa and CRP in dogs with AP and healthy controls
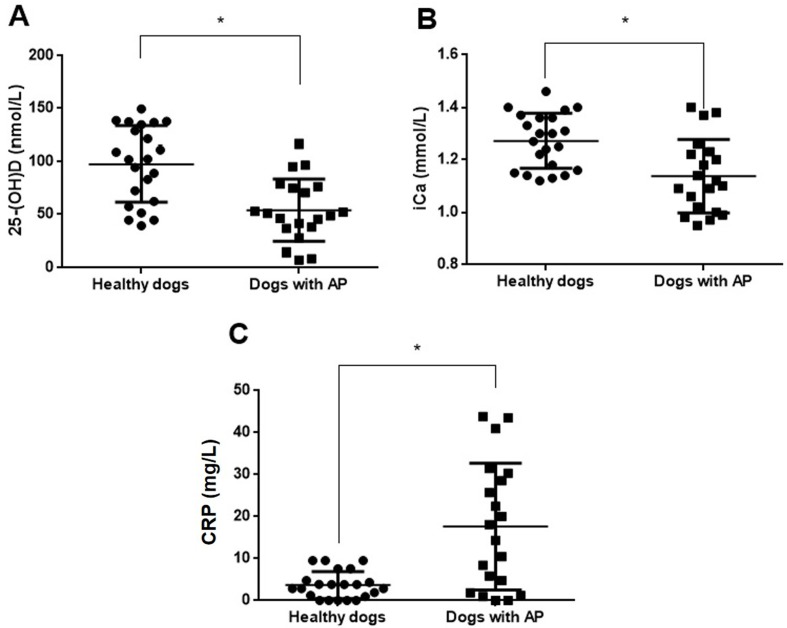
This study included 22 healthy dogs and 20 dogs with AP, and their details are shown in Table 1. Serum total calcium (95% CI for differences between means= −0.47 to −0.04; P=0.02) and albumin (95% CI for differences between means= −9.47 to −3.07; P<0.001) concentrations were significantly lower in dogs with AP than in healthy dogs. Serum amylase (95% CI for differences between means= −2,531.38 to −1,670.18; P<0.001), lipase (95% CI for differences between means= −4,498.12 to −1,962.44; P<0.001) and cPL (95% CI for differences between means= −807.060 to −329.914; P<0.001) concentrations were significantly higher in dogs with AP than in healthy dogs. Serum 25-(OH) D levels (95% CI for differences between means= −64.33 to −22.92; P<0.001) were significantly lower in dogs with AP than in healthy dogs (Fig. 1A). Concentrations of blood iCa (95% CI for differences between means= −0.21 to −0.06; P=0.001) were also significantly lower in dogs with AP than in healthy controls (Fig. 1B). By contrast, serum CRP concentrations (95% CI for differences between means=7.3 to 20.63; P<0.001) were significantly higher in dogs with AP than in healthy dogs (Fig. 1C).
Fig. 1.
Differences in the circulating levels of 25-hydroxyvitamin D [25-(OH) D] (A), ionized calcium (iCa) (B), and C-reactive protein (CRP) (C) between dogs with acute pancreatitis (AP; n=20) and healthy dogs (n=22). Horizontal bars indicate the mean. *P<0.05 (unpaired t test).
