Abstract
Purpose
The study examined the different dimensions of fatigue (general, sleep/rest, cognitive), health related quality of life (physical, emotional, cognitive, social), and the relationships between fatigue and HRQL in hospitalized children and adolescents with cancer in Brazil.
Method
Participants were recruited from a pediatric oncology inpatient unit in a comprehensive cancer care hospital in southeast Brazil. They completed the PedsQL Multidimensional Fatigue Scale and the PedsQL Inventory of Quality of Life (Generic and Cancer module) once during hospitalization.
Results
The majority (66.7%) of the participants (n=38; mean age 12.1 ± 2.9 years) had total fatigue scores < 75 on 0 to 100 scale; with the mean total fatigue score of 63.8 ± 18.5. The majority (72.2% generic; 83.3% cancer modules) had total PedsQL scores < 75 on 0 to 100 scale. The mean PedsQL score on generic module (61.1 ± 17.0) was similar to the mean PedsQL score cancer module (59.1 ± 16.7). Significant correlations were found between total fatigue and quality of life generic (r=0.63, p = 0.000) and cancer module (r=0.74, p = 0.000).
Conclusions
The study is the first to report fatigue and health related quality of life in hospitalized children and adolescents with cancer in Brazil. Similar to experiences of other children in the world, our findings indicate that children and adolescents with cancer had problems with fatigue that were associated with low HRQL. Future studies are recommended to examine interventions (exercise, leisurely activities) that may alleviate fatigue and improve HRQL in pediatric patients with cancer.
INTRODUCTION
Fatigue has multiple dimensions that include physical, psychological and cognitive aspects (Mccabe, 2009; Mota; Pimenta, 2002). Cancer related fatigue may be associated with the hypermetabolic state from different causes associated with the disease and treatments. Tumor growth that lead to competing nutrients between normal and tumor cells, the effects of chemotherapy and radiotherapy, inadequate nutritional intake related to nausea and vomiting from cancer therapy, and anemia may lead to cancer related fatigue (Wu & Mcsweeney, 2004). Psychosocial factors may also cause cancer related fatigue such as sleep disturbance, uncertainty about the future, fear of death; and loss of the roles of family maintenance (Rodriguez, et al, 2012; Zupanec, et al, 2008). Consequences of fatigue include the inability to engage in everyday activities, need for restoring energy, mood changes, sleep disturbances, impaired social relations, decrease in school attendance, lower academic achievement; and impaired quality of life (Mccabe, 2009).
The health-related quality of life (HRQL) is considered an indicator of health that encompasses physical, mental and social aspects of health. HRQOL may be perceived as the individual’s health status, as it relates to the chronic condition of cancer, the treatment effects and the symptoms, and how these interfere with daily life (Fayers; Machin, 2007). The purpose of this study was to examine fatigue and HRQL in hospitalized children and adolescents with cancer in Brazil. More specifically, we examined the different dimensions of fatigue (general, sleep/rest, and cognitive). We also examined health related quality of life (PedsQL generic and cancer modules), and compared health related quality of life in patients with and without fatigue.
REVIEW OF THE LITERATURE
Fatigue is a highly prevalent symptom in cancer patients both during and after treatments (Menezes; Camargo, 2006; Mota; Pimenta, 2002). Several studies reported that fatigue was among the most distressing symptoms in hospitalized children with cancer (Miller et al, 2011; Hinds et al, 2007a; Jalmsell et al, 2006; Theunissen et al, 2007, Walker et al, 2010; Wolfe et al, 2000), and the most debilitating symptom in children with advanced cancer (Mota; Pimenta, 2002). The experience of fatigue began at time of diagnosis and increased in frequency and intensity during treatment, especially with chemotherapy (Hinds et al., 2007a; Jalmsell et al., 2006; Poder et al., 2010; Stasi et al., 2003; Theunissen et al., 2007; Walker et al., 2010; Williams et al., 2012; Yeh et al., 2008). Fatigue persisted for long periods of time even after the end of treatment (Baggott et al., 2009), and became most intense in the last month of life (Tomlinson, et al., 2011; Ullrich et al., 2010).
Fatigue was associated with negative emotions such as feeling sad, angry and feeling sorry for oneself (Walker et al., 2010). Fatigue was often under recognized because health care professions focused mostly on cure for the disease, and paid less attention to treatment related side effects (Hockenberry-Eaton; Hinds, 2000; Gibson et al., 2005). The few studies that investigated fatigue during hospitalization were mostly in children (5 to 18 years) with brain tumors in different stages of treatment (Palmer et al., 2007), in children and adolescents (5 to 19 years) with various types of cancer receiving treatment (Erickson et al., 2011; Varni et al., 2002), and in adolescent survivors (13 to 18 years) with various types of cancers (Silva, 2009). These studies did not investigate the different aspects of fatigue (general, sleep/rest, cognitive), and did not compare health related quality of life in patients with and without fatigue.
Health-Related Quality of Life
Baggot and colleagues (2010), interviewed children (10 to18 years) with diverse cancer diagnoses, using the PedsQL, a week after the administration of myelosuppressive chemotherapy. They found significant differences between children with cancer and healthy children. Children with cancer had worse HRQL compared to healthy children, a finding similar to other studies (De Bolle, et al, 2009; Varni, et al, 2003).
The physical dimensions of HRQOL in cancer were related to pain and nausea. Pain, a symptom defined by the individual’s physical, psychological, and emotional experience (Enskar et al., 2007), was reported to be the most prevalent symptom in children with cancer (Hockenberry, Hooke, 2007), and causing fear and anxiety during the hospitalization (Jacob et al., 2008). Several factors can cause pain in children with cancer, such as metastasis, surgery, treatment related effects such as mucositis, and invasive medical procedures (Jacob et al., 2007; Hockenberry, Hooke, 2007). Nausea and vomiting, the most common side effects of chemotherapeutic medications, lead to inadequate nutritional intake, electrolyte imbalances, increasing levels of anxiety, stress and depression (Gomes, 2011). Several studies reported that nausea was among the most prevalent symptoms experienced by children with cancer (Baggott et al., 2010; Collins et al., 2000; Rheingans, 2008). In addition to chemotherapy, nausea and vomiting may also be induced by radiation, opioid use, bowel obstruction, and emotional distress (Hockenberry & Hooke, 2007; Varni et al., 2004).
The psychosocial dimensions of HRQOL were related to “procedure anxiety”, “treatment anxiety”, and “worries” over cancer treatments and invasive procedures (Ribeiro et al., 2009). Children’s concerns may also be related to treatment failure and the fear of dying (Cicogna; Nascimento; Lima, 2012).
