Abstract
Changes in stroke volume variation (SVV) and pulse pressure variation (PPV) in response to fluid infusion were experimentally evaluated during vecuronium infusion and sevoflurane anesthesia in 5 adult, mechanically ventilated, euvolemic, beagle dogs. Sequential increases in central venous pressure (CVP; 3–7[baseline], 8–12, 13–17, 18–22 and 23–27 mmHg) were produced by infusing lactated Ringer’s solution and 6% hydroxyethyl starch solution. Heart rate (beats/min), right atrial pressure (RAP, mmHg), pulmonary arterial pressure (PAP, mmHg), pulmonary capillary wedge pressure (PCWP, mmHg), transpulmonary thermodilution cardiac output (TPTDCO, l/min), stroke volume (SV, ml/beat), arterial blood pressure (ABP, mmHg), extravascular lung water (EVLW, ml), pulmonary vascular permeability index (PVPI, calculated), SVV (%), PPV (%) and systemic vascular resistance (SVR, dynes/sec/cm5) were determined at each predetermined CVP range. Heart rate (P=0.019), RAP (P<0.001), PAP (P<0.001), PCWP (P<0.001), TPTDCO (P=0.009) and SV (P=0.04) increased and SVR (P<0.001), SVV (P<0.001) and PPV (P<0.001) decreased associated with each stepwise increase in CVP. Arterial blood pressure, EVLW, PVPI and the arterial partial pressures of oxygen and carbon dioxide did not change. The changes in SVV and PPV directly reflected the fluid load and the minimum threshold values for detecting fluid responsiveness were SVV ≥11% and PPV ≥7% in dogs.
Keywords: dog, fluid infusion, pulse pressure variation, stroke volume variation
Hypotension during general anesthesia and critical care has to be treated appropriately to maintain peripheral circulation. Increases in cardiac preload, cardiac contractility and/or heart rate may increase arterial blood pressure by increasing cardiac output [28, 34]. Fluid therapy is an indispensable medical treatment for maintaining and increasing the cardiac preload [3, 34]. However, fluid overload resulting in pulmonary edema can be a consequence of thoughtless excessive fluid administration [3, 12, 26]. Therefore, the evaluation of patient’s intravascular volume status and likelihood of response to a fluid challenge (i.e., fluid responsiveness) before fluid administration is a very effective measure for appropriate fluid administration.
Central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP) are standard traditional techniques for assessing cardiac preload [7]. The CVP is a major determinant of right ventricular filling and has been assumed to be an indicator of right ventricular preload [16]. The PCWP provides an indirect estimate of left atrial pressure and provides an accurate estimate of the cardiac preload under most circumstances [7]. In healthy humans, it is possible to make reasonable assumptions about the relationship between the CVP and the left atrial pressure and manipulate the circulation according to the measurements of the CVP [24]. However, in critically ill patients and patients with cardiovascular disease, no such assumptions can be made, and measurements of CVP may be invalid for assessing cardiac preload [24]. Unfortunately, the measurement of PCWP is uncommon in the small animal veterinary practice, because it is invasive, technically challenging and requires the insertion of a balloon-tipped, multi-lumen catheter into a branch of the pulmonary artery through the right atrium and ventricle [8].
Fluid responsiveness is a term that defines an ability of the left ventricle to increase its stroke volume in response to fluid administration [39]. Positive pressure ventilation is known to reduce cardiac preload by increasing pleural pressure during the inspiratory phase of mechanical ventilation causing decreases in both cardiac preload and stroke volume [30]. The changes in stroke volume between the inspiratory and expiratory phases of positive pressure ventilation, the so-called stroke volume variation (SVV), are considered to be an indicator of fluid responsiveness in human critical care medicine [2, 29]. In addition, changes in the arterial pulse pressure (pulse pressure variation: PPV), which is the difference between the maximum and minimum arterial pulse pressure during one mechanical breath, have been shown to be an accurate predictor of fluid responsiveness in critically ill human patients [31, 32]. The SVV and PPV have been experimentally shown to be a good indicator of hemodynamic state during hypovolemia produced by severe hemorrhage in ventilated dogs [1]. However, the optimal threshold value of SVV and PPV for a prediction of fluid responsiveness has not been determined in dogs.
Pulse contour cardiac output (PiCCO) can be used to monitor real-time continuous cardiac output through pulse contour analysis, SVV, PPV, extravascular lung water (EVLW) and pulmonary vascular permeability index (PVPI) in humans and dogs [17, 22, 27, 37, 38, 40, 41, 44]. The purpose of the present study was to determine the ability of SVV and PPV measured by the PiCCO system, to detect an increase in vascular volume by producing graded sequential increases in CVP. These data were used to derive optimal threshold SVV and PPV values for predicting fluid responsiveness in mechanically ventilated dogs. We hypothesized that SVV and PVV would detect fluid administration and would decrease at the stage of hypervolemia in normal euvolemic sevoflurane anesthetized mechanically ventilated dogs.
MATERIALS AND METHODS
Experimental animals
Three male and two female beagle dogs, 7 to 8 years of age (7.4 ± 0.5 [mean ± standard deviation] years old) and weighing from 9 to 19.2 kg (13.9 ± 4.1 kg), were used. The dogs were judged to be in good to excellent health without cardiac disease based upon a physical examination, complete blood cell count, chemical analysis and echocardiographic examination. The dogs were cared for according to the principles of the “Guide for the Care and Use of Laboratory Animals” prepared by Rakuno Gakuen University. The Animal Care and Use Committee of Rakuno Gakuen University approved this study (approved No.: VH23B14). In each dog, the minimum alveolar concentration (MAC) of sevoflurane was determined using a tail clamp method [45] at least 1 month in advance of the fluid load experiment.
