Key Points
A decline in T-cell immunity is one of the most consistent and most profound deficiencies of the elderly. Therapeutic correction of this decline often restores immune responsiveness and immune defence.
T-cell immune decline in the elderly has at least two underpinnings: a drop in the responsiveness of naive T cells to stimulation (cell-autonomous defects) and a reduction in naive T-cell numbers and diversity that leads to a dominant memory T-cell pool (T-cell population imbalance).
This article discusses two key causes of age-related T-cell population imbalance: homeostatic cycling or proliferative expansion in the peripheral T-cell pool, and latent persistent infections, which repeatedly stimulate the T-cell pool over the lifetime of the individual.
The reduction in production of naive T cells by the thymus forces the ageing organism to rely on compensatory homeostatic mechanisms to maintain the balance between naive and memory T-cell pools. Although this may be initially successful, recent evidence suggests that late in life these mechanisms exhaust their usefulness and actually contribute to a further demise of the remaining naive T cells.
Latent persistent infections, particularly with herpesviruses, lead to life-long periodic restimulation of the immune system, here, evidence is presented for the role of viral reactivation in this restimulation using a mouse model of herpesvirus infection and ageing.
Relative roles and the interplay between the homeostatic and viral factors are discussed, with the former having a surprisingly prominent role. Finally, modes of immune rejuvenation and anti-ageing intervention are debated in light of these advances in our knowledge.
A decline in T-cell immunity is a major cause of morbidity and mortality from infectious diseases in the elderly. Janko Nikolich-Žugich weighs up the relative roles of and the interplay between homeostatic factors and persistent viruses in immune senescence.
Abstract
A diverse and well-balanced repertoire of T cells is thought to be crucial for the efficacious defence against infection with new or re-emerging pathogens throughout life. In the last third of the mammalian lifespan, the maintenance of a balanced T-cell repertoire becomes highly challenging because of the changes in T-cell production and consumption. In this Review, I question whether latent persistent pathogens might be key factors that drive this imbalance and whether they determine the extent of age-associated immune deficiency.
Main
Ageing of the immune system involves a complex set of changes that are collectively termed immune senescence (reviewed in Refs 1, 2, 3). These changes affect many components of the innate and adaptive immune system, often resulting in a common, but variable and still incompletely understood, state of immune deficiency. Key immunological manifestations of immune senescence include poor responsiveness to new or evolving pathogens and reduced efficacy of vaccination-induced protection against infection4,5,6. By contrast, established memory immune responses to previously encountered pathogens are much less affected1,7,8. Reduced signalling, proliferation and cytokine secretion in response to antigen9,10 result in impaired generation of effector B- and T-cell responses11,12,13. Defects that are not as well understood occur in antigen processing and presentation14 and the cytokine environment, and these have the potential to further affect both the ongoing immune response and the maintenance of lymphoid populations10,15. Clinically, these defects correlate with increased morbidity and mortality of elderly individuals from infectious diseases. Therefore, both the established pathogens (such as influenza virus, pneumococcus and varicella-zoster virus (VZV)) and the newly emerging pathogens (such as West Nile virus (WNV) and severe acute respiratory syndrome (SARS)-associated coronavirus) take a disproportionate and heavy toll in sick days and deaths in the elderly compared with younger individuals4,16.
Ageing of the immune system encompasses two types of change — primary alterations, which are a direct consequence of ageing, and secondary alterations, which represent a reaction or response to these primary changes, and which, by themselves, can also be affected by the process of ageing. Each of these types of alteration can manifest on at least two levels — the single-cell level and the tissue or cell-population level. Primary alterations encompass cellular ageing, which to a greater or lesser extent has an effect on all cells of an organism. It remains unclear whether there is one crucial type of age-related change that results in a senescent cellular phenotype, or whether, perhaps more likely, numerous types of change jointly lead to such a phenotype17,18,19. Regardless, cellular ageing can be defined as the accumulation of unrepaired and uncorrected mutations and/or alterations in macromolecules that eventually result in altered cellular responses and functions. The primary alterations result from several partially overlapping processes that include, but are not limited to, DNA damage, oxidative stress (such as peroxidation of lipids, proteins and sugars) and reduced macromolecular biosynthesis, degradation and turnover20,21. The accumulation of these primary alterations occurs because the rate of repair is failing to keep up with the rate of damage, either because the former is increased, the latter is slower or both. A set of primary alterations in cellular function could stem directly from these changes. More often, however, in response to these primary changes, homeostatic cellular and tissue mechanisms initiate secondary, compensatory changes, and ageing phenotypes present themselves as the sum of these primary and secondary changes. For example, in young cells the non-homologous end-joining DNA repair pathway would normally be capable of fully repairing DNA damage, but in ageing cells the repair might not be complete. In response to this incomplete DNA repair the cells compensate by activating p53 and this leads to either their apoptosis and removal or to their proliferative arrest and senescent phenotype. Senescence, at least in fibroblasts, manifests as altered metabolic and secretory properties of the cell22, and therefore in this situation the end result of the primary and secondary compensatory processeses is an impaired and/or altered function of the affected cell(s). One should keep in mind, however, that it remains unclear whether cellular replicative senescence (that is, the inability of a cell to divide after a certain number of divisions) actually predicts, or even correlates to, the ageing of the organism18.
Changes at the level of tissues or cell populations stem from the primary changes in cell production, renewal and death, as well as from the secondary changes in population dynamics; these changes are particularly pronounced in lymphocyte populations. Primary changes in T-cell populations stem from thymic involution and the consequent marked reduction in T-cell production. Secondary changes then ensue from a compensatory homeostatic T-cell expansion that attempts to maintain the size of the naive T-cell compartment23,24. Another set of secondary changes are due to the nature of the T-cell response to pathogens, with repeated cycles of intense proliferation and timely apoptosis, and the maintenance of lymphocytes that enter the memory pool. As discussed later, pathogens that lead to acute or persistent infection differ in how they affect T-cell expansion, contraction and memory formation. Unlike viruses that induce acute infection, pathogens that induce latent persistent infections (including the herpesviruses cytomegalovirus (CMV), Epstein–Barr virus (EBV), VZV and herpes simplex virus (HSV)) and pathogens that induce chronic persistent infections (including hepatitis C virus (HCV) and HIV) (Box 1) establish an evolving equilibrium with the host and repeatedly stimulate the lymphocyte pool.
The long-term, repeated stimulation of the immune system (particularly of T cells) by viruses that induce latent persistent infections is thought to have an important role in immune senescence25. This Review discusses the recent evidence indicating that the individual effects of and the interplay between homeostatic forces and persistent viruses may be the key factors driving T-cell ageing in humans.
T-cell differentiation and homeostasis
The topic of T-cell differentiation and T-cell homeostasis has been discussed in recent reviews (see Ref. 26 for example), and is therefore introduced only briefly here. Most αβ T-cell receptor (TCRαβ)+ T cells differentiate in the thymus from bone-marrow precursors and are then exported to secondary lymphoid organs. The rate of production of these recent thymic emigrants (RTEs) is proportional to the total thymic mass, and amounts to ∼1% of the total thymic cell content per day27,28. In mice, naive CD8+ T cells and many of the naive CD4+ T cells are selected and maintained by trophic signals that result from the interaction of their TCR with the self-peptide–MHC complexes that are expressed by other thymic cells (Fig. 1a). Naive T-cell survival also requires the common cytokine-receptor γ-chain (γc) family member interleukin-7 (IL-7; reviewed in Ref. 23) (Fig. 1a). The maintenance and survival of naive T cells is countered by the regular replacement of naive T cells by RTEs in a random manner to ensure diversity of the naive T-cell pool29; T-cell diversity is generally thought to ensure optimal responses to new, previously unencountered, infectious challenges. Under normal conditions in vivo, naive T cells undergo a very low level of spontaneous (or homeostatic) proliferation (Fig. 1a). However, homeostatic proliferation is greatly increased in lymphopaenic conditions (such as those induced by irradiation, chemotherapy or HIV infection); under these circumstances T cells sense the excess of 'space' (most likely by responding to an excess of IL-7 and IL-15) and try to fill it by antigen-independent homeostatic proliferative expansion23,24. It is unclear whether signals from peptide–MHC complexes and γc cytokines are provided by a particular cell type (such as dendritic cells or other stromal cells) (Fig. 1b) and/or whether these cells reside in a confined anatomical location, which would ensure competition for survival factors among T cells and prevent the accumulation of too many T cells. IL-2 has been shown to oppose some of the actions of IL-7 and IL-15 and its main homeostatic role is probably in the production and expansion of regulatory T cells30.