The cognitive dimensions of HRQOL were related to brain tumors and the cognitive effects of treatments (Ullrich; Embry, 2012). Previous studies have documented that the low processing speed, motor, perception and memory difficulties had compromised learning and school performance (Ellenberg et al, 2009; Rodriguez et al, 2012; Ullrich; Embry, 2012).
The social dimensions of HRQOL were related to changes in the “physical appearance” of children due to hair loss, weight changes, use of mask during neutropenic periods, as well as the presence of surgical scars and central line catheters needed for treatments and were presenting major challenges in children and adolescents with cancer. Ciacona, Nacimento and Lima (2010), reported those physical changes as distressful side effects of chemotherapy that were most frequently affecting perceptions of physical appearance in children and adolescents with cancer. Changes in self-image were consequently leading to decreased self-esteem, self-confidence, and feelings of “social misfits”. The social dimensions of HRQOL were also related to limitations or impairments in “communication” that were mostly attributed to the unwillingness to communicate, and led to social isolation. Children and adolescents were commonly not feeling well during the periods of chemotherapy treatments, a time when they preferred to remain silent, refusing to talk, sleeping or watching TV, and spending time passively (Ciacona, Nascimento and Lima, 2010). Moreover, feelings of prejudice and alienation from others may accentuate the feelings of isolation (Costa; Lima, 2002)
Fatigue and Health Related Quality of Life
Fatigue and other symptoms had significant effects on the quality of life of children and their families (Gedaly-Duff et al., 2006). Fatigue had the most significant effect on the HRQL of children with leukemia, which was attributed to fatigue which limited the child’s activity (Eddy; Cruz, 2007; Hicks et al. 2003). Meeske and colleagues (2004) reported that children with leukemia and brain tumor who had more problems with fatigue, also had lower HRQL. The HRQL was most negatively affected by the number, severity, and bothersomeness of symptoms (Baggot, et al, 2010). A study by Meeske and colleagues (2007) found that in cancer survivors (n=86; 8 to 18 years), higher levels of fatigue were associated with lower scores of quality of life in all dimensions (physical, social, emotional and school).
In a longitudinal study, Erickson and colleagues (2011) found moderate associations (r = −0.49 to −0.55, p <0.01) between fatigue, sleep and quality of life. At week 1, fatigue was associated with poor sleep quality and lower psychosocial functioning (p = −0.51, p <0.01). Daytime function (p = −0.49, p <0.01) and quality of sleep problems (p = −0.55, p <0.01) were associated with worse physical functioning. At week 2, daily function (p = −0.53, p <0.01) and cognitive fatigue (p = −0.55, p <0.01) were associated with worse HRQL. Sleep quality (p = −0.51, p <0.01) was also associated with lower HRQL. At week 4 and 5, sleep problems and daytime function were associated with worse psychosocial aspects of quality of life (p <0.01 r = −0.59).
In summary, fatigue continued to be problematic in children and adolescents with cancer, and had significant effects in HRQL. Studies to date were mostly from countries other than Brazil. It is therefore, unknown, whether fatigue experiences during hospitalization affects the HRQL in children and adolescents with cancer. Furthernmore, there is little information about the different aspects of fatigue and how different dimensions of HRQL are affect in children with and without fatigue. Therefore, we examined the different dimensions of fatigue (general, sleep/rest, and cognitive) and compared health related quality of life in pediatric patients with and without fatigue during hospitalization in Brazil.
THEORETICAL FRAMEWORK
The Symptom Management Theory (SMT) was used as the guiding framework for the study. The SMT has three interrelated components. The three components (symptom experience, symptom management, symptom outcomes) were considered to be highly interdependent and having concurrent simultaneous relationships (Dodd, Janson, et al., 2001; Humphreys et al., 2008). SMT presume that effective symptom management requires consideration of the three components. The first component is symptom experience, which consisted of the individual’s perception, evaluation, and response to a symptom. In this study, the fatigue experience during hospitalization was conceptualized as having three dimensions – general, sleep/rest, and cognitive.
The second component is symptom management, which described the “what, where, why, how much, to whom, and how” of interventions. Symptom management strategies may be designed depending on the individual symptom experiences (Dodd, Janson et al., 2001), and were intended to “avert, delay, or minimize” the symptom experience (Humphreys, et al., 2008). In this study, we did not examine interventions related to fatigue; however, understanding the different dimensions of fatigue – general, sleep/rest, and cognitive – may lead to designing specific details of the “what, where, why, how much, to whom, and how” of fatigue management strategies, that may be investigated in future studies.
The third component is symptom outcomes, which included symptom status, functional status, emotional status, self-care, costs, quality of life, morbidity and comorbidity, and mortality. These outcomes may change based on changes in the symptom experience, disease status, treatments, and symptom management strategies (Dodd, Janson, et al., 2001). In this study, we examined the functional status, emotional status, and quality of life. We also examined the relationship among fatigue experiences, functional status, and quality of life. Functional status was operationalized using the physical functioning and school functioning subscales of PedsQL. Emotional status was operationalized using psychosocial subscales of PedsQL(treatment anxiety, procedural anxiety, worries, communication).
The SMT also places symptoms in the context of the three domains of nursing science. First is the person domain that includes demographic, psychological, sociological, physiological, and developmental variables. The second is the environment domain that includes the physical, cultural, and social variables. The environment variables represent the “aggregate of conditions” in which a symptom occurs. The third is the health and illness domain that includes risk factors, health status, and disease and injury (Dodd, Janson, et al., 2001). In this study, we examined factors in the domains of person (age, gender), health/illness (cancer diagnosis, time since initiation of treatments, and reasons for hospitalization), and environment (hospital setting) that may explain variations in the symptom of fatigue and the outcomes of functional status, emotional status, and health related quality of life in children and adolescents with cancer in Brazil.
METHODS
Design
A cross-sectional study was used to collect information from children and adolescents with cancer. Children were asked to complete the PedsQL™ Multidimensional Fatigue Scale and PedsQL™ Quality of Life Inventory (generic core an cancer module) during hospitalization.
Sample & Setting
Participants were recruited from a pediatric oncology inpatient unit in a comprehensive cancer care hospital in southeast Brazil. They were included if the 1) age was 8 to 18 years, 2) parents were available for consenting, and 3) the child and parent were willing and able to complete the data collection procedures. Children were excluded if parents were previously informed that their child had prior history of neurological impairments (i.e., visual or hearing deficits, motor function deficit, and developmental delay), or if they were in the immediate postoperative period (< 5 days).
Procedures
All hospitalized children and adolescents who met the inclusion criteria were invited to participate. No child or adolescent refused to participate. Parents and children were informed about the details of the study in terms they were able to understand. They were given ample opportunity to ask questions and express concerns. Assent from the children and consent from the parents were obtained when both agreed to participate in the study. The study procedures were approved by the Institutional Review Board at the University of Sao Paulo and the participating hospital in Southeast Brazil. The child was given instructions on how to complete the fatigue and health related quality of life measures.
