Abstract
Thirty-two muskrats (Ondatra zibethicus) were captured for surveillance of avian influenza virus in wild waterfowl and mammals near Lake Chany, Western Siberia, Russia. A/muskrat/Russia/63/2014 (H2N2) was isolated from an apparently healthy muskrat using chicken embryos. Based on phylogenetic analysis, the hemagglutinin and neuraminidase genes of this isolate were classified into the Eurasian avian-like influenza virus clade and closely related to low pathogenic avian influenza viruses (LPAIVs) isolated from wild water birds in Italy and Sweden, respectively. Other internal genes were also closely related to LPAIVs isolated from Eurasian wild water birds. Results suggest that interspecies transmission of LPAIVs from wild water birds to semiaquatic mammals occurs, facilitating the spread and evolution of LPAIVs in wetland areas of Western Siberia.
Keywords: H2N2, influenza virus, muskrat, phylogenetic analysis, Siberia
Most avian influenza viruses (AIVs) have been detected in wild aquatic birds and poultry, but these viruses have also been isolated from several mammalian species, such as swine, horses, dogs and cats, and even from some rare mammalian species (dolphins, whales, seals, bats, camels, minks, rodents, etc.) [1, 23, 30, 31, 34, 35]. Antibodies to AIVs have also been detected in the sera of raccoons [7]. This suggests that AIVs can cross the interspecies barrier, efficiently replicating in “novel” hosts and occasionally evolving into pathogenic virus variants that are potentially dangerous for mammals. It has been found that rodents can be naturally infected with the AIVs of H5N1 and H9N2 subtypes. This fact has extended the range of known terrestrial mammals hosting AIV [36, 37]. The pathways of interspecies transmission of AIVs between wild birds and wild mammals within their natural habitat have been poorly studied [37]. It is currently unknown how new mammalian hosts can spread viruses to other species, such as terrestrial mammals, poultry and eventually humans [22, 24]. Muskrat (Ondatra zibethicus), the only species in genus Ondatra and tribe Ondatrini, is a semiaquatic rodent found in different parts of the world. It lives in wetlands in a wide range of climates and habitats and has a significant influence on the ecology of wetlands [13]. Muskrats are in a very close contact with wild aquatic and semiaquatic birds and pose a potential risk of AIV interspecies transmission. Moreover, AIV isolation from muskrats has been previously reported [1, 15]. This specie is a commercially significant source of fur; therefore, frequent contact with humans and potential risk of human exposure to infected animals make them an interesting subject of study.
Wild waterfowl, predominantly Anseriformes and Charadriiformes, are a natural reservoir of AIVs. To date, 16 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes have been isolated from wild water birds [11]. Rich wetland area is a perfect habitat for wild waterfowl and semiaquatic animals. Lake Chany, Russia, is the largest lake in the massive West Siberian forest-steppe that plays a key role in the migration and nesting of wild ducks and other birds from different geographical regions [33]. A large number of residential backyards adjacent to wetlands providing habitat for semiaquatic mammals make this region a perfect location for the spread and evolution of AIVs [3]. During this study conducted in August–September 2014, thirty-two muskrats (Ondatra zibethicus) were captured for our investigation of AIV at the Lake Chany region (Western Siberia, Russia). Here, we report an isolation of H2N2 influenza virus from a muskrat and the biological properties of this isolate.
Of the thirty-two captured muskrats during our study, no animals presented any clinical signs of disease. Nasopharyngeal swabs were collected and tested for AIV with the help of real-time PCR using previously published primers and probes that targeted the matrix (M) gene [26, 29]. Using this methodology, one sample tested positive for AIV. This sample was inoculated into 10-day-old embryonated chicken eggs. Allantoic fluid was collected 3 days post-infection (dpi) and frozen at −80°C for further use. The virus identified as A/muskrat/Russia/63/2014 (H2N2) was passed to 10-day-old chicken embryos and Madin–Darby canine kidney (MDCK) cells to determine the viral titer as previously described [8]. The full-length virus genome of the isolate was sequenced using MiSeq (Illumina, San Diego, CA, U.S.A.) according to the manufacturer’s instructions. Phylogenetic analysis of all viral genes was made by the maximum-likelihood method with a bootstrap test (1,000 replications) using MEGA v6.0 software.