Anesthesia and instrumentation
Food and water were withheld for 12 hr before the experiment. All dogs were mask induced using sevoflurane (Sevoflo®, Dainippon-Sumitomo Pharma, Osaka, Japan) and orotracheally intubated. Anesthesia was maintained with sevoflurane in 1.0 l/min of 100% inspired oxygen fraction (FiO2 1.0) at an end-tidal concentration (ETSEV) 1.3-fold their individual predetermined MAC value. Sevoflurane was delivered by an out-of-circuit sevoflurane vaporizer (Sevotech III, Ohmeda, Datex-Ohmeda, Tokyo, Japan) via a circle rebreathing system and anesthetic machine (Beaver 20, Kimura Medical Instrument, Tokyo, Japan). The anesthetized dog was positioned in left lateral recumbency, and a 22-gauge catheter (Supercath, Medikit Co., Tokyo, Japan) was percutaneously placed in each cephalic vein. Lactated Ringer’s solution (LRS; Solulact®, Terumo Co., Tokyo, Japan), 10 ml/kg/hr, was infused through the 22-gauge catheter placed in the left cephalic vein during instrumentation. All dogs were paralyzed by administering an intravenous (IV) bolus of 0.2 mg/kg vecuronium (Musculate®, Fuji Pharma Co., Tokyo, Japan) followed by a constant rate infusion of 0.1 mg/kg/hr vecuronium through the 22-gauge catheter placed in the right cephalic vein and artificially ventilated (12 breaths/min, 1:2 of inspiratory and expiratory time ratio, and 15 ml/kg of inspired tidal volume) using a time-cycled ventilator (Nuffield Aanesthesia Ventilation Series 200, Penlon, Abingdon Oxon, U.K.). Further adjustments of inspired gas flow rate (i.e. inspired tidal volume) were performed to maintain end-tidal partial pressures of carbon dioxide (PETCO2) between 35 and 40 mmHg during the instrumentation. Following an achievement of normocapnia (PETCO2 between 35 and 40 mmHg), the ventilator setting was fixed throughout the fluid load experiment.
The areas over the right jugular vein and inside surface of the left pelvic limb over the femoral artery were clipped and aseptically prepared for placement of thermodilution and PiCCO catheters. Approximately 0.5 ml each of 2% lidocaine (Xylocaine®, AstraZeneca, Osaka, Japan) was infiltrated into the right jugular and femoral arterial catheter sites. A 5 Fr balloon-tipped triple lumen thermodilution catheter (TC-504; Nihon Koden Co., Tokyo, Japan) was inserted through a 6 Fr catheter introducer (Catheter Introducer, Medikit Co.) placed into the right jugular vein and advanced into the right atrium and pulmonary artery under pressure waveform guidance. The left femoral artery was surgically isolated, and a guide wire and dilator were used to insert and advance a 4Fr thermistor-tipped PiCCO catheter (16 cm PiCCO Catheter PV2014L16, PULSION Medical Systems AG, Munich, Germany) towards the iliac artery.
Experimental protocol
After the completion of instrumentation, the dogs were allowed to stabilize to obtain baseline values of cardiopulmonary parameters. Sequential, stepwise fluid loading was produced by increasing the infusion rate of LRS and 6% hydroxyethyl starch solution (HES; Salinehes®, Fresenius Kabi Japan Co., Tokyo, Japan). LRS and HES were initially infused at approximately 90 and 30 ml/kg/hr, respectively; then, their infusion rates were adjusted to achieve each target CVP. Hemodynamic data were recorded at the baseline CVP (as their baseline value) and 4 predetermined target CVP values ranging from 8 to 12 mmHg (10.9 to 16.3 cmH2O), 13 to 17 mmHg (17.7 to 23.1 cmH2O), 18 to 22 mmHg (24.5 to 29.9 cmH2O) and 23 to 27 mmHg (31.3 to 36.7 cmH2O).
The LRS and HES infusions were stopped after acquiring cardiovascular data at a CVP of 23–27 mmHg, and the dogs were administered furosemide (2 mg/kg IV; Lasix®, Nichi-Iko Pharmaceutical Co., Toyama, Japan) and human atrial natriuretic peptide, carperitide (0.1 µg/kg/min IV; Hanp®, Daiichi Sankyo Co., Tokyo, Japan) until CVP returned to baseline values. The vecronium infusion was discontinued, and all dogs were administered atropine (0.025 mg/kg IV; Atropine Sulfate Injection®, Fuso Pharmaceutical Industries, Osaka, Japan) followed by neostigmine (0.06 mg/kg IV; Vagostigmine®, Shionogi Co., Osaka, Japan) in order to antagonize muscle paralysis. Once effective spontaneous ventilation that produced a normal range of PaCO2 (around 40 mmHg) was established, sevoflurane was discontinued and the dogs were allowed to recover from the anesthesia. The dogs were monitored for their general condition, food and water consumption for 24 hr after the recovery from anesthesia. Pain management was provided to each dog with an intramuscular injection of buprenorphine (0.01 mg/kg; Lepetan, Otsuka, Tokyo, Japan) and a subcutaneous injection of meloxicam (0.2 mg/kg; Metacam, Boehringer Ingelheim, Burlington, Canada) before the recovery from anesthesia.
Cardiopulmonary measurements
First of all, arterial blood pressure (ABP), HR, RAP, PAP and PCWP were recorded when the baseline CVP and each target CVP were achieved. Then, transpulmonary thermodilution cardiac output (TPTDCO) was measured, and PiCCO measurements including SV, SVV, PPV, EVLW, PVPI and SVR were recorded at the baseline CVP and each target CVP.
The RAP, PAP and PCWP were obtained by connecting pressure transducers (CDX-A90; Cobe Laboratories, Inc., Tokyo, Japan) to the appropriate ports on the thermodilution catheter. The pressure transducers were connected to a multi-parameter patient monitoring system (DS-7200, Fukuda Denshi, Tokyo, Japan). The CVP was determined using the distal port of the thermodilution catheter after the distal port was retracted into the cranial vena cava. The ABP was determined from the arterial PiCCO catheter placed in the femoral artery and connected to a pressure transducer (PiCCO Monitoring Kit PV8215, PULSION Medical Systems AG) and a PiCCO system (PiCCOplus monitor Version 6.0, PULSION Medical Systems AG). All pressure transducers for determining CVP, RAP, PAP, PCWP and ABP were calibrated to a zero reference point at the level of the manubrium. The catheters were flushed periodically with heparinized saline (Isotonic Sodium Chloride Solution®, Terumo Co.).
The TPTDCO was determined by injection of 3 ml bolus ice-cold (0–1°C) 5% dextrose (5w/v% Glucose Injection®, Terumo Co.) through the distal port of the thermodilution catheter and measuring changes in blood temperature with the arterial PiCCO catheter tip thermistor placed in the left femoral artery [15, 40]. The TPTDCO was measured at least 3 times to obtain 3 consecutive values with a difference of <10%, and the mean value was calculated. The PiCCO measurements were calibrated by each TPTDCO value determined at the baseline CVP and each target CVP. The SV, SVV and PPV were recorded when the artery pressure waveform had stabilized after the TPTDCO determination at the baseline CVP and each target CVP. The EVLW and PVPI were recorded using the PiCCO system during the TPTDCO measurement. The SVR was calculated from the ABP, RAP and TPTDCO.