Instruments
Demographic data (age, gender) and medical information (diagnosis and reasons for hospitalization) were collected from parents at the time of enrollment. The following instruments were used to collect data.
Fatigue
The PedsQL™ Multidimensional Fatigue Scale (MFS) is a self-report tool (8 to 18 years) that was used to measure fatigue (Varni, Burwinkle, Katz, Meeske, & Dickinson, 2002). It consisted of three sub-scales representing the different dimensions of fatigue (general, sleep/rest and cognitive). The three dimensions had six items each representing the different dimensions. The responses consisted of questions related to how much of a problem each item is (0=never a problem; 1=almost never a problem; 2=sometimes a problem; 3=often a problem; 4=almost always a problem). The scale took less than 5 minutes to complete.
The MFS was scored using the instructions provided by the authors (Varni, Burwinkle, Szer, 2004). The items were reverse-scored and linearly transformed to a 0 to 100 scale (0=100, 1=75, 2=50, 3=25, 4=0), with lower scores indicating that fatigue was often or almost always a problem. Internal consistency reliability and construct validity for the MFS was established previously (Varni, Burwinkle, Szer, 2004; Varni, et al, 2002), and confirmed in Brazilian version. The Brazilian psychometric validation indicated Cronbach’s alpha values ranging between 0.70 to 0.90, except for the dimension of sleep/rest fatigue (0.55). Convergent validity was higher than 0.40 and divergent validity indicated 100% adjustment (Nascimento et al., 2014).
Health-Related Quality of Life (HRQL)
The Pediatric Quality of Life Inventory – Generic Core 4.0 (PedsQL™ generic) and the PedsQL™ Cancer Module 3.0 (PedsQL Cancer) were used to measure HRQL.
The PedsQL™ Generic Core 4.0 (self-report version 8 to 18 years) is an instrument designed to measure health-related quality of life in children and adolescents (Varni, et al, 2002). It consisted of four sub-scales representing the different dimensions of HRQL (physical functioning, emotional functioning, social, functioning and school functioning). Scoring procedures for the PedsQL is similar to the procedures described above for the PedsQL™ MFS. The lower scores indicated lower HRQL. The original validation of the PedsQL was established in children with cancer in the United States (Varni, Seid, Rode, 1999; Varni et al., 2002). Kaltchoian and colleagues (2008) translated and tested the psychometric properties of the Portuguese version in Brazilian children, and found that Cronbach’s alpha values were between 0.60 and 0.90 for all dimensions, demonstrating adequate internal consistency. Patients with rheumatic diseases reported significantly lower PedsQLTM scores on all dimensions when compared to the healthy control group (p < 0.0001), and thus, confirming construct validity (Klatchoian, et al., 2008).
The PedsQL ™ 3.0 Cancer Module (self-report version 8–18 years) is a disease specific instrument designed to measure the impact of treatment on symptoms and quality of life in pediatric patients with cancer. The scale consisted of eight dimensions: 1) pain and hurts, 2) nausea, 3) anxiety related to procedure, 4) anxiety related to treatment, 5) worries, 6) cognitive impairment, 7) perceived physical appearance, and 8) communication. The administration and scoring of the PedsQL Cancer Module was similar to the PedsQL™ MFS as described above, with lower scores indicating lower HRQL. The original PedsQL was developed by Varni and colleagues (1998) in English and was translated and validated in Portuguese by Scarpelli and colleagues (2008) in Brazilian children.
Data Analyses
All data were entered into SPSS® (version 22.0, Chicago, IL). All entries were double checked by two research staff. Descriptive statistics were used to summarize total and subscale (general, sleep-rest, and cognitive) fatigue scores and HRQL scores (generic and cancer modules). Pearson correlations were used to describe the relationship between fatigue and HRQOL scores. T-tests were used to compare HRQOL in patients with and without fatigue.
RESULTS
Demographic and Clinical Characteristics
A total of 38 children (8 to 12 years) and adolescents (13 to 18 years) participated. The mean age was 12.1 ± 2.9 years. About half of the participants were adolescents (52.6%) and were predominantly male (65.8%). The most frequent cancer diagnoses were sarcomas (34.2%) and leukemias/lymphomas (31.6%). The main reason for hospitalization was chemotherapy (52.6%); the majority had chemotherapy initiated within the previous 3 months (Table 1).
Table 1.
Sample demographic and clinical characteristics (n=38)
| Age (mean ± SD) | 12.1 ± 2.9 |
| Children (n=18; 8–12 years) | 9.7 ± 1.3 |
| Adolescents (n=20; 13–17 years) | 14.8 ± 1.4 |
| Gender | |
| Male | 25 (65.8%) |
| Female | 13 (34.2%) |
| Diagnoses | |
| Leukemias/Lymphomas | 12 (31.6%) |
| Sarcomas | 13 (34.2%) |
| Brain tumor | 6 (15.8%) |
| Others | 7 (18.4%) |
| Treatment | |
| Chemotherapy | 22 (57.9%) |
| Chemo + Surgery | 4 (10.5%) |
| Chemo + Radiotherapy | 2 (5.3%) |
| Chemo + Radiotherapy + Surgery | 6 (15.8%) |
| Surgery | 3 (7.9%) |
| Awaiting Decision | 1 (2.6%) |
| Time in chemotherapy | |
| < 1 month | 19 (50.0%) |
| 1 – 3 months | 9 (18.4%) |
| 4 – 6 months | 2 (5.3%) |
| 7 – 12 months | 3 (7.9%) |
| > 13 months | 3 (7.9%) |
| No chemo | 4 (10.5%) |
| Reason For Hospitalization | |
| Chemotherapy | 20 (52.6%) |
| Fever/Neutropenia/Infection | 8 (21.1%) |
| Other | 10 (26.3%) |
Fatigue
The mean total fatigue score was 63.8 ± 18.5 on 0 to 100 scale. The majority (66.7%) had scores below 75, suggesting that fatigue was “often or almost always a problem” (Table 2).
Table 2.
Fatigue scores
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| General fatigue | 66.6 ± 18.7 | 16.7 | 100 |
| Sleep/rest fatigue | 58.7 ± 20.8 | 0 | 95.8 |
| Cognitive fatigue | 66.7 ± 25.1 | 8.3 | 100 |
| Total fatigue | 63.8 ± 18.5 | 11.1 | 94.4 |
Scores range between 0 = “Always a Problem” to 100 = “No Problem at All”
Health Related Quality of Life
The mean HRQL (generic) total score was 61.1 ± 17.0 (Table 3). Scores were lower in school functioning, emotional, and physical functioning. The mean total HRQL (cancer) was 59.1 ± 16.7 (Table 4). The lowest score was “worries” (45.6 ± 30.5).