A/muskrat/Russia/63/2014 (H2N2) from the allantoic cavities was 64 HA units (HAU) per 50 µl and 107.0 50% egg infectious dose (EID50) per ml. The virus did not cause the death of the chicken embryos. We also evaluated the virus replication in MDCK cells. Most influenza A viruses can replicate well on MDCK cells in the presence of trypsin. However, some avian strains poorly replicate and do not form plaques on MDCK cells without prior adaptation [32]. The virus could replicate with high titers of 32 HAU and 108.0 50% tissue culture infectious dose (TCID50) per ml at 4 dpi.
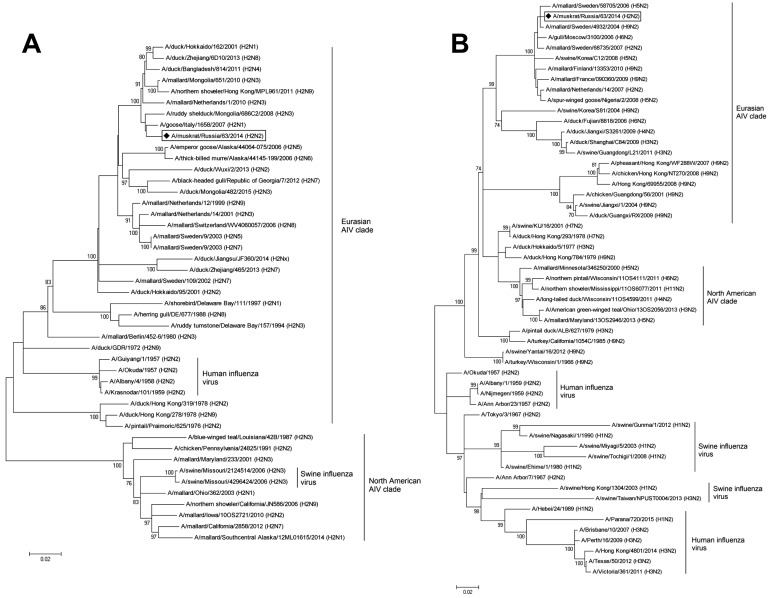
Phylogenetic and BLAST search analyses of HA and NA genes showed that the genes of A/muskrat/Russia/63/2014 (H2N2) were related to the corresponding genes of low pathogenic AIVs (LPAIVs) A/goose/Italy/1658/2007 (H2N1) and A/mallard/Sweden/4932/2004 (H9N2), respectively (Fig. 1 and Table 1). The internal genes of this H2N2 virus were also related to LPAIVs isolated in Eurasian countries (Mongolia, Sweden, China, Germany and India) (Table 1). According to the phylogenetic analysis of HA gene, A/muskrat/Russia/63/2014 (H2N2) was similar to the viruses isolated from birds in the Eurasian clade (Fig. 1A). In addition, A/swine/Missouri/2124514/2006 (H2N3) and A/swine/Missouri/4296424/2006 (H2N3) were similar to those from birds in the North American clade. Phylogenetic tree of N2 gene also showed that influenza viruses previously isolated from mammalian species and birds had similarity in NA gene (Fig. 1B). The A/swine/Korea/C12/2008 (H5N2), as well as A/muskrat/Russia/63/2014 (H2N2), was phylogenetically similar to the AIVs isolated previously in Europe. In addition, A/swine/Guangdong/L21/2011 (H3N2), A/swine/Jiangxi/1/2004 (H9N2) and A/Hong Kong/69955/2008 (H9N2) were phylogenetically close to the AIVs circulating in China.
Fig. 1.
Phylogenetic trees of the H2 HA (A) and N2 NA (B) genes. Full length sequences of the H2 HA and N2 NA genes were phylogenetically analyzed by the maximum-likelihood (ML) method using MEGA v6.0 (http://www.megasoftware.net/). Horizontal distances are proportional to the minimum number of nucleotide differences required to join nodes and sequences. Numbers at each node indicate the probability of confidence level in a bootstrap analysis with 1,000 replications. A/muskrat/Russia/63/2014 (H2N2), which was isolated from a wild muskrat in present study, is highlighted using bold lozenge.