Figure 1. T-cell homeostasis.
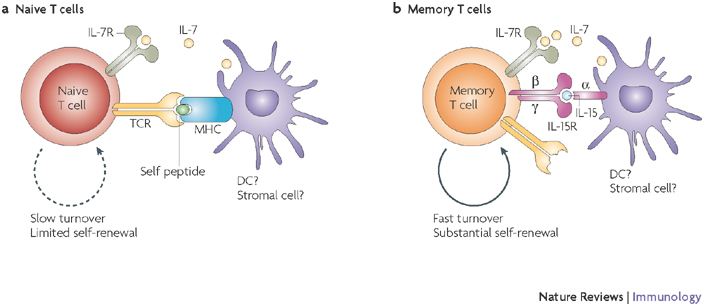
Key factors important for survival and maintenance of naive (a) and memory (b) T cells are summarized. Interleukin-7 (IL-7; for naive T cells and to a lesser extent for memory T cells), IL-15 (for memory T cells) and self-peptide–MHC complexes (for naive T cells) are known to be the key factors for the maintenance of different T-cell subsets. The cellular source of these signals is not defined, but could regulate the overall T-cell numbers by restricted physical distribution and/or numbers. The rate and the extent of self-renewal are different for naive (low) and memory (high) T cells. Question marks indicate speculative or insufficiently documented cellular or molecular interactions. R, receptor; TCR, T-cell receptor.
Following encounter with antigen, naive T cells first generate effector T cells (CD69hiCD25hiCCR7lowCD62LlowCD127lowCD27low) and then memory T cells, which can be broadly divided into central memory T cells (CD69−CD25−CCR7hiCD62LhiCD127hiCD27hi) and effector memory T cells (CD69−CD25−CCR7hiCD62LlowCD127lowCD27low)31. Memory T cells are less dependent on contact with specific peptide–MHC complexes for survival32 (Fig. 1b); they cycle and self-renew in vivo three- to fourfold faster than naive T cells and are capable of vigorous proliferation in lymphopaenic conditions23. In mice, homeostatic proliferative expansion, as well as homeostatic memory T-cell maintenance, depends on IL-15 or, in its absence, on IL-7 (Ref. 33) (Fig. 1b). Therefore, in the face of repeated pathogen challenges, homeostatic forces must balance antigen-driven expansion of naive antigen-specific T cells, generation of the effector T-cell population, its subsequent contraction and the recruitment of the surviving cells into the memory T-cell compartment. This simultaneously preserves T-cell repertoire diversity to combat new pathogens and enables a vigorous recall response to reinfection. In young and adult mice of the same strain, the numbers and diversity of peripheral T cells are remarkably similar23,34,35, suggesting that clonal expansion and contraction must be tightly regulated in adulthood (reviewed in Ref. 35). However, this balance probably depends on the stable naive T-cell pool, which in turn requires the production of new T cells by the thymus28,36 and also the absence of manifest pathogen challenge. So, T-cell homeostasis is regulated by the availability of, and the response of T cells to, environmental trophic and survival signals. Although we have precise information on the role of cytokines (primarily IL-7, IL-15 and IL-2) and self-peptide–MHC complexes, it is less clear how metabolic signals37 and possibly other as-yet-unknown signals are involved.
T-cell senscence and T-cell population imbalance
Although immune senescence affects many aspects of innate and adaptive immunity, defects in T-cell immunity are the best documented and the most marked (reviewed in Refs 1, 2, 38). Indeed, in elderly humans and animals, the restoration of T-cell population balance and numbers alone often correlates with an improvement in the response to antigen or pathogen challenge39,40,41. As mentioned, age-related changes in T-cell immunity can occur at a single-cell level and at a cell-population level. Cell-autonomous defects involve disrupted signalling through the TCR and co-stimulatory receptors (Fig. 2a); these changes have been described in detail elsewhere42,43 and are therefore not discussed further here. Instead, I focus on the set of age-associated changes that result in disruption of T-cell population balance (Fig. 2b) — an area that is now beginning to be understood. This disruption is the result of four partially interrelated events: involution of the thymus; decline of naive T-cell numbers; reduction in T-cell repertoire diversity; and accumulation of memory T cells that are specific for persisting pathogens (also known as memory inflation). All of these phenomena have the potential to reduce the reserve of T cells needed for protection against new pathogens.
Figure 2. Cellular and population-based changes in T-cell senescence.
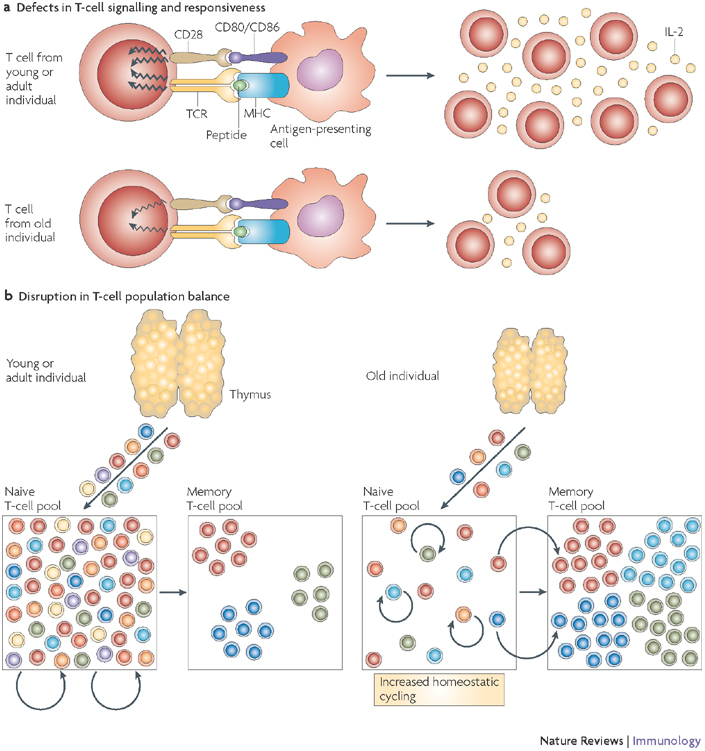
a | A decline in the T-cell response to stimulation through the T-cell receptor (TCR) and co-stimulatory pathways occurs frequently with ageing and is typically shown in ex vivo experiments using agonist T-cell ligands in the absence of antigen-presenting cells, thus showing a cell-autonomous T-cell defect. These proximal signalling defects are followed by a marked decrease in interleukin-2 (IL-2) secretion and T-cell proliferation. b | T-cell population changes result from decreased T-cell production from an involuted thymus, which critically contributes to the depletion of the naive T-cell pool. The naive T-cell pool then undergoes increased homeostatic cycling. This maintains the naive compartment for a while, but as the increased cycling (approximately once every month in mice) turns into homeostatic proliferation (four or more divisions per month, at or above the level seen in the T-cell memory pool)59,60,126 phenotypic conversion of naive T cells into memory phenotype cells has the potential to further deplete the naive T-cell compartment. This, and the conditions favourable to disrupt maintenance of the memory compartment, provide impetus for T-cell clonal expansions.