Table 3.
HRQL (Generic) scores
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Physical functioning | 57.9 ± 24.8 | 6.3 | 90.6 |
| Emotional functioning | 58.7 ± 21.6 | 15.0 | 100.0 |
| Social functioning | 73.7 ± 23.0 | 0 | 100.0 |
| Scholar functioning | 57.4 ± 22.0 | 0 | 90.0 |
| Total HRQL generic | 61.1 ± 17.0 | 32.6 | 89.1 |
Table 4.
HRQL (Cancer) scores
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Pain and hurt | 67.3 ± 29.3 | 0 | 100 |
| Nausea | 54.6 ± 23.0 | 0 | 90.0 |
| Procedural anxiety | 56.8 ± 31.6 | 0 | 100 |
| Treatment anxiety | 61.4 ± 30.0 | 0 | 100 |
| Worry | 45.6 ± 30.5 | 0 | 100 |
| Cognitive problems | 62.5 ± 22.8 | 5.0 | 100 |
| Physical appearance | 70.1 ± 24.3 | 8.3 | 100 |
| Communication | 65.0 ± 28.1 | 16.7 | 100 |
| Total HRQL cancer | 59.1 ± 16.7 | 20.4 | 90.7 |
Relationship between Fatigue and HRQL
Strong correlations were found between Total HRQL (Generic) and total fatigue (r=0.63, p <0.0001) scores. The correlations were also significant between total fatigue and the subscales of HRQL (Generic). The correlations were moderately strong in emotional functioning and school functioning (Table 5).
Table 5.
Correlations Between Fatigue and HRQL (Generic) Scores
| Total Fatigue and | r | p-values |
|---|---|---|
| Total HRQL (Generic) | 0.63 | 0.000 |
| Physical functioning | 0.38 | 0.018 |
| Social functioning | 0.38 | 0.017 |
| Emotional functioning | 0.51 | 0.001 |
| School functioning | 0.64 | 0.000 |
Strong correlations were found between Total HRQL (Cancer) and total fatigue (r=0.74, p <0.0001) scores. The correlations were also significant between total fatigue and the subscales of HRQL (Generic) for pain, nausea, both procedural and treatment anxiety, cognitive problems and communication (Table 6). The correlations were not significant for total fatigue and the “worries” and “physical appearance” subscales (Table 6).
Table 6.
Correlations Between Fatigue and HRQL (Cancer) Scores
| Total Fatigue and | r | p-values |
|---|---|---|
| Total HRQL (Cancer) | 0.74 | 0.000 |
| Pain and hurt | 0.60 | 0.000 |
| Nausea | 0.50 | 0.001 |
| Procedural anxiety | 0.62 | 0.000 |
| Treatment anxiety | 0.52 | 0.000 |
| Worries | 0.15 | 0.359 |
| Cognitive problems | 0.68 | 0.000 |
| Physical appearance | 0.06 | 0.715 |
| Communication | 0.47 | 0.004 |
HRQL in Patients With & Without Fatigue
Significant differences were found in HRQL (generic) between those with and without fatigue (total HRQL: 55.7 ± 15.5 vs 73.0 ± 14.1, p = 0.002). Significant differences were also found in the subscales: a) physical functioning (51.6 ± 26.2 vs 71.6 ± 14.4, p = 0.018), emotional functioning (52.3 ± 20.2 vs 72.5 ± 18.5, p = 0.006), and c) school functioning (49.6 ± 21.9 vs 72.9 ± 11.8, p = 0.002). No significant differences were found between those with and without fatigue in the social functioning subscale (70.4 ± 21.2 vs 80.8 ± 11.8, p = 0.198).
Significant differences were found in HRQL (Cancer) scores between those with and without fatigue (total fatigue: 54.8 ± 14.7 vs 83.4 ± 6.7, p < 0.0001, respectively) as indicated in Figure 1. There were also significant differences between those with and without fatigue in the subscales: a) general fatigue (58.7± 16.4 vs 81.9 ± 12.8, p = 0.000); b) sleep/rest fatigue (72.6 ± 13.7 vs 52.2 ± 20.6, p = 0.004), and cognitive fatigue (53.8 ± 19.4 vs 94.4, ± 6.0 p = 0.000).
Figure 1.
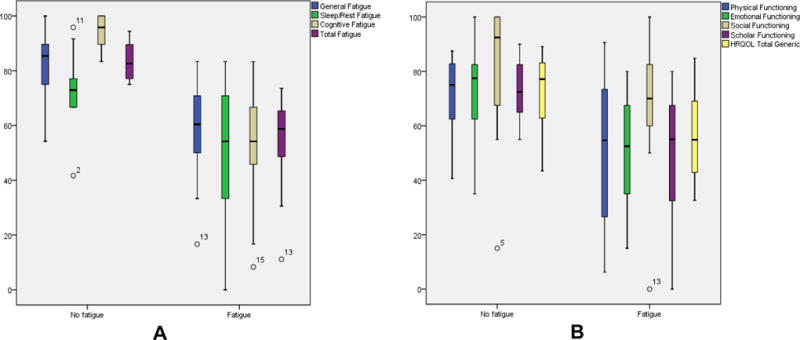
Fatigue Subscale Scores (A) and Quality of Life Subscale Scores (B) in Patient with No Fatigue and with fatigue
Explanatory Factors of Fatigue
Two models significantly explained variations in total fatigue scores. In the first model 81.1% of the variations in total fatigue scores were explained by diagnoses (p = 0.02), duration of chemotherapy (p <0.001), and total HRQL (cancer module; p = <0.001). More problems with fatigue were associated with brain tumor diagnosis (Table 7), had shorter time interval since initiation of chemotherapy (<6 months), and had lower total HRQL (cancer module).
Table 7.
Explanatory Factors of Fatigue
| Stimative | SD | Statistic | P | |
|---|---|---|---|---|
| (Intercept) | −0.73 | 9.05 | −0.081 | 0.94 |
| Sarcoma | −6.50 | 4.78 | −1.359 | 0.19 |
| Brain tumor | −14.63 | 5.72 | −2.559 | 0.02* |
| Other diagnoses | −6.91 | 5.88 | −1.175 | 0.25 |
| Time in chemotherapy | 0.03 | 0.01 | 3.089 | <0.00* |
| HRQL total cancer | 1.19 | 0.13 | 8.776 | <0,00* |
Multiple R-squared: 0.81, Adjusted R-squared: 0.77, F-statistic: 18.04 on 5 and 21 DF, p-value: 5.722e–07
In the second model, 89.6% of the variations in total fatigue were explained by cancer diagnoses (p = 0.01), duration of chemotherapy (p = 0.010), and the following subscales of HRQL (Cancer module): 1) pain and hurt (p = 0.001), 2) procedural anxiety (p <0.000), 3) cognitive problems (p = 0.001), and 4) perceived physical appearance (p = 0.010). More problems with fatigue (Table 8) was associated with sarcoma diagnoses and shorter time interval since initiation of treatment. More problems with fatigue were also associated with problems with pain, procedure anxiety, cognition, and perceived physical appearance. Age, gender, and reasons for hospitalizations were not associated with variations in total fatigue scores.
