Abstract
BACKGROUND
Effective treatment options for patients with chronic myeloid leukemia (CML) or Philadelphia‐positive (Ph+) acute lymphoblastic leukemia (ALL) who have the threonine to isoleucine mutation at codon 315 (T315I) are few. The objective of this study was to compare overall survival (OS) between patients with CML and those with Ph+ ALL who received treatment with ponatinib versus allogeneic stem cell transplantation (allo‐SCT).
METHODS
A post hoc, retrospective, indirect comparison of OS among patients who received single‐agent ponatinib in the Ponatinib Ph+ ALL and CML Evaluation (PACE) trial with those who underwent allo‐SCT as reported to the European Bone Marrow Transplant registry, stratified by CML disease phase and Ph+ ALL, was conducted. Kaplan‐Meier survival curves and multivariate Cox proportional‐hazards models were used to compare OS between intervention groups, adjusting for time from diagnosis to intervention, age, sex, and geographic region; 24‐month and 48‐month OS rates and median OS were reported.
RESULTS
After adjustment for potential confounders, 24‐month and 48‐month OS rates were significantly higher in patients with chronic‐phase CML (CP‐CML) who received ponatinib compared with those who underwent allo‐SCT (24 months: 84% vs 60.5%, respectively; P = .004; 48 months: 72.7% vs 55.8%, respectively; P = .013), with a hazard ratio (HR) of 0.37 (95% confidence interval [CI], 0.16‐0.84; P = .017). In patients who had accelerated‐phase CML, OS rates were not significantly different between the groups (HR, 0.90; 95% CI, 0.20‐4.10; P = .889). In patients who had blast‐crisis CML and those with Ph+ ALL, ponatinib was associated with shorter OS compared with allo‐SCT (blast‐crisis CML: HR, 2.29 [95% CI, 1.08‐4.82; P = .030]; Ph+ ALL: HR, 2.77 [95% CI, 0.73‐10.56; P = .146]).
CONCLUSIONS
Although allo‐SCT remains an important treatment option for patients with T315I‐positive advanced CML and Ph+ ALL, ponatinib represents a valuable alternative for patients with T315I‐positive CP‐CML. Cancer 2017;123:2875–80. © 2017 American Cancer Society.
Keywords: allogeneic stem cell transplantation (allo‐SCT), chronic myeloid leukemia (CML), Philadelphia chromosome‐positive acute lymphoblastic leukemia (Ph+ ALL), ponatinib, threonine to isoleucine mutation at codon 315 (T315I)
Short abstract
In patients who have chronic‐phase chronic myeloid leukemia (CML) with the Philadelphia chromosome threonine to isoleucine mutation at codon 315, single‐agent ponatinib is associated with significantly longer overall survival compared with allogenic stem cell transplantation. In those who have accelerated‐phase CML, blast‐crisis CML, and Philadelphia chromosome‐positive acute lymphoblastic leukemia with the T315I mutation, single‐agent ponatinib is associated with similar or shorter overall survival compared with stem cell transplantation.
INTRODUCTION
Tyrosine kinase inhibitors (TKIs) represent the standard treatment for patients with chronic myeloid leukemia (CML) and Philadelphia chromosome‐positive (Ph+) acute lymphoblastic leukemia (ALL); and, in the latter patients, TKIs are frequently used in combination with chemotherapy.1, 2 A threonine‐to‐isoleucine substitution at position 315 (T315I mutation), the gatekeeper residue of the Abelson murine leukemia viral oncogene homolog (ABL) kinase domain, is identified in approximately 20% of patients with resistant or relapsed CML3, 4 and confers resistance to most TKIs indicated for CML treatment, such as imatinib, dasatinib, bosutinib, and nilotinib.5 Ponatinib is approved in the United States and the European Union for adult patients with refractory CML or Ph+ ALL and those with the BCR‐ABL T315I mutation, and is now the only effective TKI for treating CML or Ph+ ALL in T315I‐positive patients.3, 4, 6 Recently, it was demonstrated that omacetaxine mepesuccinate, a first‐in‐class cephalotaxine, also has inhibitory activity in TKI‐resistant CML stem cells and provides a benefit to patients who have T315I‐positive chronic phase (CP)‐CML as a single agent or in combination with a TKI.7 However, omacetaxine mepesuccinate was not considered in the current analysis, which focused on a comparison between ponatinib and allogeneic stem cell transplantation (allo‐SCT).