Differences in circulating 25-(OH) D, iCa, and CRP levels among AP survivors, non-survivors, and healthy dogs
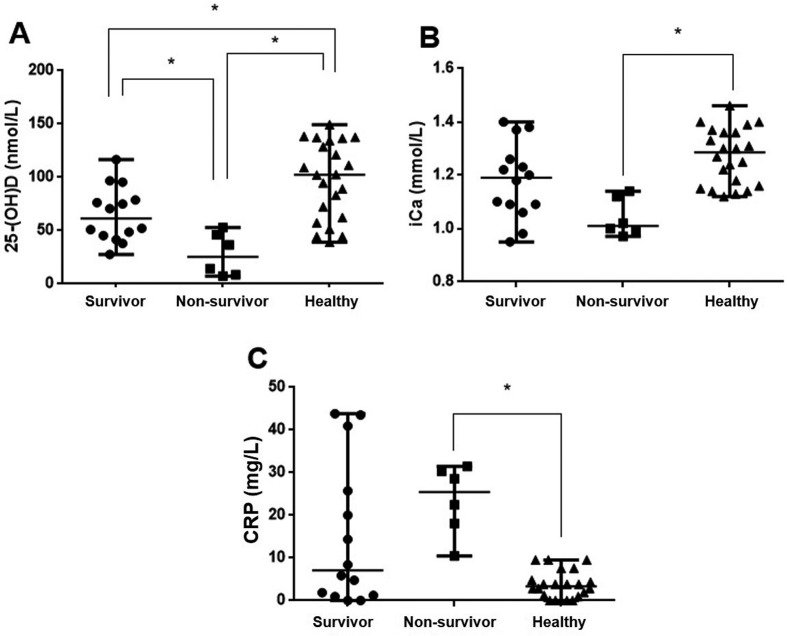
AP dogs were categorized into “survivor” (n=14) or “non-survivor” (n=6, survival duration=6.0 [2.0−16.0]) groups. There were significant difference of serum 25-(OH) D concentrations among 3 groups (H=15.66, df=2, n=42, P<0.001). Serum 25-(OH) D concentrations of AP survivors (95% CI for differences between medians= −59.60 to −7.11; P=0.0094) and non-survivors (95% CI for differences between medians= −101.70 to −36.27; P<0.001) were significantly lower than those of healthy dogs (Fig. 2A). There were significant difference of blood iCa concentrations among 3 groups (H=13.24, df=2, n=42, P=0.001). Moreover, serum 25-(OH) D levels in AP non-survivors (95% CI for differences between medians= −64.52 to −8.59; P=0.017) were significantly lower than in AP survivors (Fig. 2A). The iCa concentration in AP non-survivors (95% CI for differences between medians= −0.34 to −0.14; P<0.001) was lower than in healthy dogs, but there was no significant difference between AP survivors and non-survivors (Fig. 2B). There were significant difference of serum CRP concentrations among 3 groups (H=12.85, df=2, n=42, P=0.002). Serum CRP concentrations in AP non-survivors (95% CI for differences between medians=27.46 to 13.33; P<0.001) were significantly higher than in healthy dogs, but there was no significant difference between AP survivors and non-survivors (Fig. 2C).
Fig. 2.
Differences in circulating 25-hydroxyvitamin D [25-(OH) D] (A), ionized calcium (iCa) (B), and C-reactive protein (CRP) (C) concentrations among acute pancreatitis (AP) survivors (n=14), non-survivors (n=6), and healthy dogs. Horizontal bars indicate the median. *P<0.05 (Kruskal-Wallis tests with post hoc test using Mann-Whitney U tests).
Comparison of circulating 25-(OH) D, iCa and CRP concentrations in AP survivors before and after treatment
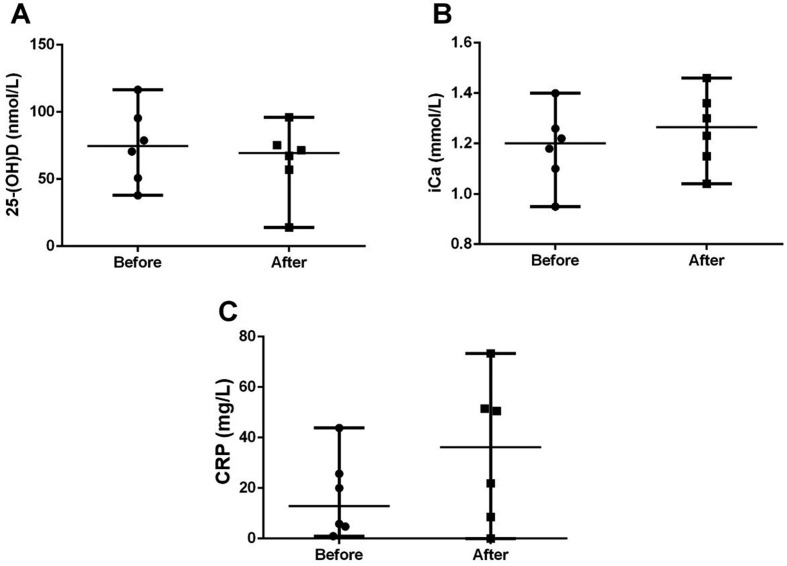
Eight of 14 AP survivors were not evaluated after treatment due to owner’s refusal. In the 6 AP survivors, there were no significant post-treatment differences in the levels of 25-(OH) D (95% CI for differences between medians= −64.87 to 20.57; P=0.44), iCa (95% CI for differences between medians= −0.06 to 0.35; P=0.84), and CRP (95% CI for differences between medians= −25.71 to 46.72; P=0.16), compared with pre-treatment levels (Fig. 3).