Table 8.
Linear regression, model 2
| estimative | SD | statistic | p | |
|---|---|---|---|---|
| (Intercept) | −7.67 | 9.93 | −0.77 | 0.451 |
| Sarcoma | −12.95 | 4.48 | −2.89 | 0.010* |
| Brain tumor | −9.66 | 5.71 | −1.69 | 0.110 |
| Other diagnoses | −1.71 | 5.79 | −0.29 | 0.771 |
| Time in chemotherapy | 0.025 | 0.01 | 2.83 | 0.011* |
| Pain and Hurt | 0.24 | 0.06 | 3.96 | 0.001* |
| Procedural anxiety | 0.41 | 0.06 | 6.52 | <0.000* |
| Cognitive problems | 0.36 | 0.09 | 3.99 | 0.001* |
| Perceived physical appearance | 0.23 | 0.08 | 2.77 | 0.013* |
Residual standard error: 7.515 on 16 degrees of freedom. Multiple R-squared: 0.8956, Adjusted R-squared: 0.8433 F-statistic: 17.15 on 8 and 16 DF, p-value: 1.746e-06
DISCUSSION
The study was the first to examine fatigue and HRQL in Brazilian children and adolescents with cancer during hospitalization. Our findings indicated that children and adolescents with cancer had problems with fatigue in the three dimensions (general, sleep/rest, cognitive). These findings were consistent with MFS scores reported during hospitalization by children and adolescents with cancer in other countries (Erickson, et al., 2011; Silva, 2009; Varni, et al., 2002 Hockenberry-Eaton et al., 2011; Miller, Jacob, Hockenberry, 2011; Walker et al., 2010; Wolfe et al., 2000; Yeh et al, 2008; Zupanec et al., 2010). It is possible that despite the cultural and socioeconomic differences of each country, fatigue continues to be problematic because the disease characteristics, disease related symptoms, treatment protocols, and treatment related effects were similar.
Several factors may explain fatigue problems during hospitalization in children and adolescents with cancer worldwide. Fatigue problems during hospitalization may be attributed to higher chemotherapy intensity and treatment related symptoms, compared with outpatient treatments (Collins, et al, 2000). Medical procedures during hospitalization such as lumbar punctures, bone marrow aspirations, and repetitive blood draws were causing pain and stress, leading to fatigue (Perdikaris et al, 2008). Adolescents reported that the hospital environment and the lack of interesting activities were the main causes of fatigue (Hockenberry-Eaton et al, 2000; Perdikaris et al., 2009). Changes in physical activity (Perdikaris et al., 2009) and negative emotional changes such as feeling anger and sadness were also reported to lead to fatigue experiences during hospitalization (Perdikaris et al, 2009; Hockenberry-Eaton et al., 1998). From the parent perspectives, it was not only the physical environment, decreased activities, and negative emotions, but also anorexia caused by chemotherapy that explained fatigue experiences in their children with cancer (Perdikaris et al., 2009).
Our findings indicated that hospitalized children and adolescents with cancer, had more problems with sleep/rest fatigue than in other dimensions, as reported in other studies (Palmer et al, 2011; Silva, 2009; Varni et al, 2002). The biggest problems with sleep/rest fatigue may be attributed to interrupted sleep caused by noises inherent to hospital, or due to presence of concomitant fatigue symptoms such as pain, nausea, vomiting, diarrhea or increased urination, which were previously reported and may be responsible for frequent interruptions in sleep (Lee et al, 2004; Lee & Landis, 2003; Morrow, 2007). Changes in the child’s sleep environment and changes in routines during hospitalization were previously described as causing disruption in the cycles of sleep/rest, and may possibly contribute to sleep/rest fatigue during hospitalization (Hinds et al. 2007b). A series of studies reported that environmental factors, such as noises from telephone rings, alarms from infusion pumps, crying infants and young children, constant activity at the nursing station, slamming doors and frequent interruptions were causing sleep disruptions and changes in sleep patterns that lead to fatigue experiences (Hinds et al., 1999; Hockenberry-Eaton et al, 1998 Hockenberry-Eaton, Hinds, 2000).
Our findings also indicated that hospitalized children and adolescents with cancer had low scores in all dimensions of HRQOL in both the generic and cancer modules. Consistent with our findings, children with cancer in other countries also reported low HRQL scores (Baggot et al., 2010; De Bolle et al., 2009; Erickson et al., 2011), compared to healthy children (Klatchoian, et al, 2010; Varni, et al, 2003). Brazilian studies using qualitative methodologies showed that after bone marrow transplant, children with cancer had life permeated by insecurity, altered body image, and physical and emotional problems (Andres, Lima, 2004), which may explain low HRQOL scores.
It is interesting that participants in our study showed greater problems in the physical, emotional, and school functioning, but less problems in social functioning. According Zareifar and colleagues (2012), disease and treatment related effects, adversely affected the HRQL, in physical, psychological, and emotional spheres. Consistent with findings in our study, Baggot and colleagues (2010) found lower scores in overall HRQL, physical functioning and school functioning in children with cancer undergoing chemotherapy, when compared to healthy children,.
The physical functioning dimension contributed to decreased HRQL due to restrictions that children/adolescents suffer during treatment. Some examples that restricted physical functioning included neutropenia related infections, chemotherapy induced anemia, chemotherapy related nausea, and fatigue (Scarpelli et al., 2008). Polo and Moraes (2009) reported that patients who were physically active had fewer symptoms, had longer survival, and showed better response to treatments when compared with those who were physically inactive and had greater symptomatology. These findings suggest the possibility that strategies for improving physical functioning may lead to improvement in HRQOL.
Consistent with findings from studies in different countries (Scarpelli et al., 2008; Erickson, et al, 2011; Van Litsenberg, et al, 2011), our findings indicated low HRQOL scores in the cancer module. Our findings indicated that children and adolescents had decreased psychosocial functioning with worse scores on “worries” and problems with “perceived physical appearance”.
The psychosocial functioning dimension were associated with frequent hospitalizations leading to negative emotional, social, and poor school performance in children. The cancer diagnoses generated negative feelings such as isolation, anger, guilt, shame, loneliness, apathy, confusion, fear of death, fear of disease recurrence, and fear of medical procedures (Pedreira; Palanca, 2013). Furthermore, the type of cancer and the recommended treatments led to feelings of distress, increased physical stress, and psychosocial limitations (Zebrack et al., 2004). Frequent hospitalizations separated children from family, school, and social environment leading to negative repercussions. In addition, medical invasive and painful procedures created anxiety, stress and feelings of helplessness (Pedreira; Palanca, 2007). School attendance were sporadic, and more frequently, the children stopped attending school, which negatively interfered with academic performance (Cicogna; Nascimento; Lima, 2010).