Before the approval of ponatinib, patients with CML and Ph+ ALL who were resistant to imatinib and harbored the T315I mutation had a poor prognosis and significantly shorter survival compared with those who did not have this mutation.8, 9 Allo‐SCT has been considered standard therapy for CML over many decades. However, ponatinib may present an alternative to allo‐SCT in T315I‐positive patients.6 No prospective trial has compared outcomes of T315I‐positive patients who received with ponatinib relative to those who underwent allo‐SCT. This study is a retrospective, post hoc comparison of overall survival (OS) among T315I‐positive patients who received ponatinib in a phase 2 trial versus those who underwent allo‐SCT as reported to the European Group for Blood and Marrow Transplantation (EBMT) registry.
MATERIALS AND METHODS
Data were pooled from the Ponatinib Ph+ ALL and CML Evaluation (PACE) trial6 and the EBMT registry10 to conduct an indirect comparison of ponatinib versus allo‐SCT. PACE is a multicenter, international, open‐label, single‐arm, phase 2 trial among patients with CML and Ph+ ALL who are resistant to or intolerant of dasatinib or nilotinib or who have the T315I mutation. Of 449 patients enrolled in PACE from September 2010 to October 2011, 128 harbored the T315I mutation at enrollment. The EBMT registry collects data on demographics, treatments, mutations, and clinical outcomes in patients who undergo SCT. EBMT data were available from 2000 through 2010. Sixty‐nine patients from the EBMT registry who underwent allo‐SCT for CML and Ph+ ALL and were identified with the T315I mutation and TKI resistance at baseline were eligible for the study.
All 128 T315I‐positive patients from PACE comprised the ponatinib group of this study. Fifty‐six patients from the EBMT database comprised the allo‐SCT group, because they had complete data for all variables used in the analysis. Both the ponatinib and allo‐SCT cohorts consisted of T315I‐positive patients aged ≥ 18 years in any phase of CML or with Ph+ ALL. Patients who underwent allo‐SCT in their second CP were excluded, and no patients who received ponatinib were in their second CP. In addition, no patients in the EBMT database had received ponatinib before undergoing allo‐SCT. The index date was defined as the date of intervention (the date of treatment initiation with ponatinib among patients from the PACE trial and the date of allo‐SCT among patients from the EBMT registry). Patients were followed from the index date until the end of observation (the earliest of death, loss to follow‐up, or the end of data availability). Baseline (pre‐index) demographic and clinical characteristics were compared between intervention groups using the Wilcoxon rank‐sum test for continuous variables and the chi‐square or Fisher's exact test for categorical variables. Adjusted Kaplan‐Meier (KM) survival curves11 and multivariate Cox proportional‐hazards models were used to compare OS between the intervention groups; 24‐month and 48‐month OS rates and median OS were reported. Comparisons were adjusted for the time from diagnosis to intervention, age, sex, and geographic region using inverse probability of treatment weights, which were estimated separately for each disease phase. It was especially important to include the adjustment variable “time from diagnosis to intervention,” because large differences in this variable (which may also serve as a proxy for residual disease) may confound the results. The inverse probability of treatment weighting method used propensity scores to build stabilized weights that balanced the distribution of covariates between intervention groups while preserving sample size.12, 13 P values were calculated using the log‐rank test for KM survival curves and the Wald chi‐square test for hazard ratios (HRs). Results were stratified by phase of CML or Ph+ ALL. All analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
One‐hundred eighty‐four patients (128 in the ponatinib group and 56 in the allo‐SCT group) were included in the analysis, consisting of 90 patients in CP‐CML (64 in the ponatinib group, 26 in the allo‐SCT group), 26 in accelerated phase (AP)‐CML (18 in the ponatinib group, 8 in the allo‐SCT group), 41 in blast crisis (BC)‐CML (24 in the ponatinib group, 17 in the allo‐SCT group), and 27 with Ph+ ALL (22 in the ponatinib group, 5 in the allo‐SCT group). Patients who received treatment with ponatinib were older on the index date (mean age, 53 vs 45 years; P = .006) and were more likely to be from North America (43.8% vs 26.8%; P = .030) than patients in the allo‐SCT group, as depicted in Table 1. Of 56 patients in the allo‐SCT group, 42 (75.0%) underwent transplantation from matched donors, and 9 (16.1%) underwent transplantation from related donors. Six patients (10.7%) underwent cord blood transplantation.