Fig. 3.
Differences in the circulating levels of 25-hydroxyvitamin D [25-(OH) D] (A), ionized calcium (iCa) (B), and C-reactive protein (CRP) (C) before and after treatment in dogs with acute pancreatitis (AP) (n=6). Horizontal bars indicate the median.
Correlations between circulating 25-(OH) D, iCa, CRP, and albumin levels in dogs with AP
Concentrations of 25-(OH) D were positively correlated with the concentrations of iCa (r=0.50, P=0.025) and albumin (r=0.65, P=0.0018) in dogs with AP (Fig. 4A and 4B). Conversely, a negative correlation was observed between serum concentrations of CRP and blood iCa (r= −0.79, P<0.001) in the AP group (Fig. 4C). However, no significant correlation was observed between CRP and 25-(OH) D levels (r= −0.30, P=0.19) in dogs with AP (Fig. 4D).
Fig. 4.
Correlations between 25-hydroxyvitamin D [25-(OH) D] and ionized calcium (iCa) (A), albumin and 25-(OH) D (B), C-reactive protein (CRP) and iCa (C), and 25-(OH) D and CRP (D) in dogs with acute pancreatitis (n=20).
DISCUSSION
The main finding of present study was that serum 25-(OH) D concentrations were reduced in dogs with AP. Moreover, 25-(OH) D concentrations were significantly lower in AP non-survivors than survivors, which supports the hypothesis that low serum 25-(OH) D concentrations might be associated with disease severity or mortality rate in dogs with AP. However, in AP survivors, there were no changes after treatment in serum 25-(OH) D concentrations.
Similar associations between low circulating 25-(OH) D and pancreatitis are observed in human studies [5, 34, 39]. Several mechanisms may explain the observed variations in 25-(OH) D. Firstly, in humans with pancreatitis, reduced intestinal uptake as a result of anorexia or starvation has been suggested as the cause of vitamin D deficiency [34]. Dogs are unable to synthesize vitamin D in the skin under the influence of ultraviolet B light and rely solely on dietary intake of vitamin D for their vitamin D requirements [20]. Therefore, as with pancreatitis in humans, decreased circulating vitamin D levels in dogs with AP may result from decreased vitamin D uptake. However, given the long half-life of 25-(OH) D of approximately three weeks and the short clinical course of AP, decreased intestinal absorption may not fully explain the pathophysiology of 25-(OH) D deficiency in dogs with AP [36, 37]. Secondly, in humans with AP, decreases in serum 25-(OH) D concentration were associated with changes in CRP, which supports the hypothesis that there may be a link between 25-(OH) D and acute inflammatory conditions [5]. Decreases in 25-(OH) D, in association with decreases in vitamin D-binding protein (VDBP), have been documented in critically ill people with sepsis [21]. Decreased VDBP is also documented in experimental endotoxemia in rats [42]. Notably, 25-(OH) D is reabsorbed from urine, and thus decreased VDBP might lead to renal loss of 25-(OH) D [21]. Considering the nature of severe inflammation in AP, although VDBP was not measured in our study, this mechanism of vitamin D deficiency could be applied to AP in dogs. Moreover, 25-(OH) D binds primarily to VDBP but also to albumin, and changes in albumin would impact 25-(OH) D levels [6]. We also found a correlation with albumin, explaining the variation in 25-(OH) D in dogs with AP. However, this possibility could be somewhat weak because the actual free concentration of 25-(OH) D might be independent of albumin changes [6].
Although vitamin D is traditionally thought to play an important role in calcium homeostasis, there is increasing evidence that vitamin D is also required for antimicrobial activity, anti-inflammation, cardioprotection, immunomodulation, and metabolic regulation in human medicine [3, 23]. Hence, it is not surprising that vitamin D deficiency is associated with comorbidities in critically ill patients. Notably, 25-(OH) D deficiency develops in 26–82% of critically ill human patients [3, 23]. Moreover, studies also reveal an association between 25-(OH) D deficiency and increased length of hospital or ICU stay, illness severity, organ dysfunction, infection, and mortality [4, 9, 24, 29]. In the present study, serum levels of 25-(OH) D were significantly lower in AP non-survivors than AP survivors. Moreover, all AP non-survivors exhibited 25-(OH) D deficiency, which was lower than the reference interval (60–215 nM/l) [37]. Although it remains unclear whether vitamin D deficiency is a cause or a consequence of severe disease, these findings suggest that vitamin D might have a beneficial role in disease, and the extent of vitamin D deficiency might be related to disease prognosis. Therefore, a further study will be necessary to clearly define whether the supplementation of vitamin D improves the prognosis of acute pancreatitis in dogs.