Table 1. Genetic identities of A/muskrat/Russia/63/2014 (H2N2) to other avian influenza viruses.
| Gene segment | Accession No.a) | Virus with the highest identityb) | Identity (%) |
|---|---|---|---|
| PB2 | KR052701.1 | A/red-crested pochard/Mongolia/463V/2009 (H3N1) | 98.9 |
| PB1 | KR052702.1 | A/mallard/Sweden/16/2002 (H12N9) | 99.1 |
| PA | KR052703.1 | A/mallard/Jiangxi/12147/2005 (H6N2) | 98.5 |
| HA | KR052704.1 | A/goose/Italy/1658/2007 (H2N1) | 98.1 |
| NP | KR052705.1 | A/mallard/Sweden/80166/2008 (H4N6) | 98.1 |
| NA | KR052706.1 | A/mallard/Sweden/4932/2004 (H9N2) | 99.4 |
| M | KR052707.1 | A/wild duck/Germany/WV2555/2006 (H3N2) | 99.3 |
| NS | KR052708.1 | A/aquatic bird/India/NIV-17095/2007 (H11N1) | 99.2 |
a) DDBJ/EMBL/GenBank accession numbers submitted in this study. b) Influenza viruses with the highest degree of nucleotide sequence identity based on the GISAID nucleotide BLAST search analysis.
To discuss the pathogenicity of A/muskrat/Russia/63/2014 (H2N2), amino acid (aa) analysis of the viral proteins was performed. We found that A/muskrat/Russia/63/2014 (H2N2), as well as similar avian H2 viruses, possess as QGG-motif in receptor-biding site (226–228) of the HA protein (H3-numbering). This motif is known to be predominant for binding of HA molecule with α 2, 3-NeuAcGal receptors expressed on avian cells [21]. The aa analysis of PB2 showed that A/muskrat/Russia/63/2014 (H2N2) possessed avian-type 627E and 701D, whereas most mammalian isolates had 627K and 701N in the PB2 protein [25]. In terms of PB1-F2, we found 66N whereas 66S has been reported as a virulence marker of H5N1 highly pathogenic AIVs [27]. On the other hand, several amino acids conferring higher pathogenicity in rodents were detected in A/muskrat/Russia/63/2014 (H2N2). We found 89V, 309D, 339K, 477G, 495V and 676T in PB2, demonstrating potentially increased polymerase activity in mice [14]. In addition, full-length PA-X protein, a virulence marker of H1N1, H5N1 and H9N2 viruses with severe inflammatory responses in mice [5, 6], was also identified in A/muskrat/Russia/63/2014 (H2N2). Moreover, some potential pathogenic markers were found in M1 (30D and 215A) [4] and NS1 (103F and 106M) [9, 10, 28] in this isolate. Taken together, it is hardly to summarize the pathogenicity of A/muskrat/Russia/63/2014 (H2N2) based on the aa analysis, because the pathogenicity of AIVs in rodents may be associated with a certain combination of several factors and depend on the fitness of the virus replication in each host. A further study on the ecology and pathogenicity of AIVs including H2 subtype in wild mammals is essential in Siberia, since the AIVs of H4N6 and H13N6 subtypes have already been identified from muskrats at the Lake Baikal, Russia in 2000 [2, 15, 16].
Along with H1 and H3, influenza viruses of H2 subtype are important concerning humans. H1 and H3 viruses are currently endemic in humans; therefore, attention has shifted to a possible recurrence of the H2 subtype due to its absence in humans for more than 50 years. The influenza H2N2 virus is known, because of the 1957 Asian flu pandemic. It originated from the wild bird influenza H2N2 virus and the human H1N1 virus, but the exact origin of the previous pandemic H2N2 virus observed in 1958 is unknown. There is little evidence that it adapted to the human population by passing through a mammalian intermediate host [12]. Since 1968, influenza H2N2 viruses have not been detected in humans; therefore, most individuals younger than 50 years lack humoral immunity to the H2 antigen [19]. Although there are no influenza H2N2 viruses in the human population at the moment, H2 influenza viruses are often isolated from wild birds and poultry [18]. The H2 influenza viruses in wild bird populations have been detected in combination with all nine NA subtypes. The AIV of the H2N3 subtype was also isolated from swine and the environment [1, 17, 20]. Therefore, it is of great interest to conduct studies on the interspecies transmission of H2 influenza viruses between avian and mammalians, because they have a high degree of genetic and antigenic similarity to the ancestral viruses that contributed genes to the 1957 H2N2 pandemic virus.