Table 1.
Baseline Characteristicsa
| All Phases: Pooled | CP‐CML | AP‐CML | BC‐CML | Ph + ALL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Ponatinib N = 128 | allo‐SCT N = 56 | P b | Ponatinib N = 64 | allo‐SCT N = 26 | P b | Ponatinib N = 18 | allo‐SCT N = 8 | P b | Ponatinib N = 24 | allo‐SCT N = 17 | P b | Ponatinib N = 22 | allo‐SCT N = 5 | P b |
| Descriptive characteristics | |||||||||||||||
| Age at diagnosis: Mean ± SD, yc | 47.5 ± 17.2 | 42.3 ± 14.6 | .064 | 47.5 ± 15.9 | 45.2 ± 14.5 | .666 | 47.0 ± 17.2 | 42.2 ± 16.5 | .460 | 42.4 ± 18.0 | 40.6 ± 15.4 | .743 | 53.3 ± 19.1 | 34.0 ± 5.4 | .098 |
| Median [IQR] | 49 [34‐61] | 42 [30‐56] | — | 47 [37‐59] | 45 [37‐56] | — | 52 [31‐56] | 43 [30‐54] | — | 38 [27‐60] | 42 [30‐58] | — | 62 [36‐69] | 37 [30‐37] | — |
| Age at index date: Mean ± SD, y | 52.5 ± 17.3 | 45.6 ± 13.2 | .006d | 53.2 ± 16.8 | 48.3 ± 12.7 | .202 | 54.6 ± 16.4 | 45.8 ± 15.8 | .244 | 46.5 ± 17.5 | 44.1 ± 13.5 | .555 | 55.3 ± 18.7 | 35.7 ± 6.3 | .087 |
| Median [IQR] | 53 [40‐67] | 45 [38‐57] | 52 [43‐66] | 48 [40‐58] | — | 54 [44‐70] | 48 [34‐57] | — | 45 [31‐63] | 43 [34‐58] | — | 64 [37‐69] | 38 [31‐40] | — | |
| Region, no. (%) | |||||||||||||||
| Europe/Asia/Australia | 72 (56.3) | 41 (73.2) | .030d | 38 (59.4) | 20 (76.9) | .115 | 12 (66.7) | 7 (87.5) | .375 | 10 (41.7) | 10 (58.8) | .279 | 12 (54.5) | 4 (80) | .618 |
| North America (US and Canada) | 56 (43.8) | 15 (26.8) | .030d | 26 (40.6) | 6 (23.1) | .115 | 6 (33.3) | 1 (12.5) | .375 | 14 (58.3) | 7 (41.2) | .279 | 10 (45.5) | 1 (20) | .618 |
| Men, no. (%) | 85 (66.4) | 43 (76.8) | .159 | 48 (75) | 21 (80.8) | .558 | 11 (61.1) | 6 (75) | .667 | 12 (50) | 13 (76.5) | .087 | 14 (63.6) | 3 (60) | 1.000 |
| Duration of follow‐up: Mean ± SD, moe | 24.1 ± 17.8 | 27.8 ± 27.2 | .745 | 34.5 ± 14.8 | 32.3 ± 31.2 | .271 | 26.8 ± 17.2 | 40.4 ± 29.5 | .186 | 9.1 ± 8.7 | 17.3 ± 16.9 | .130 | 8.2 ± 9.0 | 19.8 ± 20.6 | .201 |
| Median [IQR] | 22 [6‐43] | 11 [6‐49] | — | 42 [22‐46] | 24 [5‐52] | — | 30 [8‐43] | 45 [11‐59] | — | 7 [3‐11] | 11 [6‐29] | — | 5 [3‐9] | 8 [7‐32] | — |
| Clinical characteristics | |||||||||||||||
| Previous use of imatinib prior to index date, no. (%) | 120 (93.8) | 56 (100) | .108 | 62 (96.9) | 26 (100) | 1.000 | 18 (100) | 8 (100) | 1.000 | 22 (91.7) | 17 (100) | .502 | 18 (81.8) | 5 (100) | .561 |
| Time from T315I detection to intervention: Mean ± SD, mof | 0.4 ± 0.2 | 13.8 ± 18.8 | < .001d | 0.4 ± 0.2 | 14.0 ± 14.6 | < .001d | 0.4 ± 0.2 | 10.1 ± 16.5 | < .001d | 0.3 ± 0.2 | 19.9 ± 24.3 | < .001d | 0.4 ± 0.2 | −1.5 ± 11.3 | .052 |
| Median [IQR] | 0 [0‐1] | 8 [3‐19] | 0 [0‐1] | 9 [5‐18] | — | 0 [0‐1] | 4 [3‐7] | — | 0 [0‐0] | 10 [5‐26] | — | 0 [0‐1] | 3 [1‐5] | — | |