Consistent with findings by others (Meeske, et al, 2007; Erickson, et al, 2011), we found significant correlations between fatigue and HRQOL. There were significant differences in the physical, emotional, and school functioning, but not in social functioning, between those with and without fatigue. The different dimensions of fatigue (general, sleep/rest, cognitive) were worse in those with high fatigue scores compared with those reporting no problems with fatigue.
Limitations
The study has several limitations. First, the sample size was small, which limits generalizability of findings. Future research with larger sample sizes are needed to validate the findings. Second, data were collected from one pediatric oncology unit of a hospital in the state of São Paulo, Brazil. Therefore, generalization and representativeness to other settings and locations were limited. Third, cancer diagnoses and treatment protocols varied greatly, which may affected the reported levels of fatigue and quality of life. It was not possible to compare fatigue and HRQOL by diagnoses and treatment protocols due to the small number of participants. Fourth, the study design was cross-sectional, and therefore, it was not possible to examine changes in fatigue and HRQL over time. Future studies are needed to investigate the different aspects of fatigue and the different dimensions of HRQOL during the different phases of the chemotherapy, specifically during induction, consolidation, intensification, and maintenance. Longitudinal studies are also needed to examine fatigue at different times of day (morning, afternoon, evening) and day of week (weekdays, weekends), and the in different places (hospital, ambulatory clinics, home). These studies could lead to design of individually tailored strategies for fatigue management that could lead to improvement in quality of life in children and adolescents with cancer.
Implication for Nursing
Findings from the study support the recommendation for comprehensive fatigue assessment (general, sleep/rest, cognitive) and to incorporate individualized strategies that could lead to improvement in the different dimensions of HRQOL (physical, emotional, social, cognitive). Nurses may provide instructions to parents about the different aspects of fatigue and recommend personalized strategies for fatigue management and improving HRQOL. Strategies to reduce fatigue may include exercise or physical activity (Genc et al, 2008; Oncology Nursing Society, 2017; Rosenhagen et al, 2011; Yeh et al, 2011), leisure activities such as reading, drawing, listening to music (Genc et al, 2008), healing touch (Wong et al, 2013), and psychosocial interventions (Chang, et al, 2013). Customized interventions addressing the different aspects of fatigue and outlining the “what, where, why, how much, to whom, and how” of interventions as suggested in the Symptom Management Theory may be developed and tested in future studies. In addition, Hockenberry and colleagues (2010) proposed the symptom cluster of fatigue, nausea and vomiting, and sleep disturbances. The symptoms of fatigue and sleep disturbance clustered together, suggesting that interventions targeted to improve sleep hygiene may decrease fatigue experiences. Recommendations for sleep hygiene include the establishment of a regular uninterrupted sleep schedule during hospitalization, using daytime naps with care, and minimizing nocturnal awakenings at bedtime (Hinds, et al, 2007b). Furthermore, limiting worry, limiting exposure to light in the hours before sleep and avoiding other stimulants (television, videogames, computer use, and noise from alarms, infusion pumps, door slamming, etc) in the hours before bedtime may promote restorative and restful sleep during hospitalization.
CONCLUSION
We examined fatigue and HRQL in Brazilian children and adolescents with cancer during hospitalization. Our findings indicated that children and adolescents with cancer had problems with fatigue in the three dimensions (general, sleep/rest, cognitive) that affected HRQOL in both the generic and cancer modules. Significant differences were found in the physical, emotional, and school functioning, but not in social functioning, between those with and without fatigue. We recommend comprehensive fatigue assessment (general, sleep/rest, cognitive) and incorporation of individualized strategies that could lead to improvement in the different dimensions of HRQOL (physical, emotional, social, cognitive). Instructions may be given to parents about the different aspects of fatigue and recommend personalized strategies for fatigue management and improving HRQOL. Future studies are recommended to examine interventions (exercise, leisurely activities, sleep hygiene) that may alleviate fatigue and improve HRQL in children and adolescents with cancer in Brazil.
Highlights.
This paper presents a quantitative study about fatigue and health related quality of life in hospitalized children and adolescent with cancer.
Our data show fatigue and quality of life problems in this population.
There were significant correlations between total fatigue and quality of life (generic and cancer modules).
Although the results confirm fatigue and quality of life problems in children and adolescents with cancer, there are few studies that suggest intervention to minimize these problems.
Acknowledgments
Funding for the study was provided in part by the São Paulo Research Foundation (Nº 2010/20055-6 and Nº 2012/00091-3), National Council for Scientific and Technological Development (Nº 486239/2013-6) and research grants (E. Jacob) from the Alex’s Lemonade Stand Foundation, the UCLA Center for Vulnerable Populations Research, and National Institute of Nursing Research (Nº P30NR005041). Collaboration between Brazil and USA was made possible by the Mayday Foundation and its partnership with the Pain in Child Health, a research training initiative funded by the Canadian Institute of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baggott CR, et al. An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Supportive Care in Cancer. 2010;19(3):353–61. doi: 10.1007/s00520-010-0824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, et al. Systematic review and meta-Analysis analysis of nonpharmacological interventions for fatigue in children and adolescents with cancer. Worldviews Evidence-Based Nursing. 2013;10(4):208–17. doi: 10.1111/wvn.12007. [DOI] [PubMed] [Google Scholar]
- Cicogna EC, Nascimento LC, Lima RAG. Crianças e adolescentes com câncer: experiências com a quimioterapia. Revista Latino-Americana de Enfermagem. 2010;18(5):864–72. doi: 10.1590/s0104-11692010000500005. [DOI] [PubMed] [Google Scholar]