A great risk for interspecies transmission exists in the habitats of wild birds. Surveillance studies for AIVs in mammalian populations that come in close contact with wild waterfowl are crucial for monitoring virus evolution, its adaptation to novel species, and the development of appropriate diagnostic and treatment methods. The isolate from this study currently presents no evidence of virulence for humans or poultry; thus, it may be a muskrat-adapted virus or a transient virus in the nasal cavity of muskrats. Thus, in our opinion, muskrat could serve as a new reservoir of AIVs, posing a potential risk to other animals in the food chain, including humans.
CONFLICT OF INTEREST. Authors have no conflict of interest to declare.
Acknowledgments
This study was supported by the Ministry of Education and Science of the Russian Federation (Project # RFMEFI61315X0045) and Russian Foundation for Basic Research (17-04-01919).
REFERENCES
- 1.Bao Y., Bolotov P., Dernovoy D., Kiryutin B., Zaslavsky L., Tatusova T., Ostell J., Lipman D.2008. The influenza virus resource at the National Center for Biotechnology Information. J. Virol. 82: 596–601. doi: 10.1128/JVI.02005-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaporgina E., Danchinova G., Khasnatinov M., Pyzhyanov S., Arbatskaya E., Shulun S., Tupitsyna I., Gorin M.2007. The research results of influenza A virus circulation among birds in the South of Eastern Siberia. Bulletin ESSC SB RAMS. S3: 184–186. [Google Scholar]
- 3.De Marco M. A., Delogu M., Sivay M., Sharshov K., Yurlov A., Cotti C., Shestopalov A.2014. Virological evaluation of avian influenza virus persistence in natural and anthropic ecosystems of Western Siberia (Novosibirsk Region, summer 2012). PLOS ONE 9: e100859. doi: 10.1371/journal.pone.0100859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan S., Deng G., Song J., Tian G., Suo Y., Jiang Y., Guan Y., Bu Z., Kawaoka Y., Chen H.2009. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 384: 28–32. doi: 10.1016/j.virol.2008.11.044 [DOI] [PubMed] [Google Scholar]
- 5.Gao H., Xu G., Sun Y., Qi L., Wang J., Kong W., Sun H., Pu J., Chang K. C., Liu J.2015. PA-X is a virulence factor in avian H9N2 influenza virus. J. Gen. Virol. 96: 2587–2594. doi: 10.1099/jgv.0.000232 [DOI] [PubMed] [Google Scholar]
- 6.Gao H., Sun H., Hu J., Qi L., Wang J., Xiong X., Wang Y., He Q., Lin Y., Kong W., Seng L. G., Pu J., Chang K. C., Liu X., Liu J., Sun Y.2015. Twenty amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. J. Gen. Virol. 96: 2036–2049. doi: 10.1099/vir.0.000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horimoto T., Maeda K., Murakami S., Kiso M., Iwatsuki-Horimoto K., Sashika M., Ito T., Suzuki K., Yokoyama M., Kawaoka Y.2011. Highly pathogenic avian influenza virus infection in feral raccoons, Japan. Emerg. Infect. Dis. 17: 714–717. doi: 10.3201/eid1704.101604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilyicheva T., Susloparov I., Durymanov A., Romanovskaya A., Sharshov K., Kurskaya O., Ignashkina M., Shestopalov A.2011. Influenza A/H1N1pdm virus in Russian Asia in 2009-2010. Infect. Genet. Evol. 11: 2107–2112. doi: 10.1016/j.meegid.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Jackson D., Hossain M. J., Hickman D., Perez D. R., Lamb R. A.2008. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proc. Natl. Acad. Sci. U.S.A. 105: 4381–4386. doi: 10.1073/pnas.0800482105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao P., Tian G., Li Y., Deng G., Jiang Y., Liu C., Liu W., Bu Z., Kawaoka Y., Chen H.2008. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 82: 1146–1154. doi: 10.1128/JVI.01698-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones J. C., Baranovich T., Marathe B. M., Danner A. F., Seiler J. P., Franks J., Govorkova E. A., Krauss S., Webster R. G.2014. Risk assessment of H2N2 influenza viruses from the avian reservoir. J. Virol. 