| Time from diagnosis to index date: Mean ± SD, mog | 61.1 ± 51.5 | 47.2 ± 39.2 | .139 | 69.6 ± 50.4 | 47.3 ± 41.6 | .029d | 91.7 ± 56.7 | 50.1 ± 30.1 | .075 | 49.5 ± 50.5 | 53.5 ± 42.0 | .340 | 23.9 ± 19.0 | 21.0 ± 25.4 | .212 |
| Median [IQR] | 44 [20‐86] | 32 [19‐68] | — | 58 [33‐100] | 32 [18‐68] | — | 80 [52‐149] | 49 [24‐74] | 26 [11‐77] | 43 [26‐63] | — | 17 [12‐30] | 10 [8‐12] | — | |
| Ponatinib‐specific characteristics | |||||||||||||||
| Treatment duration: Mean ± SD, mo | 18.6 ± 18.2 | — | — | 27.6 ± 17.6 | — | — | 23.7 ± 18.0 | — | — | 3.4 ± 2.5 | — | — | 4.8 ± 8.5 | — | — |
| Median [IQR] | 7 [3‐41] | — | — | 33 [8‐44] | — | — | 25 [6‐43] | — | — | 2 [1‐6] | — | — | 3 [2‐4] | — | — |
| Treatment discontinued, no. (%) | 98 (76.6) | — | — | 38 (59.4) | — | — | 14 (77.8) | — | — | 24 (100) | — | — | 22 (100) | — | — |
| Time to treatment discontinuation: Mean ± SD, moh | 10.5 ± 12.1 | — | — | 15.7 ± 12.7 | — | — | 17.6 ± 15.4 | — | — | 3.4 ± 2.5 | — | — | 4.8 ± 8.5 | — | — |
| Median [IQR] | 5 [2‐16] | — | — | 10 [4‐28] | — | — | 8 [6‐31] | — | — | 2 [1‐6] | — | — | 3 [2‐4] | — | — |
| Allogeneic SCT‐specific characteristics | |||||||||||||||
| Transplantation type, no. (%) | |||||||||||||||
| Bone marrow | — | 21 (37.5) | — | — | 8 (30.8) | — | — | 2 (25) | — | — | 8 (47.1) | — | — | 3 (60) | — |
| Peripheral blood stem cells | — | 28 (50) | — | — | 16 (61.5) | — | — | 4 (50) | — | 6 (35.3) | — | — | 2 (40) | — | |
| Cord blood | — | 6 (10.7) | — | — | 2 (7.7) | — | — | 1 (12.5) | — | — | 3 (17.6) | — | — | 0 (0) | — |
| Unknown | — | 1 (1.8) | — | — | 0 (0) | — | — | 1 (12.5) | — | — | 0 (0) | — | — | 0 (0) | — |
| Conditioning regimen, no. (%) | |||||||||||||||
| Conventional | — | 39 (69.6) | — | — | 19 (73.1) | — | — | 4 (50) | — | — | 11 (64.7) | — | — | 5 (100) | — |
| Reduced‐intensity conditioning | — | 15 (26.8) | — | — | 7 (26.9) | — | — | 4 (50) | — | — | 4 (23.5) | — | — | 0 (0) | — |
| Unknown | — | 2 (3.6) | — | — | 0 (0) | — | — | 0 (0) | — | — | 2 (11.8) | — | — | 0 (0) | — |
| Matched donor, no. (%) | — | 42 (75) | — | — | 21 (80.8) | — | — | 5 (62.5) | — | — | 12 (70.6) | — | — | 4 (80) | — |
| Related donor, no. (%) | — | 9 (16.1) | — | — | 5 (19.2) | — | — | 0 (0) | — | — | 4 (23.5) | — | — | 0 (0) | — |
Abbreviations: ALL, acute lymphoblastic leukemia; allo‐SCT, allogeneic stem cell transplantation; AP, accelerated phase; BC, blast crisis; CML, chronic myeloid leukemia; CP, chronic phase; IQR, interquartile range; mo, months; no., numbers; Ph+, Philadelphia‐positive; SD, standard deviation; T315I, threonine to isoleucine mutation at codon 315; y, years.