Vitamin D plays a crucial role in calcium homeostasis by stimulating intestinal absorption and renal reabsorption of calcium and phosphate [14]. In dogs with AP, hypocalcemia is well documented, but the origin remains unclear [16, 19, 38]. Therefore, in the present study, we evaluated calcium status and the correlation between iCa and 25-(OH) D in dogs with AP. Blood iCa concentrations were significantly lower in dogs with AP than in healthy dogs, which might be a result of the changes in iCa in AP non-survivors. Moreover, blood iCa concentrations correlated with 25-(OH) D concentrations in dogs with AP.
Vitamin D deficiency was the most recently investigated and heavily supported mechanism for hypocalcemia in critically ill human patients [29]. In dogs with experimentally induced endotoxemia, ionized hypocalcemia was associated with hypovitaminosis D, but not hypomagnesemia, hypoparathyroidism, alkalosis, or increased calciuresis [18]. Hence, it is possible that vitamin D deficiency and inflammatory conditions play a significant role in the development of ionized hypocalcemia in dogs with AP. However, we were unable to find a correlation between 25-(OH) D and CRP concentrations, or to examine other factors of calcium regulation, such as parathyroid hormone, calcitonin, and magnesium. Therefore, further studies are required to fully understand the role of vitamin D in calcium homeostasis in dogs with AP, especially the potential associations with the severity of inflammation.
The present study analyzed circulating concentrations of 25-(OH) D, iCa, and CRP before and after treatment in dogs with AP. However, there were no significant differences in the concentrations of these analytes before and after treatment. In the present study, most dogs were fasted for pancreatic rest prior to sampling. Dogs exclusively depend on dietary intake of vitamin D for their vitamin D requirements [20]. Moreover, in dogs, 25-(OH) D has a relatively long half-life of 3 weeks in circulation in contrast to the acute clinical course of AP [36]. These facts might explain why no changes in 25-(OH) D and blood iCa were observed before and after treatment in the present study. However, in contrast to our data, mean CRP concentrations decreased from the day of diagnosis to the day of measurement 5 days later in dogs with AP [17]. This discrepancy likely resulted from the small number of dogs with AP examined in the present study; thus further studies to investigate alterations in serum CRP concentrations in canine AP are necessary.
This study was originally designed as a pilot study and it had several limitations. One limitation was the small sample size of dogs with AP, which constrains the reliability of the findings (such as the non-significant difference in iCa and CRP concentrations between survivors and non-survivors, the non-significant difference in analytes before and after treatment, and a weak correlation between 25-(OH) D and CRP in dogs with AP). Another limitation of the present study was that we were unable to study parathyroid hormone, calcitonin, serum lactate, VDBP, or extravascular calcium concentrations, which may have provided further useful information on calcium imbalances in canine AP. These limited statistical analysis using regression model to determine an association between 25-(OH) D and variables in dogs with AP. An additional limitation was that there was no histopathologic evaluation on pancreas. However, multiple pancreatic biopsy for histopathology might be an invasive procedure in sick patients and need general anesthesia [2, 30]. Therefore, the clinical importance of our findings should be interpreted with caution. Finally, although we tried to match sex, breed, ages and neuter status between the healthy group and AP group, we were unable to perfectly match dogs in terms of their sex, breed, neuter status, or undiagnosed diseases, which might influence the results [10,11,12, 41]. Future studies should avoid this limitation by matching the controls and cases rigorously. Further studies are also required to clarify the association between 25-(OH) D levels and calcium imbalances in dogs with AP. In addition, to examine 25-(OH) D based on AP severity (or hospitalization) will be also necessary.
In conclusion, dogs with AP exhibited decreased 25-(OH) D levels, which might be a consequence of inflammatory responses rather than a cause of AP. Moreover, alterations in 25-(OH) D might be associated with disease severity or mortality rate in dogs with AP. Further studies are required to confirm these results using large cohorts of dogs.