Thymic involution. Thymic involution and its cardinal manifestation — reduction in naive T-cell output from the thymus — begin as early as 1 year after birth in humans and after puberty (∼6–8 weeks) in laboratory mice. These processes remain incompletely understood2,44,45,46 partly because developing thymocytes and the thymic stroma are co-dependent, and proper thymic function depends on their crosstalk, making it difficult to precisely dissect thymocyte versus stromal-cell effects. Most of the recent studies have implicated defects in the commitment of haematopoietic stem cells to the lymphoid lineage and the migration of these lymphoid-restricted precursors (known as early T-cell progenitors47 or common lymphoid progenitor 2 (Ref. 48)) into the thymus49,50. That, however, does not exclude possible defects in thymic stromal components45,51, which are potentially precipitated by factors that include sex hormones52. In parallel with thymic involution, there is a marked decrease in the number of RTEs (Fig. 2b). The thymus seems to produce RTEs in proportion to its overall cellularity, even late in senescence28,46, and so, as thymic cellularity decreases, so does T-cell output. For example, 22-month-old mice have less than a tenth of the RTEs present in their young adult counterparts28,53. Thymic involution and the consequent marked and progressive decline in naive T-cell production put pressure on homeostatic mechanisms that are needed to maintain the naive T-cell compartment for much of adulthood and during senescence.
Reduction of T-cell renewal and TCR repertoire diversity. In elderly individuals, the few RTEs that reach the periphery are then faced with conditions that differ from those in younger counterparts: there are fewer naive T cells, and their survival and turnover is altered (Figs 2b, 3). RTEs (particularly those of the CD4+ phenotype) do not seem to survive well in the periphery of old animals28, as a result of several distinct processes. First, the naive T-cell pool in the periphery could be directly diminished by cell death and/or lifelong recruitment into the memory T-cell pool, although we lack quantitative data to discern whether these two events are important and, if they are, which one may be more important. Second, the naive T-cell pool could be indirectly affected by competition with a growing pool of memory T cells. Given that there is some overlap in the use of survival and maintenance cytokines by these two T-cell pools33,54,55, it is possible that these two populations are not independently regulated, particularly later in life, as initially postulated56. Finally, if naive T cells continued to be depleted, the consequent lymphopaenic conditions would generate an excess of survival and maintenance cytokines, which would trigger the homeostatic proliferative expansion of naive T cells. This would then eventually induce the conversion of many naive T cells into memory-phenotype T cells57, as convincingly shown in several studies58,59,60 (Figs 2b, 3a). This phenotype conversion is believed to occur because rapid proliferation, even in response to cytokines, activates a differentiation programme in T cells57. Recently, we obtained evidence in monkeys consistent with this mechanism. We found that homeostatic proliferation of naive T cells in the blood of aged rhesus macaques inversely correlates with the number and proportion of naive T cells in these animals, and that this is not accompanied by an accumulation of these cells, but rather by their rapid turnover61. Support for this mechanism has also been found for human CD4+ T cells62.
Figure 3. Disruption of T-cell homeostasis with ageing: possible causes.
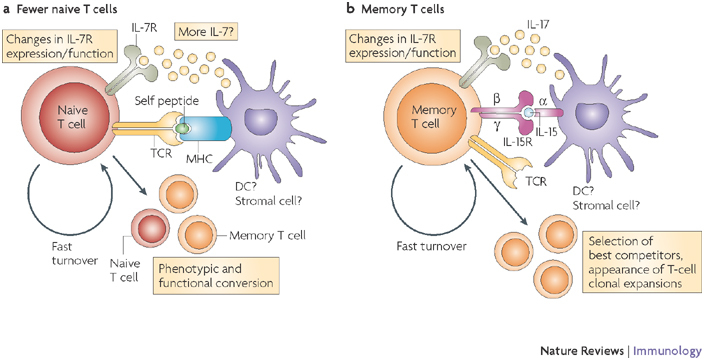
Although the thymus in young individuals produces sufficient numbers of naive T cells that express diverse T-cell receptors (TCRs) and that only infrequently cycle, the involuting, ageing thymus produces many fewer naive T cells (a) and these cells survive poorly in aged individuals28. It would be expected that fewer naive T cells would result in less consumption of interleukin-7 (IL-7) and therefore that more IL-7 would be available. This is on the proviso that IL-7 production is not affected with ageing, which remains speculative. Other known age-related alterations include altered expression and function of the IL-7 receptor (IL-7R)69, and increased turnover of naive T cells61,62, which has the potential to precipitate their conversion into memory T cells (b). This process would also support the formation of antigen-independent T-cell clonal expansions72,100. Memory T cells are known to proportionally and numerically increase with ageing1,2,3, and several processes contribute to this increase: loss of naive T cells and increased availability of and/or responsiveness to T-cell maintenance cytokines, such as IL-7 and IL-15. Other factors (not shown), including an increase in the levels of pro-inflammatory cytokines (such as IL-6 and tumour-necrosis factor) and changes in systemic and local levels of hormones (insulin-like growth factor 1, growth hormone, ghrelin and leptin) could also have a role, although this is yet to be proven. DC, dendritic cell.
Together, all of these mechanisms — reduced naive T-cell production, increased naive T-cell conversion into memory cells and accumulation of memory cells due to lifelong encounters with new pathogens — synergize to reduce TCR diversity in old individuals34,62,63,64. Importantly, this reduction affects the otherwise most diverse naive T-cell subset62, stressing the power of antigen-independent homeostatic forces in eventually precipitating age-related T-cell population imbalance61,62.
These changes in old individuals occur alongside, and are likely to be heavily influenced by, changes in the cytokine environment. It is well accepted that IL-2 production decreases with ageing65,66,67. By contrast, it is less clear whether there are age-related alterations in the levels of IL-7 or IL-15 (Refs 55, 68, 69, 70) or in the expression and function of their receptors on different T-cell subsets69,71. Analyses to understand IL-15-related alterations have been hindered by difficulties in measuring relevant IL-15 concentrations owing to its highly localized mechanism of action. Understanding whether changes in these cytokines contribute to age-related defects in T-cell homeostasis is of added interest because of the clear therapeutic potential for γc cytokines in lymphopoiesis and/or the peripheral expansion and maintenance of T-cell subsets. However, much remains to be understood about the in vivo action of exogenously administered cytokines, particularly from non-human primate and human clinical studies26,55,72,73,74.
In summary, ageing is associated with a dramatic increase in the proportion of memory T cells in the blood, with a concomitant and equivalent decrease in the proportion of circulating naive T cells. The initial event(s) that is believed to set the stage for a disruption in the balance of the T-cell population — thymic involution and loss of naive T-cell renewal — occurs early in life and is independent of pathogen exposure and antigenic experience of the individual. Subsequently, however, antigenic experience modulates this situation, with a potential to importanly influence T-cell population balance with ageing. Evidence suggests that persistent pathogen infections are particularly important in this regard.