Patients with unknown phase or age at treatment initiation (n = 5), unknown date of treatment initiation (n = 2), a last follow‐up date earlier than the date of treatment initiation (n = 1), or unknown date of diagnosis (n = 2) were removed from this analysis only (some patients had more than 1 exclusion criteria). Additional patients who had phase values of “CP2” or “CR” complete remission for stage at allo‐SCT were also removed from this analysis (n = 5).
P values were computed using Wilcoxon rank‐sum tests for continuous variables and chi‐square tests or Fisher's exact tests for categorical variables, as appropriate.
Patients with missing values were excluded (N = 1).
P < .05.
Follow‐up started at the index date and continued until the first of death, loss to follow‐up, or the study end date. A few patients who underwent allo‐SCT were missing a date of last follow‐up (n = 1), and the latest date in the database (January 27, 2015) was used.
A few patients who underwent allo‐SCT were missing the date of T315I detection (n = 2); the mean was computed among patients who had both dates known.
A few patients who underwent allo‐SCT were missing the date of diagnosis or had a date of diagnosis that was the same as the index date (n = 15), and the date they started imatinib use was used.
The time to treatment discontinuation was measured among patients who discontinued ponatinib.
In addition, we expected that patients with T315I‐positive allo‐SCT in the EBMT would be heterogeneous in their response status at intervention. Although limited information about this is available, data indicate that, of the 17 patients in the study who had BP‐CML, 5 were in complete remission (CR), 7 had blasts present in the bone marrow or blood, and 5 had unknown response status at transplantation. Similarly, 4 patients with Ph+ ALL were in CR, whereas 1 patient was blastic. All 4 patients who underwent allo‐SCT before the approval of ponatinib (specifically, during 2005‐2008) and underwent transplantation in second CR, with a median time from diagnosis to transplantation of 11.8 months (1 patient who had a time from diagnosis to transplantation of 63 months was excluded from this calculation). Among patients with CP‐CML and AP‐CML, the data did not indicate that any patients in either stratum were blastic.
Adjusted KM survival curves are provided in Figure 1A‐D. Patients with CP‐CML who received ponatinib had significantly better OS at 24 and 48 months compared with those who underwent allo‐SCT (24 months: 84% vs 60.5%; P = .004; 48 months: 72.7% vs 55.8%; P = .013). Median OS was longer for the ponatinib group (not reached vs 103.3 months; HR, 0.37; 95% confidence interval [CI], 0.16‐0.84; P = .017). OS at 24 months and 48 months did not differ significantly in patients with AP‐CML who received ponatinib versus those who underwent allo‐SCT (24 months: 77.2% vs 68.8%, P = .618; 48 months: 69% vs 68.8%; P = .889; median OS: not reached vs 55.6 months; HR, 0.90; 95% CI, 0.20‐4.10; P = .889). In patients with BC‐CML, however, ponatinib was associated with lower OS at 24 months (13.9% vs 36.3%; P = .084) and at 48 months (2% vs 26%; P = .026) compared with allo‐SCT, with an HR of 2.29 (95% CI, 1.08‐4.82; P = .030) corresponding to a shorter median OS in the ponatinib group (7.0 vs 10.5 months). Among patients who had Ph+ ALL, ponatinib was associated with lower OS at 24 and at 48 months compared with allo‐SCT (24 months: 8.8% vs 70.3%; P = .059; 48 months: 8.8% vs 32%; P = .119), with an HR of 2.77 (95% CI, 0.73‐10.56; P = .136) and median OS of 6.7 versus 32.4 months.
Figure 1.