- Collins JJ, et al. The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management. 2000;19(5):363–377. doi: 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Costa JC, Lima RAG. Children and adolescents in outpatient clinic chemotherapy: nursing implications. Revista Latino-Americana de Enfermagem. 2002;10(3):321–33. [PubMed] [Google Scholar]
- Bolle De M, et al. Self- and parental perspectives on quality of life in children with cancer. Journal of Psychosocial Oncology. 2008;26:35–47. doi: 10.1300/j077v26n02_03. [DOI] [PubMed] [Google Scholar]
- Eddy L, Cruz M. The relationship between fatigue and quality of life in children with chronic health problems: a systematic review. Journal for Specialists in Pediatric Nursing. 2007;12(2):105–14. doi: 10.1111/j.1744-6155.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Enskar K, Ljusegren G, Berglund G, Eaton N, Harding R, et al. Attitudes to and knowledge about pain and pain management, of nurses working with children with cancer: A comparative study between UK, South Africa, and Sweden. Journal of Nursing Research. 2007;12:501–515. [Google Scholar]
- Erickson JM, et al. Fatigue, sleep-wake disturbances, and quality of life in adolescents receiving chemotherapy. Journal of Pediatric Hematology/Oncology. 2011;33(1):17–25. doi: 10.1097/MPH.0b013e3181f46a46. [DOI] [PubMed] [Google Scholar]
- Fayers PM, Machin D. Quality of life the assessment, analysis and interpretation of patient-reported outcomes. 2°. Chichester, England: Jonh Wiley & Sons; 2007. p. 566. [Google Scholar]
- Gedaly-Duf FV. Pain, sleep disturbance, and fatigue in children with leukemia and their parents: a pilot study. Oncology Nursing Forum, New York. 2006;33(3):641–6. doi: 10.1188/06.ONF.641-646. [DOI] [PubMed] [Google Scholar]
- Genc RE, Conk Z. Impact of effective nursing interventions to the fatigue syndrome in children who receive chemotherapy. Cancer Nursing, New York. 2008;31(4):312–7. doi: 10.1097/01.NCC.0000305740.18711.c6. [DOI] [PubMed] [Google Scholar]
- Gibson F, et al. Heavy to carry: a survey of parents and healthcare professionals’ perceptions of cancer related fatigue in children and young people. Cancer Nursing. 2005;28:27–35. doi: 10.1097/00002820-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Hicks J, Bartholomew J, Ward-Smith P, Hutto CJ. Quality of life among childhood leukemia patients. Journal of Pediatric Oncology Nursing. 2003;20(4):192–200. doi: 10.1177/1043454203253969. [DOI] [PubMed] [Google Scholar]
- Hinds PS, et al. Comparing patient, parent and staff descriptions of fatigue in pediatric oncology patients. Cancer Nursing. 1999;22(4):277–289. doi: 10.1097/00002820-199908000-00004. [DOI] [PubMed] [Google Scholar]
- Hinds PS, et al. Clinical field testing of an enhanced-activity intervention in hospitalized children with cancer. Journal of Pain and Symptom Management, Madison. 2007a;33(6):686–97. doi: 10.1016/j.jpainsymman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Hinds PS, et al. Nocturnal awakenings, sleep environment interruptions, and fatigue in hospitalized children with cancer. Oncology Nursing Forum. 2007b;34(32):393–402. doi: 10.1188/07.ONF.393-402. [DOI] [PubMed] [Google Scholar]
- Hockenberry M, Hooke MC. Symptom clusters in children with cancer. Seminars in Oncology Nursing. 2007;23:152–157. doi: 10.1016/j.soncn.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hooke MC, Gregurich M, McCarthy K, Sambuco G, Krull K. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum. 2010;37:E16–E27. doi: 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton MJ, et al. Fatigue in children and adolescents with cancer. Journal of Pediatric Oncology Nursing. 1998;15(3):172–182. doi: 10.1177/104345429801500306. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton MJ, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. Journal of Pain and Symptom Management. 2003;25(4):319–28. doi: 10.1016/s0885-3924(02)00680-2. [DOI] [PubMed] [Google Scholar]
- Hockenberry-Eaton M, Hinds P. Fatigue in children and adolescents with cancer: evolution of a program of study. Seminars in Oncology Nursing. 2000;16:261–272. doi: 10.1053/sonu.2000.16577. [DOI] [PubMed] [Google Scholar]
- Jacob E, Hesselgrave J, Sambuco G, Hockenberry M. Variations in pain, sleep, and activity during hospitalization in children with cancer. Journal of Pediatric Oncology Nursing. 2007;24:208–219. doi: 10.1177/1043454207299875. [DOI] [PubMed] [Google Scholar]
- Jacob E, Mccarthy KS, Sambuco G, Hockenberry M. Intensity, location, and quality of pain in Spanish-speaking children with cancer. Pediatric Nursing. 2008;34:45–52. [PubMed] [Google Scholar]
- Jalmsell L, et al. Symptoms affecting children with malignancies during the last month of life: a nationwide follow-up. Pediatrics. 2006;117:1314–20. doi: 10.1542/peds.2005-1479. [DOI] [PubMed] [Google Scholar]
- Klatchoian DA, et al. Quality of life of children and adolescents from São Paulo: reliability and validity of the Brazilian version of the Pediatric Quality of Life Inventory™ version 4.0 Generic Core Scales. Jornal de pediatria. 2008;84(4):308–315. doi: 10.2223/JPED.1788. [DOI] [PubMed] [Google Scholar]
- Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Medicine Reviews. 2004;8:199–212. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lee K, Landis CA. Priorities for sleep research during the next decade. Research in Nursing and Health. 2003;26(3):175–176. doi: 10.1002/nur.10085. [DOI] [PubMed] [Google Scholar]
- Mccabe M. Fatigue in children with long-term conditions: an evolutionary concept analysis. Journal of Advanced Nursing. 2009;65(8):1735–45. doi: 10.1111/j.1365-2648.2009.05046.x. [DOI] [PubMed] [Google Scholar]
- Meeske K, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101(9):2116–25. doi: 10.1002/cncr.20609. [DOI] [PubMed] [Google Scholar]
- Meeske K, et al. Factors associated with health-related quality of life in pediatric cancer survivors. Pediatric Blood & Cancer. 2007;49(3):298–305. doi: 10.1002/pbc.20923. [DOI] [PubMed] [Google Scholar]
- Menezes MFB, Camargo TC. Cancer-related fatigue as a thematic issue in oncology nursing. Revista Latino-Americana de Enfermagem. 2006;14(3):442–7. doi: 10.1590/s0104-11692006000300020. [DOI] [PubMed] [Google Scholar]
- Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncology Nursing Forum. 2011;38(5):E382–393. doi: 10.1188/11.ONF.E382-E393. [DOI] [PubMed] [Google Scholar]
- Morrow GR. Cancer-related fatigue: causes, consequences, and management. Oncologist. 2007;12(Suppl 1):1–3. doi: 10.1634/theoncologist.12-S1-1. [DOI] [PubMed] [Google Scholar]
- Mota DDCCF, Pimenta CA. Fatigue in patients with advanced cancer: concept, assesment and management. Revista Brasileira de Cancerologia. 2002;48(4):577–583. [Google Scholar]
- Oncology Nursing Forum. Fatigue. 2017 https://www.ons.org/practice-resources/pep/fatigue (accessed 06.03.17)