Impact of infection on T-cell homeostasis
T-cell homeostasis in acute and persistent infections. The aforementioned homeostatic changes — loss of naive T-cell production and homeostatic proliferation in response to relative naive T-cell lymphopaenia — seem to have a crucial influence on T-cell ageing in laboratory mice kept under specific pathogen free (SPF) conditions. However, in pathogen-exposed mammals studied so far, specific pathogens provide another, perhaps dominant, level of influence. On pathogenic invasion, T cells specific for the epitopes of the pathogen undergo marked clonal expansion, such that one naive T cell can generate up to 105 effector T cells. Once the pathogen is eliminated, more than 90% of these cells are eliminated by apoptosis, and those that remain are recruited into the memory T-cell pool (Fig. 4a). A different situation occurs if the pathogen is not eliminated. Persistent pathogens can establish latent or chronic infection (Box 1), and these two types of infection differ by the level at which they stimulate the immune system. The replication of pathogens that establish chronic infection is never truly controlled in the host, and although the level of the pathogens in the blood is likely to be lower than that measured at the peak of acute infection, such pathogens constantly replicate and maintain a set point of pathogen load in the host (Fig. 5). Therefore, immune cells (including T cells) are constantly and systemically stimulated by these pathogens. In many, if not most, cases, these pathogens cause chronic diseases that last for years and often have poor prognosis, in part because they exhaust the T cells that are supposed to control them. Chronic infections of this type, including those caused by HIV and SIV (simian immunodeficiency virus), HCV and chronic strains of lymphocytic choriomeningitis virus (LCMV), can coincide with ageing, but are not part of the 'normal' ageing process because they do not affect the majority of the old population and because they cause clinically manifest, direct pathology. Therefore, they will not be discussed further in this Review, except to illustrate any relevant parallels between the functional consequences of these chronic infections and those that stem from T-cell ageing.
Figure 4. Adding insult to injury: the impact of persistent pathogens on T-cell population dynamics with age.

Stages of the immune response to viruses that establish acute (a) and persistent (b) infection. In both cases, the kinetics of activation and waning of innate immunity, the kinetics of infectious virus and the kinetics of the initial, primary T-cell response are essentially the same. Key differences between acute and persistent viral infections emerge subsequently: the causative agent of acute infection is eliminated and T-cell memory is therefore maintained at a more or less constant, low level, whereas latent persistent pathogens will periodically reactivate, each time then activating the innate immune system. Because innate immunity does not have the property of memory, innate cells do not accumulate. However, memory T cells accumulate following each stimulation and therefore produce memory inflation. This process is pronounced for CD8+ T cells and is more discrete for CD4+ T cells.
Figure 5. Persistent viruses and their antigenic load over time.

Relative availability of, and therefore the exposure of T cells to, viral antigens in the course of infection with different persistent viruses. Latent viruses reach fairly high antigen levels in the course of primary infection but then establish latency and reactivate intermittently, with different frequencies (cytomegalovirus (CMV) more often than herpes simplex virus (HSV)). By contrast, HIV antigens, representative of a chronic persistent virus infection, are present at very high levels initially, but importantly, they also remain at high levels in the blood during the set-point of infection. This provides T cells with constant stimulation, and eventually leads to exhaustion. Note that this illustration is mostly based on indirect immunological parameters for CMV and HSV, rather than on direct identification of the virus or viral antigen, which is difficult to achieve during the persistent phase of the infection.
In contrast to chronic infections, in latent infections the pathogen is controlled systemically and therefore cannot be detected in the blood at all times (Fig. 5). Rather, the pathogen finds a niche where it establishes latency (for example, HSV establishes latent infection in neurons, mostly in the sensory ganglia innervating the site of viral entry75) and remains largely dormant with regard to its genome replication. Occasionally, the latent pathogen will be reactivated, at which time it may or may not be systemically (or locally) detectable (Fig. 5). This, of course, will stimulate the T cells (mainly memory T cells), which will force the pathogen back into its latent state. However, this intermittent stimulation can potentially lead to expansion of pathogen-specific T-cell populations (Fig. 4b). Although the stimulation of T cells in localized latent infections is repeated, it is not likely to be continuous (at least not for all pathogen-reactive T cells) and therefore is not associated with massive exhaustion and/or deletion of parts of the T-cell repertoire, as is the case with chronic infection. Indeed, it is rare to see loss of immune control of a latent infection, even at an advanced age, unless there is concomitant immune suppression independent of ageing. Correlative experimental and clinical evidence7,25,76,77 has linked latent infections to expanded populations of T cells in advanced age, as described below.
Latent herpesvirus infections and T-cell ageing in humans: 'till death do us part'? Given the above considerations, the pathogens that are likely to have the maximum impact on the ageing immune system should be highly adapted to the host so as not to generate disease. They should be ubiquitous, and able to infect most of the human population early after birth. Although there are numerous human pathogens that persist after primary infection with no sterilizing immunity to them (including mycobacteria and helminths), the pathogen family that best corresponds to the above description is the Herpesviridae78,79 (Box 1).
Herpesviruses usually establish latency in specific cell types or tissues and deploy multiple mechanisms to evade the innate and adaptive immune systems80,81,82. HSV, EBV and CMV are probably the most prominent herpesviruses that are linked to the expansion of T cells in elderly humans25. Among them, CMV stands out because it typifies the key features of the herpesvirus family that are pertinent to T-cell ageing. First, CMV prevalence varies between 60% in industrial countries and nearly 100% in developing countries83, with an increasing prevalence in older humans (up to 90%) even in industrial countries. Second, the CD8+ T-cell response against CMV is unusually broad, targeting numerous viral peptides84,85. This response also displays an unusual feature of progressive, long-term expansion of antigen-specific CD8+ memory T cells84,86,87 that is known as memory inflation88. Indeed, the CD8+ T-cell response to individual epitopes of this virus can reach up to 20% of the total memory T-cell compartment, and the response to all of its epitopes in humans is estimated to occupy 50% or more of the entire CD8+ memory T-cell pool84. Finally, this pronounced and progressive response has been associated with an accumulation of dysfunctional CMV-specific T cells89, as well as with shorter lifespan in octogenarians90, although the nature of the link between these associations is not clear at present.
Interesting findings were reported recently by Hadrup et al.91 on the complexity of the immune response to CMV in very old humans. Large CD8+ T-cell clonal expansions specific for the immunodominant CMV peptides were seen in most octogenarians and nonagenarians in a Swedish population study, and these tended to become progressively more prominent with age91. However, the individuals that survived into very old age exhibited smaller T-cell responses to CMV, and even showed loss of certain T-cell clonotypes responding to this virus. This raises the possibility that survival into very old age (centenarians and older) may be associated with an immune system that is no longer overtly preoccupied with persisting infections.
Unfortunately, longitudinal studies in relevant human populations, although they are invaluable in providing information on immune ageing in the most relevant physiological model, suffer from ethical and practical constraints that prevent definitive experimentation. Therefore, it remains unclear whether latent pathogens are the cause of memory inflation, and if so whether they cause it by directly stimulating relevant T cells, indirectly through the action of virus-induced type I interferons or other mediators of innate immunity, or by a combination of these and other mechanisms. Experiments showing that CD8+ T cells specific for some viral antigens accumulate88,92,93, whereas others do not85,94,95,96, and that this correlates to the expression of these antigens or proteins in latency, would suggest that viral antigen expression during early reactivation and/or latency is driving the process of memory inflation, but this remains to be formally proven.
It is also possible that the aged immune system may be inefficient at controlling latent virus reactivation (and gene expression) owing to T-cell-intrinsic defects, allowing for prolonged persistence of viral antigens that then further stimulate an already expanded, but inefficient, pool of virus-specific T cells. In this scenario, age-related T-cell defects could be the primary cause for their own accumulation via a positive-feedback loop, although the presence of latent viral infection would be needed to expose it. Finally, it is unclear whether the expanded T cells in humans resemble the spontaneously arising T-cell clonal expansions seen in mice34,64, and whether they depend on viral antigen for survival and proliferation.