88: 1175–1188. doi: 10.1128/JVI.02526-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawaoka Y., Krauss S., Webster R. G.1989. Avian-to-human transmission of the PB1 gene of influenza A viruses in the 1957 and 1968 pandemics. J. Virol. 63: 4603–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keddy P.2010. Wetland Ecology: Principles and Conservation, 2nd ed., Cambridge University Press, Cambridge. [Google Scholar]
- 14.Li J., Ishaq M., Prudence M., Xi X., Hu T., Liu Q., Guo D.2009. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 144: 123–129. doi: 10.1016/j.virusres.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 15.L’vov D., Gorin O., Yamnikova S., Zlobin V., lvov, N., Khasnatinov, M., Fedyakina, I., Chumakov V., Nepoklonov, E. and Aliper, T. 2001. Isolation of Influenza A viruses from wild birds and muskrat in Western Part of Eastern-Asian migration flyway.Voprosi Virusologii. N4: 35–39. [Google Scholar]
- 16.L’vov D., Yamnikova S., Fedyakina I.2004. Evolution of H4, H5 influenza viruses in natural ecosystems in Northern Eurasia (2000–2002). pp. 169–173. In: Proceedings of the International Conference on Options for the Control of Influenza V., Okinawa.
- 17.Ma W., Vincent A. L., Gramer M. R., Brockwell C. B., Lager K. M., Janke B. H., Gauger P. C., Patnayak D. P., Webby R. J., Richt J. A.2007. Identification of H2N3 influenza A viruses from swine in the United States. Proc. Natl. Acad. Sci. U.S.A. 104: 20949–20954. doi: 10.1073/pnas.0710286104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makarova N. V., Kaverin N. V., Krauss S., Senne D., Webster R. G.1999. Transmission of Eurasian avian H2 influenza virus to shorebirds in North America. J. Gen. Virol. 80: 3167–3171. doi: 10.1099/0022-1317-80-12-3167 [DOI] [PubMed] [Google Scholar]
- 19.Nabel G. J., Wei C. J., Ledgerwood J. E.2011. Vaccinate for the next H2N2 pandemic now. Nature 471: 157–158. doi: 10.1038/471157a [DOI] [PubMed] [Google Scholar]
- 20.Pappas C., Yang H., Carney P. J., Pearce M. B., Katz J. M., Stevens J., Tumpey T. M.2015. Assessment of transmission, pathogenesis and adaptation of H2 subtype influenza viruses in ferrets. Virology 477: 61–71. doi: 10.1016/j.virol.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappas C., Viswanathan K., Chandrasekaran A., Raman R., Katz J. M., Sasisekharan R., Tumpey T. M.2010. Receptor specificity and transmission of H2N2 subtype viruses isolated from the pandemic of 1957. PLoS ONE 5: e11158. doi: 10.1371/journal.pone.0011158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reperant L. A., Kuiken T., Osterhaus A. D.2012. Adaptive pathways of zoonotic influenza viruses: from exposure to establishment in humans. Vaccine 30: 4419–4434. doi: 10.1016/j.vaccine.2012.04.049 [DOI] [PubMed] [Google Scholar]
- 23.Romero Tejeda A., Aiello R., Salomoni A., Berton V., Vascellari M., Cattoli G.2015. Susceptibility to and transmission of H5N1 and H7N1 highly pathogenic avian influenza viruses in bank voles (Myodes glareolus). Vet. Res. (Faisalabad) 46: 51. doi: 10.1186/s13567-015-0184-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Runstadler J., Hill N., Hussein I. T., Puryear W., Keogh M.2013. Connecting the study of wild influenza with the potential for pandemic disease. Infect. Genet. Evol. 17: 162–187. doi: 10.1016/j.meegid.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya K., Makino A., Ozawa M., Kim J. H., Sakai-Tagawa Y., Ito M., Le Q. M., Kawaoka Y.2009. Ostrich involvement in the selection of H5N1 influenza virus possessing mammalian-type amino acids in the PB2 protein. J. Virol. 83: 13015–13018. doi: 10.1128/JVI.01714-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivay M. V., Sayfutdinova S. G., Sharshov K. A., Alekseev A. Y., Yurlov A. K., Runstadler J., Shestopalov A. M.2012. Surveillance of influenza A virus in wild birds in the Asian portion of Russia in 2008. Avian Dis. 56: 456–463. doi: 10.1637/9868-080111-Reg.1 [DOI] [PubMed] [Google Scholar]