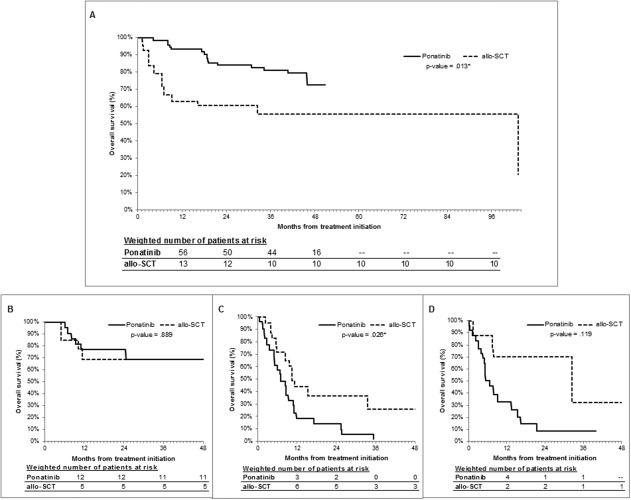
Adjusted overall survival analysis is illustrated for patients who received treatment with ponatinib (solid line) or underwent stem cell transplantation (allo‐SCT) (dashed line) stratified by phase. (A) Chronic‐phase chronic myeloid leukemia (CML), (B) accelerated‐phase CML, (C) blast‐crisis CML, and (D) Philadelphia chromosome‐positive acute lymphoblastic leukemia are illustrated. Patients were censored at the end of follow‐up or at the end of the study, whichever occurred first. Kaplan‐Meier curves were adjusted by standardizing each treatment group sample to the characteristics of the combined study population. P values comparing adjusted overall survival were computed at the 48‐month mark using log‐rank tests. The numbers of patients at risk at each 12‐month interval are indicated below the corresponding figure and were obtained from adjusted Kaplan‐Meier curves weighted by stabilized inverse probability of treatment weights.
DISCUSSION
This study is the first demonstrating that patients with T315I‐positive CP‐CML who received ponatinib alone had significantly longer OS than patients who underwent allo‐SCT. Conversely, patients with BC‐CML or Ph+ ALL who underwent allo‐SCT had better survival than those who received ponatinib alone, which was expected because multiple genes are activated and contribute to progression in these settings. Thus, although allo‐SCT remains standard therapy for patients who have BC‐CML at diagnosis or after TKI treatment, our results suggest that ponatinib alone is a valuable alternative to transplantation for prolonging survival in patients with T315I‐positive CP‐CML.
Our study has several limitations, including very small sample sizes in each stratum (especially for the allo‐SCT group in AP‐CML and Ph+ ALL, rendering results for these phases inconclusive); residual confounding, because only variables that were common between PACE and the EBMT database could be adjusted for (eg, we lacked data on previous therapies and residual disease before intervention); selection bias; and missing data in the EBMT database to implement inclusion/exclusion criteria common to PACE or to examine cause of death and adverse events. Data on progression‐free survival also were unavailable for analysis. In addition, the majority of allo‐SCTs in the EBMT data occurred during the preponatinib era. This is an important limitation of our indirect comparison, because treatment paradigms may have changed over time. For instance, recently, ponatinib in combination with chemotherapies demonstrated a significant improvement in 24‐month event‐free OS (up to 81% in patients with Ph+ ALL).14 Longer follow‐up also may help us understand differences in OS between the intervention groups.15 Prospective randomized trials comparing ponatinib with allo‐SCT in patients with T315I‐positive CML and Ph+ ALL are needed to confirm our findings but are difficult to achieve.
FUNDING SUPPORT
Funding for this study was provided by ARIAD Pharmaceuticals, Inc.