Acknowledgments
The authors would like to thank all the owners of the dogs included in this study. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, which was funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1A1A1A05005395).
REFERENCES
- 1.Abdel-Wareth L., Haq A., Turner A., Khan S., Salem A., Mustafa F., Hussein N., Pallinalakam F., Grundy L., Patras G., Rajah J.2013. Total vitamin D assay comparison of the Roche Diagnostics “Vitamin D total” electrochemiluminescence protein binding assay with the Chromsystems HPLC method in a population with both D2 and D3 forms of vitamin D. Nutrients 5: 971–980. doi: 10.3390/nu5030971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrian A. M., Twedt D. C., Kraft S. L., Marolf A. J.2015. Computed tomographic angiography under sedation in the diagnosis of suspected canine pancreatitis: a pilot study. J. Vet. Intern. Med. 29: 97–103. doi: 10.1111/jvim.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amrein K., Venkatesh B.2012. Vitamin D and the critically ill patient. Curr. Opin. Clin. Nutr. Metab. Care 15: 188–193. doi: 10.1097/MCO.0b013e32834f0027 [DOI] [PubMed] [Google Scholar]
- 4.Arnson Y., Gringauz I., Itzhaky D., Amital H.2012. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM 105: 633–639. doi: 10.1093/qjmed/hcs014 [DOI] [PubMed] [Google Scholar]
- 5.Bang U. C., Novovic S., Andersen A. M., Fenger M., Hansen M. B., Jensen J. E.2011. Variations in serum 25-hydroxyvitamin D during acute pancreatitis: an exploratory longitudinal study. Endocr. Res. 36: 135–141. doi: 10.3109/07435800.2011.554937 [DOI] [PubMed] [Google Scholar]
- 6.Bikle D. D., Gee E., Halloran B., Kowalski M. A., Ryzen E., Haddad J. G.1986. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 63: 954–959. doi: 10.1210/jcem-63-4-954 [DOI] [PubMed] [Google Scholar]
- 7.Bradley E. L., 3rd.1993. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch. Surg. 128: 586–590. doi: 10.1001/archsurg.1993.01420170122019 [DOI] [PubMed] [Google Scholar]
- 8.Cook A. K., Breitschwerdt E. B., Levine J. F., Bunch S. E., Linn L. O.1993. Risk factors associated with acute pancreatitis in dogs: 101 cases (1985-1990). J. Am. Vet. Med. Assoc. 203: 673–679. [PubMed] [Google Scholar]
- 9.Flynn L., Zimmerman L. H., McNorton K., Dolman M., Tyburski J., Baylor A., Wilson R., Dolman H.2012. Effects of vitamin D deficiency in critically ill surgical patients. Am. J. Surg. 203: 379–382, discussion 382. doi: 10.1016/j.amjsurg.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 10.Gerber B., Hässig M., Reusch C. E.2003. Serum concentrations of 1,25-dihydroxycholecalciferol and 25-hydroxycholecalciferol in clinically normal dogs and dogs with acute and chronic renal failure. Am. J. Vet. Res. 64: 1161–1166. doi: 10.2460/ajvr.2003.64.1161 [DOI] [PubMed] [Google Scholar]
- 11.Gerber B., Hauser B., Reusch C. E.2004. Serum levels of 25-hydroxycholecalciferol and 1,25-dihydroxycholecalciferol in dogs with hypercalcaemia. Vet. Res. Commun. 28: 669–680. doi: 10.1023/B:VERC.0000045954.71403.74 [DOI] [PubMed] [Google Scholar]
- 12.Gow A. G., Else R., Evans H., Berry J. L., Herrtage M. E., Mellanby R. J.2011. Hypovitaminosis D in dogs with inflammatory bowel disease and hypoalbuminaemia. J. Small Anim. Pract. 52: 411–418. doi: 10.1111/j.1748-5827.2011.01082.x [DOI] [PubMed] [Google Scholar]
- 13.Guillot X., Semerano L., Saidenberg-Kermanac’h N., Falgarone G., Boissier M. C.2010. Vitamin D and inflammation. Joint Bone Spine 77: 552–557. doi: 10.1016/j.jbspin.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 14.Hazewinkel H. A., Tryfonidou M. A.2002. Vitamin D3 metabolism in dogs. Mol. Cell. Endocrinol. 197: 23–33. doi: 10.1016/S0303-7207(02)00275-7 [DOI] [PubMed] [Google Scholar]