- 27.Smith A. M., McCullers J. A.2013. Molecular signatures of virulence in the PB1-F2 proteins of H5N1 influenza viruses. Virus Res. 178: 146–150. doi: 10.1016/j.virusres.2013.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spesock A., Malur M., Hossain M. J., Chen L. M., Njaa B. L., Davis C. T., Lipatov A. S., York I. A., Krug R. M., Donis R. O.2011. The virulence of 1997 H5N1 influenza viruses in the mouse model is increased by correcting a defect in their NS1 proteins. J. Virol. 85: 7048–7058. doi: 10.1128/JVI.00417-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swayne D. E., Glisson J. R., Jackwood M. W., Pearson J. E., Reed W. M.2006. pp. 74–80, 150–163, 235–240. In: Laboratory Manual for the Isolation and Identification of Avian Pathogens, 4th ed., Am. Assoc. Avian Pathol., International Book Distributing Co., Lucknow. [Google Scholar]
- 30.Tong S., Li Y., Rivailler P., Conrardy C., Castillo D. A., Chen L. M., Recuenco S., Ellison J. A., Davis C. T., York I. A., Turmelle A. S., Moran D., Rogers S., Shi M., Tao Y., Weil M. R., Tang K., Rowe L. A., Sammons S., Xu X., Frace M., Lindblade K. A., Cox N. J., Anderson L. J., Rupprecht C. E., Donis R. O.2012. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. U.S.A. 109: 4269–4274. doi: 10.1073/pnas.1116200109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong S., Zhu X., Li Y., Shi M., Zhang J., Bourgeois M., Yang H., Chen X., Recuenco S., Gomez J., Chen L. M., Johnson A., Tao Y., Dreyfus C., Yu W., McBride R., Carney P. J., Gilbert A. T., Chang J., Guo Z., Davis C. T., Paulson J. C., Stevens J., Rupprecht C. E., Holmes E. C., Wilson I. A., Donis R. O.2013. New world bats harbor diverse influenza A viruses. PLoS Pathog. 9: e1003657. doi: 10.1371/journal.ppat.1003657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda Y., Isoda N., Sakoda Y., Kida H.2009. Factors responsible for plaque formation of A/duck/Siberia/272/1998 (H13N6) influenza virus on MDCK cells. Virus Res. 140: 194–198. doi: 10.1016/j.virusres.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 33.Veen J., Yurlov A., Delany S., Mihantiev A., Selivanova M., Boere G. 2005. An Atlas of Movements of Southwest Siberian Waterbirds. Wetlands International, Wageningen. [Google Scholar]
- 34.Webster R. G., Bean W. J., Gorman O. T., Chambers T. M., Kawaoka Y.1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56: 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon K. J., Schwartz K., Sun D., Zhang J., Hildebrandt H.2012. Naturally occurring Influenza A virus subtype H1N2 infection in a Midwest United States mink (Mustela vison) ranch. J. Vet. Diagn. Invest. 24: 388–391. doi: 10.1177/1040638711428349 [DOI] [PubMed] [Google Scholar]
- 36.Yu Z., Cheng K., Sun W., Xin Y., Cai J., Ma R., Zhao Q., Li L., Huang J., Sang X., Li X., Zhang K., Wang T., Qin C., Qian J., Gao Y., Xia X.2014. Lowly pathogenic avian influenza (H9N2) infection in Plateau pika (Ochotona curzoniae), Qinghai Lake, China. Vet. Microbiol. 173: 132–135. doi: 10.1016/j.vetmic.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Sun W., Wang J., Guo J., Yin W., Wu N., Li L., Yan Y., Liao M., Huang Y., Luo K., Jiang X., Chen H.2009. Characterization of the H5N1 highly pathogenic avian influenza virus derived from wild pikas in China. J. Virol. 83: 8957–8964. doi: 10.1128/JVI.00793-09 [DOI] [PMC free article] [PubMed] [Google Scholar]