A mouse model of latent herpesvirus infection and T-cell ageing. To answer some of the questions concerning persistent pathogens and immune ageing, we have developed a model of latent viral infection in old mice. We sought to mimic the human situation, with an early infection in youth and persistence throughout life. HSV was administered locally or systemically to young mice, and expansion, contraction and maintenance of the T-cell response were followed in a longitudinal manner. Following intraocular administration, we observed the expected T-cell expansion until day 8–9 (Ref. 97), followed by contraction and stable maintenance of ∼1–2% virus-specific CD8+ T cells in the blood, measured using MHC class I tetramers98. This set point for memory maintenance was stable over the next 16 months, at which point, in most mice that were now bona fide old, we observed a slow, progressive and variable accumulation of HSV-specific T cells. In some of the mice (∼20–30%), the HSV-specific T-cell numbers reached levels similar to, or even higher than, those seen at the peak of the acute infection. These expanded virus-specific T cells had a central memory phenotype, with no signs of acute activation98. Moreover, continuous administration of antiviral drugs did not alter the course of this accumulation of T cells. Altogether, these observations suggest that in a localized HSV infection, viral reactivation and repeated antigenic stimulation have a minor, if any, role in the accumulation of virus-specific CD8+ T cells. Rather, the phenotype of the accumulating cells (central memory T cells) raises the possibility that these expanded T cells arise as a consequence of homeostatic processes that are similar to those that drive the onset of spontaneous T-cell clonal expansions in uninfected mice71,99. In this regard, spontaneously arising (antigen-independent) T-cell clones express high levels of receptors for the survival-promoting γc cytokines IL-7 and IL-15 (Ref. 71), and similar features were observed in HSV-specific CD8+ T cells that accumulated with age in our model of localized HSV infection98. This is consistent with the idea that, rather than repeated antigenic stimulation, old age and its homeostatic disturbances, are the key factors in the onset of T-cell expansions in this model of localized infection with a latent virus.
Another line of evidence supporting this view comes from a recent and important paper by Woodland and colleagues100, who found that a T-cell clone specific for an acute respiratory virus (Sendai virus), which was cleared in youth, can markedly expand in old age in the absence of signs of antigen-driven activation. We have confirmed these results with a different acute virus (WNV)98, and suggest that this is a general phenomenon in SPF mice, and that any T cell from the memory pool, if adequately responsive to cytokine or other survival factors, can be selected for clonal expansion, regardless of its antigenic specificity or antigenic stimulation. Of note, children and young adults often mount strong CMV-specific immune responses101,102 but memory inflation does not occur until later in life, and the most pronounced abnormalities are most prominent in, although not exclusive to, the elderly. This too would be consistent with an important role of age-related homeostatic disturbances, although the direct progeroid influence of CMV cannot be excluded.
A different kinetic of T-cell expansion was previously described by Reddehase and colleagues93, and subsequently by Klenerman and colleagues88, using the mouse model of CMV infection (murine CMV (MCMV)). In this model, an early accumulation of MCMV-specific effector memory T cells (that is, memory inflation88) was seen as early as 2–3 months after primary infection, and this accumulation matched or exceeded the levels of antigen-specific T cells seen at the peak of primary infection (which are rather low in primary CMV infection). In contrast to our observations using the HSV model, these studies raised a possibility that MCMV and HSV-1 differed in the fundamental ability to induce memory inflation, or that other differences (dose, route or other parameters) may account for the fact that HSV-1 did not induce memory inflation in our model. To test these possibilities, we immunized adult mice intraperitoneally, thereby matching the route used in the MCMV experiments. In these experiments, we saw early and pronounced memory inflation starting at 3 months post infection (A. Lang et al., unpublished observations). The cells exhibited the phenotype and the distribution typical of effector memory T cells (in the blood and tissues, but not secondary lymphoid organs). This memory inflation could be delayed or prevented with antiviral drugs, and T-cell transfers into virus-free or virus-infected hosts formally showed that direct viral stimulation, establishment of latency and reactivation from latency were all necessary to produce memory inflation (A. Lang et al., unpublished observations). Nevertheless, this does not resolve the basic mechanisms involved in the onset and maintenance of memory inflation, and decisive experiments are needed to evaluate the roles of viral antigen and of virally induced innate immune response factors in this process.
Overall, questions still remain related to the evolution of memory inflation in old age. Will the cells undergoing memory inflation retain the effector memory phenotype and function? Will they convert into central memory cells, perhaps indicating that homeostatic cytokines or factors, rather than antigenic stimulation, may be more important in advanced ageing? And will that be similar or distinct to the situation observed in humans?
Persistent infections, replicative senescence and T-cell exhaustion. As mentioned above, chronic infections, such as HIV, HCV and chronic strains of LCMV, have been shown to lead to T-cell clonal exhaustion. The discovery that these chronically stimulated and exhausted T cells express programmed cell death 1 (PD1), a B7-family molecule that may have a role in dampening signals through the co-stimulatory pathway103, led to attempts to improve T-cell function by blocking PD1-mediated signalling. Such blockade104, as well as the blockade of IL-10, a cytokine often associated with inhibition of type 1 immunity105, can result in a marked reversal of function in exhausted T cells in mice. Two other markers were associated with exhaustion, KLRG1 (killer-cell lectin-like receptor subfamily G, member 1), a natural-killer-cell marker that is often associated with low proliferative ability106, and CD57, which has been shown in HIV-infected subjects to mark replicatively senescent cells likely to undergo apoptosis107, but in other in vivo studies expression of these markers did not correlate precisely with functional exhaustion108,109. So far, attempts to reverse the exhausted phenotype of human T cells using blockade of PD1 have been met with far more modest success (reviewed in Ref. 104), suggesting that this pathway and the concepts of exhaustion need to be better understood in humans. Nevertheless, a key question is whether similar molecular and functional changes occur with ageing and with latent infections.
Several published manuscripts have described an accumulation of dysfunctional or hypofunctional cells with a 'terminally differentiated' phenotype that is associated with human ageing76,110,111. These terminally differentiated cells share features with effector memory cells, being CD69−CD25−CD62LlowCD127lowCCR7lowCD27low, and, in humans, are also CD28− and re-express CD45RA (which is normally expressed by naive T cells). Therefore, they have been termed T effector memory, re-expressing CD45RA (or T-EMRA) cells87,112. Although these cells can be stained with MHC class I tetramers, confirming their specificity for viral antigens, in some situations they show impaired, or absent, in vitro proliferation and IL-2 production in response to antigen or agonist TCR-specific antibodies, and variably reduced secretion of interferon-γ, although their cytolytic capacity can be intact87,106,113. This functional phenotype of T cells in aged individuals has been taken to indicate exhaustion, but are these cells really exhausted and are they counterparts of the similar population observed in chronic viral infections? Several observations urge caution in equating these cell populations. First, typical expression of PD1 and its ligand PDL1 is several-fold lower on the cells responding to CMV in aged individuals compared to those responding to chronic infection with HIV or HCV104,114,115 (A. Lang and J.N-.Ž., unpublished observations). Second, similar T-cell populations in non-human primates, although possibly hyporesponsive in vitro, appear to turn over quite vigorously in vivo, as measured by labelling with bromodeoxyuridine61,116,117. It is likely that in vitro conditions simply do not provide a replica of the environment that allows these cells to accumulate with ageing. Third, as mentioned above, there is little to no clinical evidence that such 'exhausted' T-cell populations indeed allow reactivation of the latent herpesviruses that they are supposed to control, and the protective function of this subset has rarely been addressed directly. In some cases, proliferative capacity118 and even protective effect119 of the so-called terminally differentiated and/or exhausted cells was strong. Indeed, profound immunosuppression is necessary to reveal clinical reactivation of herpesvirus infections in the elderly, suggesting that they are adequately controlled by the ageing immune system. Fourth, one should be mindful of another physiological possibility: that cells arising by memory inflation should really not be allowed to further proliferate. Indeed, from a homeostatic standpoint, it would seem economical to curtail the proliferation of an already expanded population. Therefore, ensuring that expanded memory T cells remain hypoproliferative, would be an elegant way to reduce further expansion of these cells. Of course, it remains possible that these cells usurp the space and potentially constrict the T-cell repertoire needed for the response to new microbial invasion, as was shown in the case of spontaneously arising antigen-independent T-cell clonal expansions in SPF mice120. Another possibility was recently raised in adult mice; persistent herpesviruses cross-protected against bacterial infections via innate immune mechanisms121, and so it will be interesting to determine whether this extends to old animals and humans as well. Therefore, the role of latent persistent herpesviruses in ageing of the immune system (and indeed of the individual) will remain a rewarding problem to dissect mechanistically for the foreseeable future.