The ETSEV, PETCO2, HR and the electrocardiogram (lead II) were determined by a patient monitoring system (BP-508V, Omron Colin Co., Tokyo, Japan). Peak inspiratory pressure (PIP) was measured and recorded by a manometer within the circle rebreathing system included in the rebreathing circuit. Arterial blood samples were anaerobically withdrawn from the PiCCO catheter and were collected into a plastic syringe heparinized with liquid containing 1,000 unit/ml of sodium heparin (Novo-heparin for injection, Mochida Pharmaceutical Co., Tokyo, Japan) by an evacuation technique to minimize the sample dilution [13]. Air bubbles in the syringe were immediately expelled following the blood collection. These blood samples were analyzed immediately after the collection (<5 min) to measure partial pressures of arterial oxygen (PaO2), partial pressures of arterial carbon dioxide (PaCO2), packed cell volume (PCV) and hemoglobin (Hb) using a blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Tokyo, Japan).
Statistical analysis
The data are presented as mean ± standard deviation (SD). All cardiopulmonary measurements were analyzed by Shapiro-Wilk test and Kolmogorov-Smirnov test, two-way repeated measures analysis of variance (ANOVA), and Tukey’s studentized range test. Pearson correlation was calculated between CVP and hemodynamic measurements (HR, mean ABP, RAP, PAP, PCWP, SV, SVV and PPV). A value of P<0.05 was considered significant.
Dogs that increased their stroke volume by ≥15% in response to fluid administration, at each target CVP stage, were considered to be responders, and those that did not were defined as non-responders. The cut off value for a SV increase of ≥15% was based on previous findings in human patients showing the ability of SVV and PPV to predict fluid responsiveness [11, 36]. Responders and non-responders hemodynamic measurements recorded before and after the target CVP range were compared by Student’s t-test for normally distributed continuous variables or by the Mann-Whitney test for non-normally distributed variables. Receiver operating characteristic (ROC) curves were generated to assess the ability of a variable to identify responders and non-responders. The areas under the ROC curves (AUC) were calculated and compared as previously described [9]. Optimal threshold values (the value that maximizes the sum of both sensitivity and specificity) for PCWP, RAP, SV, HR, MABP, CVP, PAP, SVV and PPV were determined from the ROC curves.
RESULTS
The mean ± SD for sevoflurane MAC in the dogs was 2.47 ± 0.35% (range: 2.07 to 2.92%). Consequently, anaesthesia was maintained with an ETSEV of 3.22 ± 0.47% (1.3 MAC) throughout the fluid load experiment. The target ranges for CVP, produced by LRS and HES administration, were achieved at 62 ± 3 min for 8 to 12 mmHg (mean ± SD: 11.9 ± 0.2 mmHg), 109 ± 8 min for 13 to 17 mmHg (16.4 ± 0.4 mmHg), 163 ± 8 min for 18 to 22 mmHg (21.1 ± 0.5 mmHg) and 220 ± 9 min for 23 to 27 mmHg (25.3 ± 1.1 mmHg) after the hemodynamic data were obtained at the baseline CVP (3 to 7 mmHg, 5.3 ± 1.5 mmHg). Total volumes of LRS and HES administered were 3,176 ± 480 ml (243 ± 87 ml/kg) and 1,071 ± 131 ml (78 ± 27 ml/kg), respectively.
Cardiopulmonary measurements changed at each stage of fluid loading (Table 1). Fluid loading increased CVP (P<0.001), HR (P=0.019), RAP (P<0.001), PAP (P<0.001), PCWP (P<0.001), TPTDCO (P=0.009) and SV (P=0.040) and decreased SVR (P<0.001), SVV (P<0.001), PPV (P<0.001), PCV (P<0.001) and Hb (P<0.001). There were no significant changes in mean ABP (MABP), EVLW, PVPI, PaO2, PaCO2 and PIP.
Table 1. Cardiopulmonary values induced by sequential increases in central venous pressure (CVP) in euvolemic, mechanically ventilated, sevoflurane anesthetized dogs.
| Measurements | Target range of CVP | ||||
|---|---|---|---|---|---|
| 3–7 mmHg (baseline) (4.1–9.5 cmH2O) |
8–12 mmHg (10.9–16.3 cmH2O) |
13–17 mmHg (17.7–23.1 cmH2O) |
18–22 mmHg (24.5–29.9 cmH2O) |
23–27 mmHg (31.3–36.7 cmH2O) |
|
| CVP (mmHg) | 5.3 ± 1.5 | 11.9 ± 0.2 | 16.4 ± 0.4 | 21.1 ± 0.5 | 25.3 ± 1.1 |
| Heart rate (beats/min) | 108 ± 15 | 128 ± 18 | 131 ± 13 | 134 ± 12a) | 138 ± 9a) |
| MABP (mmHg) | 88 ± 10 | 85 ± 12 | 87 ± 7 | 88 ± 8 | 90 ± 4 |
| RAP (mmHg) | 4 ± 2 | 11 ± 2 a) | 16 ± 2a) | 19 ± 2a) | 24 ± 2a) |
| PAP (mmHg) | 15 ± 2 | 21 ± 2 a) | 25 ± 1a) | 30 ± 1a) | 35 ± 2a) |
| PCWP (mmHg) | 7 ± 2 | 15 ± 1 a) | 20 ± 2a) | 24 ± 3a) | 30 ± 2a) |
| TPTDCO (l/min) | 1.8 ± 0.3 | 3.0 ± 0.8 | 3.1 ± 0.7 | 3.3 ± 0.7a) | 3.4 ± 0.8a) |
| SVR (dynes/sec/cm5) | 3,686 ± 273 | 2,049 ± 616 | 1,942 ± 502a) | 1,728 ± 417a) | 1,608 ± 361a) |
| SV (ml/beat) | 17 ± 2 | 23 ± 3 | 23 ± 3 | 24 ± 4 | 25 ± 4a) |
| SVV (%) | 14.3 ± 1.4 | 7.6 ± 2.9a) | 7.3 ± 0.8a) | 5.6 ± 1.9a) | 5.7 ± 1.1a) |
| PPV (%) | 10.8 ± 2.9 | 6.0 ± 2.1a) | 3.5 ± 0.9a) | 3.2 ± 0.3a) | 2.9 ± 0.6a) |
| EVLW (ml) | 198 ± 83 | 199 ± 115 | 199 ± 104 | 227 ± 125 | 244 ± 138 |
| PVPI | 2.1 ± 0.2 | 1.9 ± 0.5 | 1.8 ± 0.4 | 1.9 ± 0.6 | 2.1 ± 0.7 |
| PaO2 (mmHg) | 519 ± 68 | 501 ± 50 | 472 ± 113 | 496 ± 47 | 417 ± 92 |
| PaCO2 (mmHg) | 40 ± 3 | 38 ± 2 | 37 ± 2 | 39 ± 3 | 40 ± 3 |
| PIP (cmH2O) | 10 ± 1 | 11 ± 1 | 11 ± 1 | 12 ± 1 | 13 ± 1 |
CVP: central venous pressure, MABP: mean arterial blood pressure, RAP: right atrial pressure, PAP: pulmonary artery pressure, PCWP: pulmonary capillary wedge pressure, TPTDCO: transpulmonary thermodilution cardiac output, SVR: systemic vascular resistance, SV: stroke volume, SVV: stroke volume variation, PPV: pulse pressure variation, EVLW: extravascular lung water, PVPI: pulmonary vascular permeavility index, PaO2: partial pressure of arterial oxygen, PaCO2: partial pressure of arterial carbon dioxide. a) significantlly statistical difference (P<0.05) compared with the baseline value recorded at the CVP of 3–7 mmHg.