- Palmer SN, et al. The PedsQL Brain Tumor Module: initial reliability and validity. Pediatric Blood & Cancer. 2007;49(3):287–293. doi: 10.1002/pbc.21026. [DOI] [PubMed] [Google Scholar]
- Pedreira JL, Palanca I. Psicooncología pediátrica. 2013 http://www.psicooncologia.org/profesionales.php (accessed 29.07.13)
- Perdikaris P, et al. Changes in children’s fatigue during the course of treatment for paediatric cancer. International Nursing Review. 2008;55(4):412–19. doi: 10.1111/j.1466-7657.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- Perdikaris P, et al. Evaluating cancer related fatigue during treatment according to children’s, adolescents’ and parents’ perspectives in a sample of Greek young patients. European Journal of Oncology Nursing. 2009;13(5):399–408. doi: 10.1016/j.ejon.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Poder U, Ljungman G, Von Essen L. Parents’ perceptions of their children’s cancer-related symptoms during treatment: a prospective, longitudinal study. Journal of Pain and Symptom Management. 2010;40(5):661–670. doi: 10.1016/j.jpainsymman.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Polo LHV, de Moraes MW. The Zubrod performance status and the Karnofsky index in quality of life evaluation of children with cancer. Einstein. 2009;7:314–21. [Google Scholar]
- Rheingans JL. Pediatric oncology nurses’ management of patients’ symptoms. Journal of Pediatric Oncology Nursing. 2008;25:303–311. doi: 10.1177/1043454208323294. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, et al. Cancer-related sources of stress for children with cancer and their parents. Journal of Pediatric Psychology. 2012;37(2):185–197. doi: 10.1093/jpepsy/jsr054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhagen A, et al. Implementation of structured physical activity in the pediatric stem cell transplantation. Klinische Paediatrie. 2011;223(3):147–51. doi: 10.1055/s-0031-1271782. [DOI] [PubMed] [Google Scholar]
- Scarpelli AC, et al. The Pediatric Quality of Life Inventory™ (PedsQL™) family impact module: reliability and validity of the Brazilian version. Health and Quality of Life Outcomes. 2008;6(1):35. doi: 10.1186/1477-7525-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RZM. Evaluation of fatigue in young cancer survivors and the correlation with depressive symptoms, sleep disturbances and variable clinics [Tesis] Fundação Antônio Prudente. São Paulo: 2009. p. 111. [Google Scholar]
- Stasi R, et al. Fadiga relacionada ao câncer: evolução conceitos em avaliação e tratamento. Cancer. 2003;98:1786–1801. [Google Scholar]
- Sung L, et al. Identification of paediatric cancer patients with poor quality of life. Britsh Journal of Cancer. 2009;100:82–88. doi: 10.1038/sj.bjc.6604826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen JM, et al. Symptoms in the palliative phase of children with cancer. Pediatric Blood & Cancer. 2007;49:160–5. doi: 10.1002/pbc.21042. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, et al. Parent reports of quality of life for pediatric patients with cancer with no realistic chance of cure. Journal of Clinical Oncology. 2011;29(6):639–45. doi: 10.1200/JCO.2010.31.4047. [DOI] [PubMed] [Google Scholar]
- Ullrich CK, et al. Fatigue in children with cancer at the end of life. Journal of Pain and Symptom Management. 2010;40(4):483–494. doi: 10.1016/j.jpainsymman.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Ullrich NJ, Embry L. Neurocognitive dysfunction in survivors of childhood brain tumors. Seminars in Pediatric Neurology. 2012;19(1):35–42. doi: 10.1016/j.spen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Van Litsenburg RRL, et al. Impaired sleep affects quality of life in children during maintenance treatment for acute lymphoblastic leukemia: an exploratory study. Health and Quality of Life Outcomes. 2011;9(1):25–31. doi: 10.1186/1477-7525-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varni JW, et al. The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94:2090–106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical Care. 1999;37:126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Katz ER. The PedsQL™ in pediatric cancer pain: A prospective longitudinal analysis of pain and emotional distress. Developmental and Behavioral Pediatrics. 2004;25:239–246. doi: 10.1097/00004703-200408000-00003. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™ 4.0 as a Pediatric Population Health Measure: Feasibility, Reliability, and Validity. Ambulatory Pediatrics. 2003;3(6):329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Walker AJ, Gedaly-Duff V, Miaskowski C, Nail L. Differences in symptom occurrence, frequency, intensity, and distress in adolescents prior to and one week after the administration of chemotherapy. Journal Pediatric Oncology Nursing. 2010;25(5):259–65. doi: 10.1177/1043454210365150. [DOI] [PubMed] [Google Scholar]
- Williams PD, et al. A symptom checklist for children with cancer: the Therapy-Related Symptom Checklist-Children. Cancer Nursing. 2012;35(2):89–98. doi: 10.1097/NCC.0b013e31821a51f6. [DOI] [PubMed] [Google Scholar]
- Wolfe J, et al. Symptoms and suffering at the end of life in children with cancer. New England Journal of Medicine. 2000;342:326–33. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- Wong J, et al. The impact of healing touch on pediatric oncology patients. Integrative Cancer Therapy. 2013;12:25–30. doi: 10.1177/1534735412446864. [DOI] [PubMed] [Google Scholar]
- Wu HS, Mcsweeney M. The assessment and measurement of fatigue in people with cancer. In: Armes J, Krishnasamy M, Higginson I, editors. Fatigue in cancer. Oxford: Oxford University; 2004. pp. 193–221. [Google Scholar]
- Yeh CH, et al. Clinical factors associated with fatigue over time in paediatric oncology patients receiving chemotherapy. Britsh Journal of Cancer. 2008;99(1):23–29. doi: 10.1038/sj.bjc.6604434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, et al. A pilot study to examine the feasibility and effects of a home-based aerobic program on reducing fatigue in children with acute lymphoblastic leukemia. Cancer Nursing. 2011;34(1):3–12. doi: 10.1097/NCC.0b013e3181e4553c. [DOI] [PubMed] [Google Scholar]
- Zareifar S, et al. Evaluation of Health Related Quality of Life in 6–18 Years Old Patients with Acute Leukemia during Chemotherapy. Indian Journal of Pediatrics. 2012;79(2):177–182. doi: 10.1007/s12098-011-0483-0. [DOI] [PubMed] [Google Scholar]
- Zebrack BJ, et al. Resultados psicológicos em longo prazo sobreviventes de câncer cerebral na infância: um relatório do estudo sobrevivente do câncer infantil. Journal of Clinical Oncology. 2004;22(6):999–1006. [Google Scholar]
- Zupanec S, Jones H, Stremler R. Sleep habits and fatigue of children receiving maintenance chemotherapy for all and their parents. Journal of Pediatric Oncology Nursing. 2010;27(4):217–28. doi: 10.1177/1043454209358890. [DOI] [PubMed] [Google Scholar]