Perspectives for intervention
Mechanisms that lead to T-cell senescence at the cellular and population level are becoming better defined. Along with this, we are approaching the design of realistic treatments to prevent or treat T-cell senescence and/or to ameliorate its consequences. Here, I briefly mention several approaches that have the potential to achieve therapeutic goals in T-cell senescence.
Vaccination has been at the forefront of the fight against infectious diseases for several centuries and remains among the best strategies available. Problems associated with vaccination of the elderly are, unfortunately, numerous, and they stem from the same defects that impair the primary response of an aged immune system to a pathogen. Live-attenuated vaccines have been associated with a high rate of complications in the elderly, and several of them, such as FluMist (MedImmune, Inc.), an inhalable influenza virus vaccine, and the live-attenuated yellow fever vaccine, are not recommended for this population122. By contrast, heat-killed, fully attenuated vaccines, being weaker, tend to elicit at best moderate humoral and CD4+ T-cell responses, and these responses are heavily compromised in the elderly. Therefore, by some estimates, up to 60% of the elderly receive no benefit from annual vaccination against influenza virus. Modification of the existing vaccines is therefore needed to improve vaccination outcomes. However, it is not clear whether such modifications should primarily act on T cells, antigen-presenting cells or some other element of the innate immune system. It is possible that not all age-related defects will prove to be universal, necessitating some sort of screening to evaluate who will need which type of therapy, and we remain far from having useful biomarkers for such screening112. Clearly, if the T-cell reserve is fatally depleted such that the vaccine antigen has no cells to stimulate, modification of the vaccine is unlikely to afford any benefit to the recipient. It is therefore an even higher imperative to screen and select individuals in whom immune reconstitution will be the primary course of action. Unfortunately, we do not fare much better with regard to the screening tools in this case, although some studies have used immune risk phenotype measurements as a prognostic factor for longevity and other age-related parameters90,123. Modalities of intervention can globally be divided into thymic rejuvenation and improvement in maintaining peripheral naive T cells (reviewed in Ref. 26). However, it is likely that the true anti-ageing interventions in the realm of T-cell immunity will need to aim for the former.
If persistent viruses are indeed key factors in precipitating immune ageing, then antiviral therapy could be another potentially desirable intervention. This idea is largely hypothetical at present and needs further verification in small animal models. Moreover, although there are antiviral drugs available against herpesviruses, their efficacy is rarely absolute and side effects would have to be considered before they could be recommended for administration to the elderly. It is pertinent to note that here the goal would not be to achieve sterilizing immunity (which is anyway all but impossible to reach with herpesvirus-specific drugs), but rather to keep these viruses from (frequent) reactivation.
Finally, nutritional intervention by caloric restriction was recently shown to improve the production, maintenance and function of naive T cells in rhesus macaques124, corroborating prior data in rodents41,125. If parlayed into a pharmacological treatment, this could be a powerful way to enhance T-cell immunity in the elderly.
Concluding remarks
On the one hand, the most recent results tell us that persistent pathogens are probably important contributors to some of the immunological abnormalities seen in aged humans, by driving the expansion and accumulation of effector and memory T cells. However, it still remains to be seen whether they decisively influence immune defence and, by extension, longevity, and an answer to these questions will have to await controlled experiments in appropriate animal models. On the other hand, results from rodents98,99,100, as well as from non-human primates61 and humans62, strongly support the decisive role of the homeostatic forces in shaping the immune system in old individuals. Future experiments will illuminate whether this influence is more pronounced than that of latent infections.
Box 1 | Biology of persistent pathogens and possible relevance to ageing.
Unlike acute infections, which are cleared after mobilization of the immune system, persistent infections do not result in sterilizing immunity. In general, virologists that study herpesviruses tend to think that they all become latent. By contrast, hepatitis C virus and HIV are considered to be chronic persistent viruses. However, it is difficult to place these infections in a specific category, as infection tends to be more of a continuum: from latency (with no viral re-activation), through increasing frequencies of viral re-activation, to low-level persistent replication (with constant viral re-activation), to increasing levels of continuous viral replication, ending with high-level continuous replication.
Acknowledgements
I thank the members of my laboratory for stimulating discussions (in particular, L. Cicin-Sain, A. Lang and B. Rudd) and A. Townsend for help with the original illustrations. I also acknowledge support from the US Public Health Service awards AG20719, AG21384, AG23664 and N0150027 from the National Institute on Aging and National Institute of Allergy and Infectious Diseases and RR 0163 (the Core National Primate Research Center award to Oregon National Primate Research Centre) from the National Institutes of Health, USA.
Glossary
- Oxidative stress
The cellular state in which there is an imbalance between the production and neutralization of reactive oxygen species (ROS), such as H2O2, OH−, HOO− and superoxide. Excess ROS can generate oxidative reactions that can result in disrupted cellular signalling, injury and death.
- Non-homologous end-joining
A pathway that rejoins DNA strand breaks without relying on significant homology. The main known pathway uses the Ku-end binding complex and is regulated by DNA protein kinase. The pathway is often used in mammalian cells to repair strand breaks caused by DNA-damaging agents, and some of the same enzymes are used during the strand-joining steps of V(D)J recombination.
- Thymic involution
The age-dependent decrease of thymic epithelial volume and decreased production of T cells.
- T-cell homeostasis
The maintenance of relatively stable numbers of naive and memory T cells and of balanced T-cell receptor diversity, as well as the ability of the T-cell pool to restore these numbers and diversity to prior resting state after acute antigen challenge.
- Common cytokine-receptor γ-chain family
A family of cytokine receptors in which each receptor complex is composed of two or three subunits, with one of those subunits being the common cytokine-receptor γ-chain (γc).
- Regulatory T cells
A subset of CD4+ T cells that has suppressive regulatory activity towards effector T cells and other immune cells. This subset includes cells that express high levels of CD25 (the interleukin-2 receptor α-chain) and the transcription factor forkhead box P3 (FOXP3). Other regulatory T-cell populations can be CD25−FOXP3− and produce the regulatory cytokine interleukin-10, which suppresses the functions of T-helper-1-type effector T cells and other immune cells. The absence or dysfunction of regulatory T cells is associated with severe autoimmunity.
- Central memory T cells
Antigen-experienced CD8+ T cells that lack immediate effector function but can mediate rapid recall responses by virtue of quick and sustained cell division and rapid development into effector or effector memory cells after restimulation with antigen. Central memory T cells retain the migratory properties of naive cells and therefore circulate through the secondary lymphoid organs.
- Effector memory T cells
Terminally differentiated T cells that lack most, but not all, lymph-node homing receptors, but express receptors that enable them to home to inflamed tissues. Effector memory cells contain perforin and can exert immediate effector functions without the need of further differentiation.
- Specific pathogen free (SPF) conditions
Vivarium conditions for rodents whereby an increasing number of pathogens are excluded or eradicated from the colony. These animals are maintained in the absence of most of the known chronic and latent persistent pathogens. Although this enables better control of experimental conditions related to immunity and infection, it also sets apart such animal models from pathogen-exposed humans or non-human primates, whose immune systems are in constant contact with infection.