Hemodynamic measurements before and after the attainment of target CVP values in the responders and non-responders differed (Table 2). A total of 20 data points were obtained for each hemodynamic measurement from the 5 dogs during fluid loading. There were 5 responders at the CVP 8 to 12 mmHg stage, 2 responders and 3 non-responders at the CVP 13 to 17 mmHg stage and 5 non-responders at the CVP 18 to 22 mmHg and 23 to 27 mmHg stages. The SVV and PPV were significantly increased in responders only (P=0.009 and P=0.008, respectively). The CVP, RAP, PAP and PCWP were significantly increased in both responders (P=0.004, P=0.004, P=0.001 and P=0.002, respectively) and non-responders (P=0.002, P=0.009, P=0.004 and P=0.010, respectively). There was no significant change in HR and MABP in both responders and non-responders.
Table 2. Hemodynamic measurements before and after the attainment of target central venous pressure (CVP) in responders and non-responders.
| Responders (n=7) | Non-responders (n=13) | ||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Heart rate (beats/min) | 114 ± 8 | 130 ± 16 | 130 ± 11 | 134 ± 9 | |
| MABP (mmHg) | 85 ± 8 | 85 ± 9 | 87 ± 8 | 88 ± 6 | |
| CVP (mmHg) | 7 ± 3 | 13 ± 1a) | 17 ± 3 | 21 ± 3a) | |
| RAP (mmHg) | 6 ± 3 | 12 ± 2a) | 15 ± 3 | 19 ± 3a) | |
| PAP (mmHg) | 16 ± 2 | 22 ± 2a) | 26 ± 2 | 30 ± 2a) | |
| PCWP (mmHg) | 9 ± 3 | 16 ± 3a) | 20 ± 3 | 25 ± 4a) | |
| SV (ml/beat) | 20 ± 3 | 26 ± 3a) | 27 ± 3 | 28 ± 3 | |
| SVV (%) | 13.9 ± 2.1 | 7.8 ± 2.8a) | 6.1 ± 1.6 | 5.8 ± 1.3 | |
| PPV (%) | 10.2 ± 2.7 | 5.4 ± 2.2a) | 3.7 ± 0.9 | 3.2 ± 0.6 | |
Dogs increasing their stroke volume by ≥15% in response to fluid administration at each target CVP stage were considered to be responders, and others were defined as non-responders. MABP: mean arterial blood pressure, RAP: right atrial pressure, PAP: pulmonary artery pressure, PCWP: pulmonary capillary wedge pressure, SV: stroke volume, SVV: stroke volume variation, PPV: pulse pressure variation. a) significantlly statistical difference (P<0.05) compaered with the hemodynamic measurement recorded before attained the CVP.
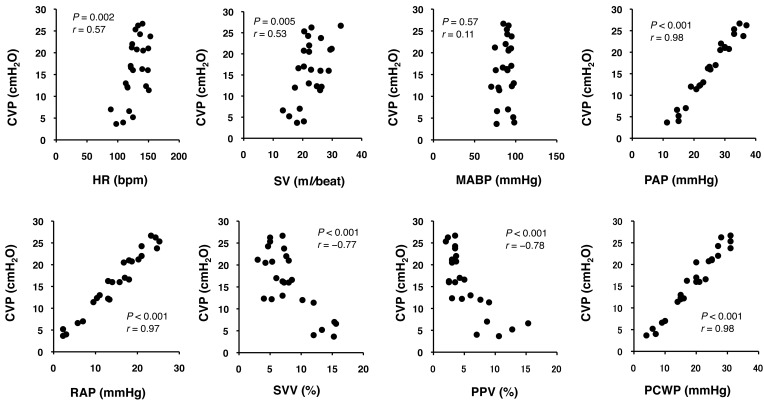
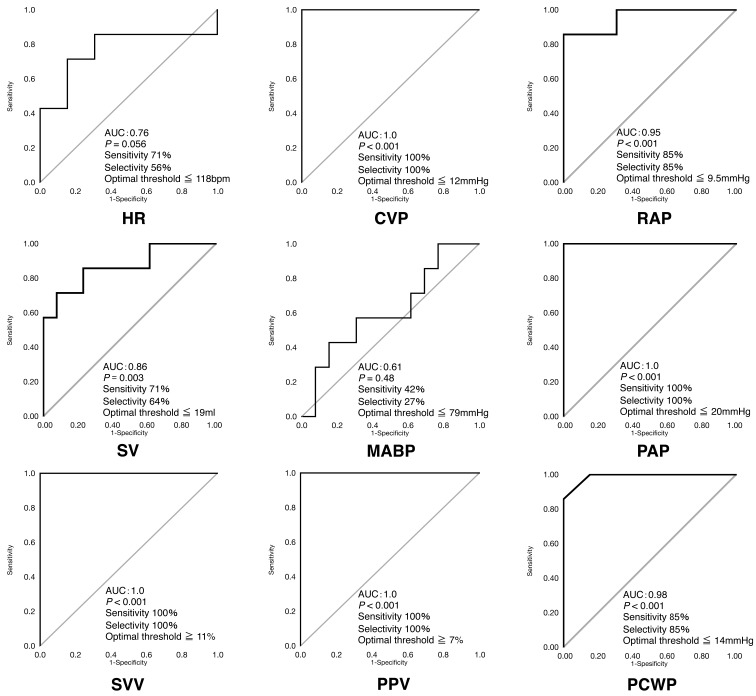
The CVP was significantly correlated with HR (r=0.57, P=0.002), RAP (r=0.97, P<0.001), PAP (r=0.98, P<0.001), SV (r=0.53, P=0.005), SVV (r= −0.77, P<0.001), PPV (r= −0.78, P<0.001) and PCWP (r=0.98, P<0.001) (Fig. 1). The SVV (AUC=1.0, P<0.001), PPV (AUC=1.0, P<0.001), CVP (AUC=1.0, P<0.001) and PAP (AUC=1.0, P<0.001) produced the greatest AUC for detecting a SV ≥15% due to the fluid loading (Fig. 2). High AUCs were also seen for PCWP (AUC=0.98, P<0.001), RAP (AUC=0.95, P<0.001) and SV (AUC=0.86, P=0.003). HR (AUC=0.76, P=0.056) and MABP (AUC=0.61, P=0.480) failed to predict fluid responsiveness. These data yielded optimal threshold values for fluid responsiveness of SVV ≥11% (sensitivity 100%; specificity 100%), PPV ≥7% (sensitivity 100%; specificity 100%), CVP ≤12 mmHg (16.3 cmH2O) (sensitivity 100%; specificity 100%), PAP ≤20 mmHg (sensitivity 100%; specificity 100%), PCWP ≤14 mmHg (sensitivity 85%; specificity 85%), RAP ≤9.5 mmHg (sensitivity 85%; specificity 85%) and SV ≤19 ml/beat (sensitivity 71%; specificity 64%).