- 15.Hecht S., Henry G.2007. Sonographic evaluation of the normal and abnormal pancreas. Clin. Tech. Small Anim. Pract. 22: 115–121. doi: 10.1053/j.ctsap.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 16.Holm J. L., Chan D. L., Rozanski E. A.2003. Acute pancreatitis in dogs. J. Vet. Emerg. Crit. Care (San Antonio) 13: 201–213. doi: 10.1111/j.1534-6935.2003.00113.x [DOI] [Google Scholar]
- 17.Holm J. L., Rozanski E. A., Freeman L. M., Webster C. R. L.2004. C-reactive protein concentrations in canine acute pancreatitis. J. Vet. Emerg. Crit. Care (San Antonio) 14: 183–186. doi: 10.1111/j.1534-6935.2004.04010.x [DOI] [Google Scholar]
- 18.Holowaychuk M. K., Birkenheuer A. J., Li J., Marr H., Boll A., Nordone S. K.2012. Hypocalcemia and hypovitaminosis D in dogs with induced endotoxemia. J. Vet. Intern. Med. 26: 244–251. doi: 10.1111/j.1939-1676.2012.00886.x [DOI] [PubMed] [Google Scholar]
- 19.Holowaychuk M. K., Hansen B. D., DeFrancesco T. C., Marks S. L.2009. Ionized hypocalcemia in critically ill dogs. J. Vet. Intern. Med. 23: 509–513. doi: 10.1111/j.1939-1676.2009.0280.x [DOI] [PubMed] [Google Scholar]
- 20.How K. L., Hazewinkel H. A., Mol J. A.1994. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen. Comp. Endocrinol. 96: 12–18. doi: 10.1006/gcen.1994.1154 [DOI] [PubMed] [Google Scholar]
- 21.Jeng L., Yamshchikov A. V., Judd S. E., Blumberg H. M., Martin G. S., Ziegler T. R., Tangpricha V.2009. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 7: 28. doi: 10.1186/1479-5876-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laflamme D.1997. Development and validation of a body condition score system for dogs. Canine Pract. 22: 10–15. [Google Scholar]
- 23.Lee P.2011. Vitamin D metabolism and deficiency in critical illness. Best Pract. Res. Clin. Endocrinol. Metab. 25: 769–781. doi: 10.1016/j.beem.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 24.Lucidarme O., Messai E., Mazzoni T., Arcade M., du Cheyron D.2010. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 36: 1609–1611. doi: 10.1007/s00134-010-1875-8 [DOI] [PubMed] [Google Scholar]
- 25.Mansfield C.2012. Pathophysiology of acute pancreatitis: potential application from experimental models and human medicine to dogs. J. Vet. Intern. Med. 26: 875–887. doi: 10.1111/j.1939-1676.2012.00949.x [DOI] [PubMed] [Google Scholar]
- 26.Mansfield C.2012. Acute pancreatitis in dogs: advances in understanding, diagnostics, and treatment. Top. Companion Anim. Med. 27: 123–132. doi: 10.1053/j.tcam.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Maruotti N., Cantatore F. P.2010. Vitamin D and the immune system. J. Rheumatol. 37: 491–495. doi: 10.3899/jrheum.090797 [DOI] [PubMed] [Google Scholar]
- 28.McCord K., Morley P. S., Armstrong J., Simpson K., Rishniw M., Forman M. A., Biller D., Parnell N., Arnell K., Hill S., Avgeris S., Gittelman H., Moore M., Hitt M., Oswald G., Marks S., Burney D., Twedt D.2012. A multi-institutional study evaluating the diagnostic utility of the spec cPL™ and SNAP® cPL™ in clinical acute pancreatitis in 84 dogs. J. Vet. Intern. Med. 26: 888–896. doi: 10.1111/j.1939-1676.2012.00951.x [DOI] [PubMed] [Google Scholar]
- 29.McNally J. D., Menon K., Chakraborty P., Fisher L., Williams K. A., Al-Dirbashi O. Y., Doherty D. R., Canadian Critical Care Trials Group2012. The association of vitamin D status with pediatric critical illness. Pediatrics 130: 429–436. doi: 10.1542/peds.2011-3059 [DOI] [PubMed] [Google Scholar]