- MHC class I tetramers
Biotinylated monomeric MHC molecules that are folded with a specific peptide in the binding groove and tetramerized with a fluorescently labelled streptavidin molecule. These tetramers will bind to T cells that express T-cell receptors specific for the cognate peptide, allowing direct enumeration of antigen-specific T cells.
- Clonal exhaustion
A state of non-reactivity in which all precursor lymphocytes responding to persistent antigen(s) differentiate into effector cells which are driven into the state of functional exhaustion, such that they no longer can productively engage and control the pathogen. The end result of clonal exhaustion is purging the immune-response repertoire of a particular specificity (or specificities).
- Bromodeoxyuridine
(5-Bromo-2-deoxyuridine; BrdU). A thymidine analogue that is incorporated into DNA on replication, allowing tracking of cells that have divided using BrdU-specific antibodies and flow cytometry.
- Immune risk phenotype
A set of empirical immune system (mainly T-cell) measurements that are thought to be biomarkers (although in some circumstances this still needs to be verified) of immune senescence. These include: reduced naive T-cell numbers, inverted CD4:CD8 ratios (>1 in youth, ≥1 in ageing), accumulation of CD28− (effector memory) T cells, reduced proliferative ability, reduced interleukin-2 production and presence of cytomegalovirus infection.
Biography
Janko Nikolich-Žugich is Professor and Chairman of the Department of Immunobiology and Co-Director of the Arizona Center on Aging at the University of Arizona in Tucson, Arizona, USA. He obtained his M.D. and Ph.D. in immunology from the University of Belgrade, Serbia, and postdoctoral training from the Scripps Research Institute, La Jolla, California, USA. Before the University of Arizona, he held positions at the Memorial Sloan-Kettering Cancer Center and Cornell University in New York, USA, and at the Oregon Health and Science University in Portland, Oregon, USA. His main interest is in understanding CD8+ T-cell biology, homeostasis and senescence, and the relationship between immunosenescence and ageing. His goal is to apply this knowledge to immune system rejuvenation and to improvements of vaccination and immune defence against infection, particularly in the elderly.
References
- 1.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 2.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 3.Cambier J. Immunosenescence: a problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol. Rev. 2005;205:5–6. doi: 10.1111/j.0105-2896.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 4.Brody JA, Brock DB. Handbook of the Biology of Aging. 1985. Epidemiologic and statistical characteristics of the United States elderly population; pp. 3–42. [Google Scholar]
- 5.Robinson KA, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 6.Gardner ID. The effect of aging on susceptibility to infection. Rev. Infect. Dis. 1980;2:801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- 7.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr. Opin. Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl Acad. Sci. USA. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J. Immunol. 2001;166:3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- 10.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common γ chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J. Exp. Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ershler WB, Sun WH, Binkley N. The role of interleukin-6 in certain age-related diseases. Drugs Aging. 1994;5:358–365. doi: 10.2165/00002512-199405050-00005. [DOI] [PubMed] [Google Scholar]
- 12.Ginaldi L, et al. The immune system in the elderly: II. Specific cellular immunity. Immunol. Res. 1999;20:109–115. doi: 10.1007/BF02786467. [DOI] [PubMed] [Google Scholar]
- 13.Haynes L, Eaton SM, Swain SL. The defects in effector generation associated with aging can be reversed by addition of IL-2 but not other related γc-receptor binding cytokines. Vaccine. 2000;18:1649–1653. doi: 10.1016/S0264-410X(99)00501-0. [DOI] [PubMed] [Google Scholar]
- 14.Pamer EG. Antigen presentation in the immune response to infectious diseases. Clin. Infect. Dis. 1999;28:714–716. doi: 10.1086/515207. [DOI] [PubMed] [Google Scholar]
- 15.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 16.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guney I, Sedivy JM. Cellular senescence, epigenetic switches and c-Myc. Cell Cycle. 2006;5:2319–2323. doi: 10.4161/cc.5.20.3348. [DOI] [PubMed] [Google Scholar]
- 18.Bird J, Ostler EL, Faragher RG. Can we say that senescent cells cause ageing? Exp. Gerontol. 2003;38:1319–1326. doi: 10.1016/j.exger.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Gavrilov LA, Gavrilova NS. Evolutionary theories of aging and longevity. Scientific WorldJournal. 2002;2:339–356. doi: 10.1100/tsw.2002.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- 21.Masoro EJ. Physiology of aging. Int. J. Sport Nutr. Exerc. Metab. 2001;11:S218–S222. doi: 10.1123/ijsnem.11.s1.s218. [DOI] [PubMed] [Google Scholar]
- 22.Campisi J. Between scylla and charybdis: p53 links tumor suppression and aging. Mech. Ageing Dev. 2002;123:567–573. doi: 10.1016/S0047-6374(02)00006-4. [DOI] [PubMed] [Google Scholar]
- 23.Surh CD, Sprent J. Regulation of naive and memory T-cell homeostasis. Microbes Infect. 2002;4:51–56. doi: 10.1016/S1286-4579(01)01509-X. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 25.Pawelec G, et al. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Zúñiga-Pflücker JC, van den Brink MR. Giving T cells a chance to come back. Semin. Immunol. 2007;19:279. doi: 10.1016/j.smim.2007.11.001. [DOI] [Google Scholar]
- 27.Scollay R, Butcher E, Weissman I. Thymus migration: quantitative studies on the rate of migration of cells from the thymus to the periphery in mice. Eur. J. Immunol. 1980;10:210. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 28.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl Acad. Sci. USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berzins SP, Boyd RL, Miller JFAP. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nature Rev. Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Caserta S, Zamoyska R. Memories are made of this: synergy of T cell receptor and cytokine signals in CD4+ central memory cell survival. Trends Immunol. 2007;28:245–248. doi: 10.1016/j.it.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Tan J, et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 35.Freitas AA, Rocha B. Population biology of lymphocytes: the fight for survival. Annu. Rev. Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Berzins SP, Godfrey DI, Miller JFAP, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc. Natl Acad. Sci. USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nature Rev. Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 38.Nikolich-Žugich J. T cell aging: naive but not young. J. Exp. Med. 2005;201:837–840. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J. Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes L, Eaton SM, Buns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J. Exp. Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Effros RB, Walford RL, Weindruch R, Mitcheltree C. Influences of dietary restriction on immunity to influenza in aged mice. J. Gerontol. 1991;46:B142–B147. doi: 10.1093/geronj/46.4.B142. [DOI] [PubMed] [Google Scholar]
- 42.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol. Rev. 1997;160:79–90. doi: 10.1111/j.1600-065X.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 43.Miller RA, et al. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol. Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 44.Montecino-Rodriquez E, Min. H, Dorshkind K. Reevaluating current models of thymic involution. Semin. Immunol. 2005;17:356–361. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Zediak VP, Bhandoola A. Aging and T cell development: interplay between progenitors and their environment. Semin. Immunol. 2005;17:337–346. doi: 10.1016/j.smim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J. Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allman D, et al. Thymopoiesis independent of common lymphoid progenitors. Nature Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 48.Martin CH, et al. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nature Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 49.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J. Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 50.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J. Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 51.Utsuyama M, Kasai M, Kurashima C, Hirokawa K. Age influence on the thymic capacity to promote differentiation of T cells: induction of different composition of T cell subsets by aging thymus. Mech. Ageing Dev. 1991;58:267–277. doi: 10.1016/0047-6374(91)90098-K. [DOI] [PubMed] [Google Scholar]
- 52.Chidgey AP, Boyd RL. Stemming the tide of thymic aging. Nature Immunol. 2006;7:1013–1016. doi: 10.1038/ni1006-1013. [DOI] [PubMed] [Google Scholar]
- 53.Heng TS, et al. Effects of castration on thymocyte development in two different models of thymic involution. J. Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 54.Picker LJ, et al. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fry TJ, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 56.Tanchot C, Lemonnier FA, Pérarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 57.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nature Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 58.Ge Q, Hu H, Eisen HN, Chen J. Naive to memory T-cell differentiation during homeostasis-driven proliferation. Microbes Infect. 2002;4:555–558. doi: 10.1016/S1286-4579(02)01572-1. [DOI] [PubMed] [Google Scholar]