Fig. 1.
Correlation between central venous pressure (CVP) and heart rate (HR), stroke volume (SV), mean arterial blood pressure (MABP), pulmonary artery pressure (PAP), right atrial pressure (RAP), stroke volume variation (SVV), pulse pressure variation (PPV) and pulmonary capillary wedge pressure (PCWP). Significant correlations with CVP were detected in HR (r=0.57, P=0.002), RAP (r=0.97, P<0.001), PAP (r=0.98, P<0.001), SV (r=0.53, P=0.005), SVV (r= −0.77, P<0.001), PPV (r= −0.78, P<0.001) and PCWP (r=0.98, P<0.001).
Fig. 2.
Receiver operating characteristic (ROC) and (AUC) curves for predicting the ability of changes in heart rate (HR), central venous pressure (CVP), stroke volume (SV), mean arterial blood pressure (MABP), pulmonary artery pressure (PAP), right atrial pressure (RAP), stroke volume variation (SVV), pulse pressure variation (PPV) and pulmonary capillary wedge pressure (PCWP) to detect fluid responsiveness. The straight line indicates the line of identity. AUC=0.5 predicts that fluid responsiveness is equal to chance. AUC=1.0 indicates that SV ≥15% increases with fluid loading. SVV, PPV, CVP and PAP provided the best predictions. HR and MABP failed to predict fluid responsiveness.
DISCUSSION
Fluid infusions of LRS and HES successfully produced stepwise increases in CVP. Fluid responsiveness as indicated by a SV increase ≥15% was observed during earlier stages of fluid loading. Fluid responsiveness was lost when CVP was increased to values >12 mmHg. The optimal threshold values for predicting fluid responsiveness were SVV ≥11%, PPV ≥7%, CVP ≤12 mmHg (16.3 cmH2O), PAP ≤20 mmHg, PCWP ≤14 mmHg, RAP ≤9.5 mmHg and SV ≤19 ml/beat in our dogs. As hypothesized, SVV and PPV provided the best diagnostic performance for identifying fluid responsiveness. To our knowledge, this is the first data reporting the optimal threshold values for PPV and SVV in euvolemic sevoflurane anesthetized dogs.
Currently, the thermodilution is the standard clinical method of measuring cardiac output. However, this technique requires the placement of a thermodilution catheter into pulmonary artery through the right atrium and ventricle, which increases the risk for possible complications [8]. The TPTDCO employs a central venous catheter for thermal indicator injection, and a thermistor-tipped catheter placed in the femoral artery to detect thermal dilution [17, 42]. Therefore, TPTDCO facilitates cardiac output measurement while reducing or eliminating the complications associated with catheterization through the right atrium and ventricle. Our laboratory and others have previously reported that TPTDCO measured by the PiCCO system exhibited good agreement with cardiac output measured by thermodilution or lithium dilution methods in anesthetized dogs [15, 40]. SV values were calculated from HR and TPTDCO measured by the PiCCO system. We considered fluid responsiveness to be an increase in SV ≥15% based upon previous reports in human patients [11, 36].
Changes in CVP, RAP, PAP and PCWP have traditionally been used to assess cardiac preload in dogs [3, 16, 25]. Their normal values range from 0 to 5 cmH2O for CVP [16], from 2 to 5 mmHg for RAP [25], from 8 to 20 mmHg for PAP [25] and from 5 to 12 mmHg for PCWP [25] in dogs. The CVP, RAP, PAP and PCWP pressure values increased linearly and significantly in all dogs (both fluid responders and non-responders) at all stages of fluid loading from euvolemic, making it impossible to differentiate fluid responders and non-responders in these pressure parameters. Our data suggest that, similar to human patients, these pressure parameters are indicative of cardiac preload but are not predictors of fluid responsiveness [7, 21, 24].
Both PPV and SVV have been shown to be reliable predictors of fluid responsiveness in conscious or anesthetized, septic, hemorrhaged or traumatized, mechanically ventilated human patients [2, 4, 6, 11, 27, 29,30,31,32, 37, 38, 44, 45, 47]. Their optimal threshold values have been reported to be 10% for PPV and 10% for SVV in 25 adult patients before coronary artery bypass grafting [2], 13.5% for PPV and 12.5% for SVV in 40 adult patients undergoing off-pump coronary artery bypass grafting [11], 8% for PPV and 10% for SVV in 25 adult patients with obstructive jaundice [46], 12% for PPV and 10% for SVV in 46 adult patients with sepsis [6] and 13% for PPV and 12% for SVV in 11 adult patients undergoing major abdominal surgery [4]. It was also reported that the optimal threshold values for predicting fluid responsiveness in 20 healthy awake human volunteers breathed constantly at 15 breaths/min were 8% for PPV and 13% for SVV [47]. The optimal threshold values for predicting fluid responsiveness in 12 anesthetized healthy piglets ventilated with normal tidal volume (12ml/kg) were 9.5% for PPV and 8.5% for SVV [5]. The optimal threshold values for predicting fluid responsiveness in our dogs were 7% for PPV and 11% for SVV. The SVV threshold value in our healthy dogs was similar to those in human patients [2, 4, 6, 11, 46] and the healthy human volunteers [47]. The threshold value for PPV in our dogs was similar to those in the healthy human volunteers [47] and closer to those in the healthy piglets [5]. The optimal threshold values of 7% for PPV and 11% for SVV may be valid for predicting fluid responsiveness in healthy euvolemic anesthetized dogs. Further investigation should be conducted to establish clinical relevant optimal threshold values for PPV and SVV in healthy and diseased dogs.