- 30.Newman S., Steiner J., Woosley K., Barton L., Ruaux C., Williams D.2004. Localization of pancreatic inflammation and necrosis in dogs. J. Vet. Intern. Med. 18: 488–493. doi: 10.1111/j.1939-1676.2004.tb02572.x [DOI] [PubMed] [Google Scholar]
- 31.Paek J., Kang J. H., Kim H. S., Lee I., Seo K. W., Yang M. P.2014. Serum adipokine concentrations in dogs with acute pancreatitis. J. Vet. Intern. Med. 28: 1760–1769. doi: 10.1111/jvim.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pápa K., Máthé A., Abonyi-Tóth Z., Sterczer A., Psáder R., Hetyey C., Vajdovich P., Vörös K.2011. Occurrence, clinical features and outcome of canine pancreatitis (80 cases). Acta Vet. Hung. 59: 37–52. doi: 10.1556/AVet.59.2011.1.4 [DOI] [PubMed] [Google Scholar]
- 33.Penninck D.2008. Pancreas. pp. 319–337. In: Atlas of Small Animal Ultrasonography (Penninck, D. and d’Anjou, M-A. eds.). Wiley-Blackwell, Ames. [Google Scholar]
- 34.Reddy S. V., Ramesh V., Bhatia E.2013. Double blind randomized control study of intramuscular vitamin D3 supplementation in tropical calcific pancreatitis. Calcif. Tissue Int. 93: 48–54. doi: 10.1007/s00223-013-9726-6 [DOI] [PubMed] [Google Scholar]
- 35.Ruaux C. G., Atwell R. B.1998. A severity score for spontaneous canine acute pancreatitis. Aust. Vet. J. 76: 804–808. doi: 10.1111/j.1751-0813.1998.tb12331.x [DOI] [PubMed] [Google Scholar]
- 36.Rucker R. B., Morris J. G.1997. The vitamins. pp. 703–739. In: Clinical Biochemistry of Domestic Animals (Kaneko, J. J., Harvey, J. W. and Bruss, M. L. eds.), Academic Press, San Diego. [Google Scholar]
- 37.Schenck P. A., Chew D. J., Nagode L. A., Rosol T. J.2006. Disorders of calcium: hypercalcemia and hypocalcemia. pp. 120–194. In: Fluid, Electrolyte, and Acid-Base Disorders in Small Animal Practice, 3rd ed. (DiBartola, S. P. ed.), Elsevier, St. Louis. [Google Scholar]
- 38.Steiner J. M.2010. Canine pancreatic disease. pp. 1695–1704. In: Textbook of Veterinary Internal Medicine, 7th ed. (Ettinger, S. J. and Feldman, E. C. eds.), Elsevier Saunders, Philadelphia. [Google Scholar]
- 39.Teichmann J., Mann S. T., Stracke H., Lange U., Hardt P. D., Klör H. U., Bretzel R. G.2007. Alterations of vitamin D3 metabolism in young women with various grades of chronic pancreatitis. Eur. J. Med. Res. 12: 347–350. [PubMed] [Google Scholar]
- 40.Trivedi S., Marks S. L., Kass P. H., Luff J. A., Keller S. M., Johnson E. G., Murphy B.2011. Sensitivity and specificity of canine pancreas-specific lipase (cPL) and other markers for pancreatitis in 70 dogs with and without histopathologic evidence of pancreatitis. J. Vet. Intern. Med. 25: 1241–1247. doi: 10.1111/j.1939-1676.2011.00793.x [DOI] [PubMed] [Google Scholar]
- 41.Wakshlag J. J., Rassnick K. M., Malone E. K., Struble A. M., Vachhani P., Trump D. L., Tian L.2011. Cross-sectional study to investigate the association between vitamin D status and cutaneous mast cell tumours in Labrador retrievers. Br. J. Nutr. 106Suppl 1: S60–S63. doi: 10.1017/S000711451100211X [DOI] [PubMed] [Google Scholar]
- 42.Watt G. H., Ashton S. H., Cook J. A., Wise W. C., Halushka P. V., Galbraith R. M.1989. Alterations in plasma levels and complexing of Gc (vitamin D-binding protein) in rats with endotoxic shock. Circ. Shock 28: 279–291. [PubMed] [Google Scholar]
- 43.Xenoulis P. G., Steiner J. M.2012. Canine and feline pancreatic lipase immunoreactivity. Vet. Clin. Pathol. 41: 312–324. doi: 10.1111/j.1939-165X.2012.00458.x [DOI] [PubMed] [Google Scholar]