- 59.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol. Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 61.Cicin-Sain L, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc. Natl Acad. Sci. USA. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naylor K, et al. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 63.Posnett DN, Sinha S, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J. Exp. Med. 1994;179:609–617. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LeMaoult J, et al. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J. Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 65.Miller RA, Stutman O. Limiting dilution analysis of IL-2 production: studies of age, genotype, and regulatory interactions. Lymphokine Res. 1982;1:79–86. [PubMed] [Google Scholar]
- 66.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell. Immunol. 1983;81:298–305. doi: 10.1016/0008-8749(83)90237-X. [DOI] [PubMed] [Google Scholar]
- 67.Thoman ML, Weigle WO. Lymphokines and aging: interleukin-2 production and activity in aged animals. J. Immunol. 1981;127:2102–2106. [PubMed] [Google Scholar]
- 68.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 2002;37:455–463. doi: 10.1016/S0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 69.Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7Rα expression with aging and the potential implications of IL-7 therapy on CD8+ T cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.El Kassar N, et al. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 71.Messaoudi I, Warner J, Nikolich-Žugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J. Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 72.Moniuszko M, et al. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J. Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips JA, et al. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J. Immunol. 2004;173:4867–4874. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 74.Nikolich-Žugich J. Non-human primate models of T-cell reconstitution. Semin. Immunol. 2007;19:310–317. doi: 10.1016/j.smim.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 76.Almanzar G, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8 T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olsson J, et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 2000;121:187–201. doi: 10.1016/S0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 78.Mocarski ES, Courcelle CT. Fields Virology. 2001. Cytomegaloviruses and their replication; pp. 2629–2674. [Google Scholar]
- 79.Murphy BL, Maynard JE, Krushak DH, Fields RM. Occurrence of a carrier state for Herpesvirus tamarinus in Marmosets. Appl. Microbiol. 1971;21:50–52. doi: 10.1128/am.21.1.50-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson DC, Hill AB. Herpesvirus evasion of the immune system. Curr. Top. Microbiol. Immunol. 1998;232:149–177. doi: 10.1007/978-3-642-72045-1_8. [DOI] [PubMed] [Google Scholar]
- 81.Stevenson PG. Immune evasion by gamma-herpesviruses. Curr. Opin. Immunol. 2004;16:456–462. doi: 10.1016/j.coi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 82.Novak N, Peng WM. Dancing with the enemy: the interplay of herpes simplex virus with dendritic cells. Clin. Exp. Immunol. 2005;142:405–410. doi: 10.1111/j.1365-2249.2005.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pass RF. Fields Virology. 2001. Cytomegalovirus; pp. 2675–2706. [Google Scholar]
- 84.Sylwester AW, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munks MW, et al. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J. Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 86.Reddehase MJ. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 2000;12:390–396. doi: 10.1016/S0952-7915(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 87.Ouyang Q, et al. Dysfunctional CMV-specific CD8+ T cells accumulate in the elderly. Exp. Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 88.Karrer U, et al. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 89.Ouyang Q, et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 2003;23:247–257. doi: 10.1023/A:1024580531705. [DOI] [PubMed] [Google Scholar]
- 90.Wikby A, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 91.Hadrup SR, et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 92.Podlech J, Holtappels R, Wirtz N, Steffens HP, Reddehase MJ. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J. Gen. Virol. 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 93.Holtappels R, Pahl-Seibert MF, Thomas D, Reddehase MJ. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62Llo memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J. Virol. 2000;74:11495–11503. doi: 10.1128/JVI.74.24.11495-11503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holtappels R, Thomas D, Podlech J, Reddehase MJ. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 2002;76:151–164. doi: 10.1128/JVI.76.1.151-164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holtappels R, et al. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J. Virol. 2000;74:1871–1884. doi: 10.1128/JVI.74.4.1871-1884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sierro S, Rothkopf R, Klenerman P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 2005;35:1113–1123. doi: 10.1002/eji.200425534. [DOI] [PubMed] [Google Scholar]
- 97.Lang A, Nikolich-Žugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J. Immunol. 2005;174:2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 98.Lang A, Brien JD, Messaoudi I, Nikolich-Žugich J. Age-related dysregulation of CD8+ T-cell memory specific for a persistent virus is independent of viral replication. J. Immunol. 2008;180:4848–4857. doi: 10.4049/jimmunol.180.7.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Messaoudi I, Warner J, Nikolich-Žugich D, Fischer M, Nikolich-Žugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J. Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 100.Ely KH, et al. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J. Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- 101.Komatsu H, et al. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun. Ageing. 2006;3:11. doi: 10.1186/1742-4933-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komatsu H, et al. Bone marrow transplantation from a pediatric donor with a high frequency of cytomegalovirus-specific T-cells. J. Med. Virol. 2006;78:1616–1623. doi: 10.1002/jmv.20746. [DOI] [PubMed] [Google Scholar]
- 103.Petrovas C, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riley JL, June CH. The road to recovery: translating PD-1 biology into clinical benefit. Trends Immunol. 2007;28:48–50. doi: 10.1016/j.it.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 105.Brooks DG, et al. Interleukin-10 determines viral clearance or persistence in vivo. Nature Med. 2006;12:1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ouyang Q, et al. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp. Gerontol. 2003;38:911–920. doi: 10.1016/S0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 107.Brenchley JM, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 108.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 109.Casazza JP, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 111.Maini MK, Casorati G, Dellabona P, Wack A, Beverley PC. T-cell clonality in immune responses. Immunol. Today. 1999;20:262–266. doi: 10.1016/S0167-5699(99)01472-3. [DOI] [PubMed] [Google Scholar]
- 112.Beverley PC, Grubek-Loebenstein B. Is immune senescence reversible? Vaccine. 2000;18:1721–1724. doi: 10.1016/S0264-410X(99)00514-9. [DOI] [PubMed] [Google Scholar]
- 113.Globerson A, Effros R. Aging of lymphocytes and lymphocytes in the aged. Immunol. Today. 2000;21:515–521. doi: 10.1016/S0167-5699(00)01714-X. [DOI] [PubMed] [Google Scholar]
- 114.Sauce D, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 115.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 116.Pitcher CJ, et al. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 117.Jankovic V, Messaoudi I, Nikolich-Žugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD8 and CD4 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 118.Dunne PJ, et al. Epstein–Barr virus-specific CD8+ T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–940. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 119.Northfield JW, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. J. Virol. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Žugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J. Exp. Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 122.Betts RF, Treanor JJ. Approaches to improved influenza vaccination. Vaccine. 2000;18:1690–1695. doi: 10.1016/S0264-410X(99)00508-3. [DOI] [PubMed] [Google Scholar]
- 123.Wikby A, et al. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech. Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 124.Messaoudi I, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc. Natl Acad. Sci. USA. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen J, Astle CM, Harrison DE. Delayed immune aging in diet-restricted B6CBAT6 F1 mice is associated with preservation of naive T cells. J. Gerontol. A Biol. Sci. Med. Sci. 1998;53:B330–B337. doi: 10.1093/gerona/53A.5.B330. [DOI] [PubMed] [Google Scholar]
- 126.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]