Fluid loading in euvolemic dogs did not produce a significant change in MABP, but significantly increased HR, TPTDCO and SV and significantly decreased SVR. Fluid loading increased atrial and ventricular volumes and myocardial wall stress as evidenced by increases in RAP and PCWP. Increases in HR were attributed to stimulation of the sinoatrial node via the Bainbridge reflex while increases in ventricular pressure, as evidenced by an increase in PCWP, augmented cardiac contractile force resulting in increased TPTDCO via the Frank-Starling mechanism [20]. In addition, decreases in afterload, as suggested by the decreased SVR, are known to be associated with fluid loading, increases in atrial natriuretic peptide (ANP) and increases in cardiac output [14, 33]. We surmised that increases in plasma ANP might have contributed to vasodilation and the decrease in SVR and the resultant changes in TPTDCO. Importantly, MABP was a poor indicator of fluid loading in our dogs. Muir et al. [35] and Valverde et al. [43] reported that arterial blood pressure was a poor indicator of fluid responsiveness in euvolemic, normotensive or hypotensive isoflurane anesthetized dogs. Our study also illustrated that arterial blood pressure is a poor indicator of fluid responsiveness in hypervolemic dogs.
A previous experimental study in dogs has suggested that PCWP >25 mmHg caused hydrostatic pulmonary edema in dogs [18]. The PCWP increased to 30 ± 2 mmHg at the highest CVP stage in dogs in our present study. However, there was no significant change in EVLW, PVPI, PaO2 and PaCO2. Increases in EVLW imply that fluid is accumulating within the lung but outside the vasculature [17, 22, 41]. Abnormal findings indicating pulmonary edema on chest radiographs were observed in dogs when EVLW increased over 130% from the normal condition [10, 42]. The PVPI is indicative of pulmonary microvascular permeability and used to differentiate increased permeability pulmonary edema from hydrostatic pulmonary edema [18]. A PVPI >2.6 provides a definitive diagnosis of acute lung injury/acute respiratory distress syndrome in humans [23]. The EVLW values recorded at the highest CVP stage (244 ± 138 ml) were not significantly different from their baseline values (198 ± 83 ml), and PVPI remained less than 2.6 throughout the period of fluid loading. In addition, there was no apparent impairment of blood gas values. It was considered that the hydrostatic pulmonary edema was not developed in our dogs.
Our study has several limitations. First, our data and threshold values were obtained from a small group of euvolemic healthy dogs that were fluid loaded. Although fluid infusions are commonly administered during elective surgical procedures, our results may have been different if collected from hypovolemic, hypotensive or septic dogs. We must be cautious extrapolating these results to critically ill patients where multiorgan involvement is frequently observed. Second, different mechanical ventilation settings, in particular tidal volume setting, may influence venous return and cardiac output altering SVV and PPV values [5, 19]. It was experimentally reported that larger tidal volume caused higher changes in SVV and PPV [5, 19]. In the present study, the ventilator setting had been fixed throughout the fluid load experiment, since normocapnia (PETCO2 between 35 and 40 mmHg) was achieved during the instrumentation. The PIP was remained in a narrow range from 10 ± 1 cmH2O at the baseline CVP to 13 ± 1 cmH2O at the highest target CVP without statistical difference. We inferred that a change in tidal volume was minimum in our dogs throughout the fluid load experiment. However, we cannot confirm this interpretation, because we did not measure the tidal volume in the present study. Finally, the level of anesthesia could have altered our results. Rapid-rate administration of a large volume of isotonic crystalloid was ineffective in restoring hemodynamics in euvolemic dogs at a deep plane of isoflurane (1.6 MAC) anesthesia [43]. Fluid responsiveness returned when the isoflurane concentration was reduced to 1.3 MAC. Our inhalant anesthetic concentration was set at 1.3 MAC, a concentration that is typically used during elective surgical procedures. Further studies adopting a larger population of dogs, including clinical patients, will be necessary to determine the clinical relevance of our threshold values for PPV and SVV in response to fluid loading.
In conclusion, changes in SVV and PPV are useful predictors of fluid load in dogs. Both SVV and PVV could detect fluid responsiveness at an earlier stage of fluid load than conventional static hemodynamic measures. Fluid responsiveness is lost in hypervolemic dogs. The proposed optimal threshold values for a prediction of fluid responsiveness were SVV ≥11% and PPV ≥7% in euvolemic, mechanically ventilated dogs under general anesthesia.
REFERENCES
- 1.Berkenstadt H., Friedman Z., Preisman S., Keidan I., Livingstone D., Perel A.2005. Pulse pressure and stroke volume variations during severe haemorrhage in ventilated dogs. Br. J. Anaesth. 94: 721–726. doi: 10.1093/bja/aei116 [DOI] [PubMed] [Google Scholar]
- 2.Cannesson M., Musard H., Desebbe O., Boucau C., Simon R., Hénaine R., Lehot J. J.2009. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth. Analg. 108: 513–517. doi: 10.1213/ane.0b013e318192a36b [DOI] [PubMed] [Google Scholar]
- 3.Davis H., Jensen T., Johnson A., Knowles P., Meyer R., Rucinsky R., Shafford H., American Association of Feline PracticionersAmerican Animal Hospital Association2013. 2013 AAHA/AAFP fluid therapy guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 49: 149–159. doi: 10.5326/JAAHA-MS-5868 [DOI] [PubMed] [Google Scholar]
- 4.Derichard A., Robin E., Tavernier B., Costecalde M., Fleyfel M., Onimus J., Lebuffe G., Chambon J. P., Vallet B.2009. Automated pulse pressure and stroke volume variations from radial artery: evaluation during major abdominal surgery. Br. J. Anaesth. 103: 678–684. doi: 10.1093/bja/aep267 [DOI] [PubMed] [Google Scholar]
- 5.Díaz F., Erranz B., Donoso A., Salomon T., Cruces P.2015. Influence of tidal volume on pulse pressure variation and stroke volume variation during experimental intra-abdominal hypertension. BMC Anesthesiol. 15: 127–136. doi: 10.1186/s12871-015-0105-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drvar Z., Pavlek M., Drvar V., Tomasević B., Baronica R., Perić M.2013. [Stroke volume and pulse pressure variation are good predictors of fluid responsiveness in sepsis patients]. Acta Med. Croatica 67: 407–414 (in Croatian). [PubMed] [Google Scholar]
- 7.Gödje O., Peyerl M., Seebauer T., Lamm P., Mair H., Reichart B.1998. Central venous pressure, pulmonary capillary wedge pressure and intrathoracic blood volumes as preload indicators in cardiac surgery patients. Eur. J. Cardiothorac. Surg. 13: 533–539, discussion 539–540. doi: 10.1016/S1010-7940(98)00063-3 [DOI] [PubMed] [Google Scholar]
- 8.Gore J. M., Goldberg R. J., Spodick D. H., Alpert J. S., Dalen J. E.1987. A community-wide assessment of the use of pulmonary artery catheters in patients with acute myocardial infarction. Chest 92: 721–727. doi: 10.1378/chest.92.4.721 [DOI] [PubMed] [Google Scholar]
- 9.Hanley J. A., McNeil B. J.1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148: 839–843. doi: 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 10.Hirakawa A., Sakamoto H., Misumi K., Kamimura T., Shimizu R.1993. Assessment of pulmonary edema based on extravascular thermal volume in dogs. J. Vet. Med. Sci. 55: 995–1000. doi: 10.1292/jvms.55.995 [DOI] [PubMed] [Google Scholar]
- 11.Hofer C. K., Müller S. M., Furrer L., Klaghofer R., Genoni M., Zollinger A.2005. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest 128: 848–854. doi: 10.1378/chest.128.2.848 [DOI] [PubMed] [Google Scholar]
- 12.Holte K., Sharrock N. E., Kehlet H.2002. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 89: 622–632. doi: 10.1093/bja/aef220 [DOI] [PubMed] [Google Scholar]
- 13.Hopper K., Rezende M. L., Haskins S. C.2005. Assessment of the effect of dilution of blood samples with sodium heparin on blood gas, electrolyte, and lactate measurements in dogs. Am. J. Vet. Res. 66: 656–660. doi: 10.2460/ajvr.2005.66.656 [DOI] [PubMed] [Google Scholar]
- 14.Houshmand F., Faghihi M., Zahediasl S.2015. Role of atrial natriuretic Peptide in oxytocin induced cardioprotection. Heart Lung Circ. 24: 86–93. doi: 10.1016/j.hlc.2014.05.023 [DOI] [PubMed] [Google Scholar]
- 15.Itami T., Endo Y., Hanazono K., Ishizuka T., Tamura J., Miyoshi K., Sano T., Yamashita K.2016. Comparison of cardiac output measurements using transpulmonary thermodilution and conventional thermodilution techniques in anaesthetized dogs with fluid overload. Vet. Anaesth. Analg. 43: 388–396. doi: 10.1111/vaa.12331 [DOI] [PubMed] [Google Scholar]
- 16.Jennings P. B., Anderson R. W., Martin A. M., Jr1967. Central venous pressure monitoring: a guide to blood volume replacement in the dog. J. Am. Vet. Med. Assoc. 151: 1283–1293. [PubMed] [Google Scholar]
- 17.Jozwiak M., Silva S., Persichini R., Anguel N., Osman D., Richard C., Teboul J. L., Monnet X.2013. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit. Care Med. 41: 472–480. doi: 10.1097/CCM.0b013e31826ab377 [DOI] [PubMed] [Google Scholar]
- 18.Katzenelson R., Perel A., Berkenstadt H., Preisman S., Kogan S., Sternik L., Segal E.2004. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit. Care Med. 32: 1550–1554. doi: 10.1097/01.CCM.0000130995.18334.8B [DOI] [PubMed] [Google Scholar]
- 19.Kawazoe Y., Nakashima T., Iseri T., Yonetani C., Ueda K., Fujimoto Y., Kato S.2015. The impact of inspiratory pressure on stroke volume variation and the evaluation of indexing stroke volume variation to inspiratory pressure under various preload conditions in experimental animals. J. Anesth. 29: 515–521. doi: 10.1007/s00540-015-1995-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobirumaki-Shimozawa F., Inoue T., Shintani S. A., Oyama K., Terui T., Minamisawa S., Ishiwata S., Fukuda N.2014. Cardiac thin filament regulation and the Frank-Starling mechanism. J. Physiol. Sci. 64: 221–232. doi: 10.1007/s12576-014-0314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A., Anel R., Bunnell E., Habet K., Zanotti S., Marshall S., Neumann A., Ali A., Cheang M., Kavinsky C., Parrillo J. E.2004. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit. Care Med. 32: 691–699. doi: 10.1097/01.CCM.0000114996.68110.C9 [DOI] [PubMed] [Google Scholar]
- 22.Kushimoto S., Endo T., Yamanouchi S., Sakamoto T., Ishikura H., Kitazawa Y., Taira Y., Okuchi K., Tagami T., Watanabe A., Yamaguchi J., Yoshikawa K., Sugita M., Kase Y., Kanemura T., Takahashi H., Kuroki Y., Izumino H., Rinka H., Seo R., Takatori M., Kaneko T., Nakamura T., Irahara T., Saito N.2013. The PiCCO Pulmonary Edema Study Group: Relation ship between extravascular lung water and severity categories of acute respiratory distress syndrome by the Berlin definition. Crit. Care 17: R132–141. doi: 10.1186/cc12811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kushimoto S., Taira Y., Kitazawa Y., Okuchi K., Sakamoto T., Ishikura H., Endo T., Yamanouchi S., Tagami T., Yamaguchi J., Yoshikawa K., Sugita M., Kase Y., Kanemura T., Takahashi H., Kuroki Y., Izumino H., Rinka H., Seo R., Takatori M., Kaneko T., Nakamura T., Irahara T., Saito N., Watanabe A., PiCCO Pulmonary Edema Study Group2012. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit. Care 16: R232. doi: 10.1186/cc11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magder S.2015. Understanding central venous pressure: not a preload index? Curr. Opin. Crit. Care 21: 369–375. doi: 10.1097/MCC.0000000000000238 [DOI] [PubMed] [Google Scholar]
- 25.Mandel D. C.2015. Pulmonary artery catheterization. pp. 1043–1049. In: Small Animal Critical Care Medicine, 2nd ed. (Silverstein, D. G. and Hopper, K. eds.), Elsevier Saunders, St. Louis. [Google Scholar]
- 26.Marjanovic G., Villain C., Juettner E., zur Hausen A., Hoeppner J., Hopt U. T., Drognitz O., Obermaier R.2009. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann. Surg. 249: 181–185. doi: 10.1097/SLA.0b013e31818b73dc [DOI] [PubMed] [Google Scholar]
- 27.Marx G., Cope T., McCrossan L., Swaraj S., Cowan C., Mostafa S. M., Wenstone R., Leuwer M.2004. Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur. J. Anaesthesiol. 21: 132–138. doi: 10.1097/00003643-200402000-00009 [DOI] [PubMed] [Google Scholar]
- 28.Mazzaferro E., Wanger A. E.2001. Hypotension during anesthesia in dogs and cats: recognition, causes, and treatment. Compend. Contin. Educ. Pract. Vet. 23: 728–737. [Google Scholar]
- 29.Mesquida J., Kim H. K., Pinsky M. R.2011. Effect of tidal volume, intrathoracic pressure, and cardiac contractility on variations in pulse pressure, stroke volume, and intrathoracic blood volume. Intensive Care Med. 37: 1672–1679. doi: 10.1007/s00134-011-2304-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michard F.2005. Changes in arterial pressure during mechanical ventilation. Anesthesiology 103: 419–428, quiz 449–5. doi: 10.1097/00000542-200508000-00026 [DOI] [PubMed] [Google Scholar]
- 31.Michard F., Boussat S., Chemla D., Anguel N., Mercat A., Lecarpentier Y., Richard C., Pinsky M. R., Teboul J. L.2000. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am. J. Respir. Crit. Care Med. 162: 134–138. doi: 10.1164/ajrccm.162.1.9903035 [DOI] [PubMed] [Google Scholar]
- 32.Michard F., Chemla D., Richard C., Wysocki M., Pinsky M. R., Lecarpentier Y., Tebol J. L.1999. Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of PEEP. Am. J. Respir. Crit. Care Med. 159: 935–939. doi: 10.1164/ajrccm.159.3.9805077 [DOI] [PubMed] [Google Scholar]
- 33.Monge García M. I., Guijo González P., Gracia Romero M., Gil Cano A., Oscier C., Rhodes A., Grounds R. M., Cecconi M.2015. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 41: 1247–1255. doi: 10.1007/s00134-015-3898-7 [DOI] [PubMed] [Google Scholar]
- 34.Muir W. W., 3rd, Kijtawornrat A., Ueyama Y., Radecki S. V., Hamlin R. L.2011. Effects of intravenous administration of lactated Ringer’s solution on hematologic, serum biochemical, rheological, hemodynamic, and renal measurements in healthy isoflurane-anesthetized dogs. J. Am. Vet. Med. Assoc. 239: 630–637. doi: 10.2460/javma.239.5.630 [DOI] [PubMed] [Google Scholar]
- 35.Muir W. W., Ueyama Y., Pedraza-Toscano A., Vargas-Pinto P., Delrio C. L., George R. S., Youngblood B. L., Hamlin R. L.2014. Arterial blood pressure as a predictor of the response to fluid administration in euvolemic nonhypotensive or hypotensive isoflurane-anesthetized dogs. J. Am. Vet. Med. Assoc. 245: 1021–1027. doi: 10.2460/javma.245.9.1021 [DOI] [PubMed] [Google Scholar]
- 36.Renner J., Broch O., Gruenewald M., Scheewe J., Francksen H., Jung O., Steinfath M., Bein B.2011. Non-invasive prediction of fluid responsiveness in infants using pleth variability index. Anaesthesia 66: 582–589. doi: 10.1111/j.1365-2044.2011.06715.x [DOI] [PubMed] [Google Scholar]
- 37.Reuter D. A., Kirchner A., Felbinger T. W., Weis F. C., Kilger E., Lamm P., Goetz A. E.2003. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit. Care Med. 31: 1399–1404. doi: 10.1097/01.CCM.0000059442.37548.E1 [DOI] [PubMed] [Google Scholar]
- 38.Rex S., Brose S., Metzelder S., Hüneke R., Schälte G., Autschbach R., Rossaint R., Buhre W.2004. Prediction of fluid responsiveness in patients during cardiac surgery. Br. J. Anaesth. 93: 782–788. doi: 10.1093/bja/aeh280 [DOI] [PubMed] [Google Scholar]
- 39.Roy S., Couture P., Qizilbash B., Toupin F., Levesque S., Carrier M., Lambert J., Denault A. Y.2013. Hemodynamic pressure waveform analysis in predicting fluid responsiveness. J. Cardiothorac. Vasc. Anesth. 27: 676–680. doi: 10.1053/j.jvca.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 40.Shih A., Maisenbacher H. W., Bandt C., Ricco C., Bailey J., Rivera J., Estrada A.2011. Assessment of cardiac output measurement in dogs by transpulmonary pulse contour analysis. J. Vet. Emerg. Crit. Care (San Antonio) 21: 321–327. doi: 10.1111/j.1476-4431.2011.00651.x [DOI] [PubMed] [Google Scholar]
- 41.Tagami T., Nakamura T., Kushimoto S., Tosa R., Watanabe A., Kaneko T., Fukushima H., Rinka H., Kudo D., Uzu H., Murai A., Takatori M., Izumino H., Kase Y., Seo R., Takahashi H., Kitazawa Y., Yamaguchi J., Sugita M., Takahashi H., Kuroki Y., Kanemura T., Morisawa K., Saito N., Irahara T., Yokota H.2014. Early-phase changes of extravascular lung water index as a prognostic indicator in acute respiratory distress syndrome patients. Ann. Intensive Care 4: 27–37. doi: 10.1186/s13613-014-0027-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda A., Okumura S., Miyamoto T., Hagio M., Fujinaga T.1995. Comparison of extravascular lung water volume with radiographic findings in dogs with experimentally increased permeability pulmonary edema. J. Vet. Med. Sci. 57: 481–485. doi: 10.1292/jvms.57.481 [DOI] [PubMed] [Google Scholar]
- 43.Valverde A., Gianotti G., Rioja-Garcia E., Hathway A.2012. Effects of high-volume, rapid-fluid therapy on cardiovascular function and hematological values during isoflurane-induced hypotension in healthy dogs. Can. J. Vet. Res. 76: 99–108. [PMC free article] [PubMed] [Google Scholar]
- 44.Wiesenack C., Fiegl C., Keyser A., Prasser C., Keyl C.2005. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur. J. Anaesthesiol. 22: 658–665. doi: 10.1017/S0265021505001092 [DOI] [PubMed] [Google Scholar]
- 45.Yamashita K., Iwasaki Y., Umar M. A., Itami T.2009. Effect of age on minimum alveolar concentration (MAC) of sevoflurane in dogs. J. Vet. Med. Sci. 71: 1509–1512. doi: 10.1292/jvms.001509 [DOI] [PubMed] [Google Scholar]
- 46.Zhao F., Wang P., Pei S., Mi W., Fu Q.2015. Automated stroke volume and pulse pressure variations predict fluid responsiveness in mechanically ventilated patients with obstructive jaundice. Int. J. Clin. Exp. Med. 8: 20751–20759. [PMC free article] [PubMed] [Google Scholar]
- 47.Zöllei E., Bertalan V., Németh A., Csábi P., László I., Kaszaki J., Rudas L.2013. Non-invasive detection of hypovolemia or fluid responsiveness in spontaneously breathing subjects. BMC Anesthesiol. 13: 40–47. doi: 10.1186/1471-2253-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]