CONFLICT OF INTEREST DISCLOSURES
Frank E. Nicolini reports personal fees and honoraria from ARIAD Pharmaceuticals, Novartis, and Bristol‐Myers Squibb and a research grant from Novartis. Grzegorz W. Basak reports honoraria from Astellas, Sanofi, and Pierre‐Fabre and personal fees from Merck Sharp & Dohme. Dong‐Wook Kim reports personal fees, honoraria, and research grants from Pfizer, Bristol‐Myers Squibb, Novartis, and Il‐Yang. Javier Pinilla‐Ibarz reports personal fees from Bristol‐Myers Squibb, Novartis, ARIAD Pharmaceuticals, and Pfizer. Jane F. Apperley reports personal fees from ARIAD Pharmaceuticals, Pfizer, Novartis, and Bristol‐Myers Squibb. Timothy Hughes reports honoraria and research grants from Novartis, Bristol‐Myers Squibb, and ARIAD Pharmaceuticals. Dietger Niederwieser reports personal fees from Novartis. Michael J. Mauro reports personal fees from ARIAD Pharmaceuticals, Pfizer, Novartis, and Bristol‐Myers Squibb and research grants from Novartis. Charles Chuah reports honoraria from Bristol‐Myers Squibb, Novartis, and Chiltern International. Andreas Hochhaus reports honoraria and research grants from Pfizer, Novartis, Bristol‐Myers Squibb, and ARIAD Pharmaceuticals. Giovanni Martinelli reports personal fees from Novartis, Merck Sharp & Dohme, Pfizer, ARIAD Pharmaceuticals, Roche, Bristol‐Myers Squibb, and Amgen. Jorge E. Cortes reports personal fees from Pfizer, Bristol‐Myers Squibb, Novartis, ARIAD Pharmaceuticals, Astellas, Ambit, and Janssen and research grants from Pfizer, Bristol‐Myers Squibb, BerGenBio AS, Teva, Novartis, ARIAD Pharmaceuticals, Astellas, Ambit, Arog, and Celator. Lisa J. McGarry is an employee of ARIAD Pharmaceuticals. Maral DerSarkissian and Mei Sheng Duh are employees of Analysis Group, Inc., which has received research funds from ARIAD Pharmaceuticals. The remaining authors made no disclosures.
AUTHOR CONTRIBUTIONS
Franck E. Nicolini: Designed the research, member of the European Group for Blood and Marrow Transplantation (EBMT), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, contributed to writing–initial draft, and writing–critical review. Grzegorz W. Basak: Coordinated the research within the EBMT Chronic Malignancies Working Party, member of the EBMT, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Dong‐Wook Kim: Investigator in the Ponatinib Philadelphia‐Positive ALL and CML Evaluation (PACE) clinical trial, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Eduardo Olavarria: Coordinated the research within the EBMT Chronic Malignancies Working Party, member of the EBMT, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Javier Pinilla‐Ibarz: Investigator in the PACE clinical trial, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Jane F. Apperley: Investigator in the PACE clinical trial, member of the EBMT, supervised and contributed to data collection for the EBMT registry (UK), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Timothy Hughes: Investigator in the PACE clinical trial, member of the PACE Steering Committee, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Dietger Niederwieser: Member of the EBMT, supervised and contributed to data collection for the EBMT registry (Germany), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Michael J. Mauro: Investigator in the PACE clinical trial, assisted with data interpretation and data review/approval, and writing–critical review. Charles Chuah: Investigator in the PACE clinical trial, supervised and contributed to data collection for the EBMT registry (Singapore), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Andreas Hochhaus: Investigator in the PACE clinical trial, member of the PACE Steering Committee, supervised and contributed to data collection for the EBMT registry (Germany), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Giovanni Martinelli: Supervised and contributed to data collection for the EBMT registry (Italy), cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Maral DerSarkissian: Designed the research, assisted with data interpretation and data review/approval, contributed to writing–initial draft, and writing–critical review. Mei Sheng Duh: Designed the research, assisted with data interpretation and data review/approval, contributed to writing–initial draft, and writing–critical review. Lisa J. McGarry: Designed the research, assisted with data interpretation and data review/approval, contributed to writing–initial draft, and writing–critical review. Hagop M. Kantarjian: Investigator in the PACE clinical trial, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review. Jorge E. Cortes: Investigator in the PACE clinical trial, member of the PACE Steering Committee, cared for patients and collected clinical data, assisted with data interpretation and data review/approval, and writing–critical review.
Presented in part at the 57th Annual Meeting of the American Society of Hematology; December 5‐8, 2015; Orlando, FL
We thank the patients, families, and their caregivers for participating in the Ponatinib Philadelphia‐Positive Acute Lymphoblastic Leukemia and Chronic Myeloid Leukemia Evaluation (PACE) trial and the PACE investigators and team members at each site. In addition, we thank patients for participating in the European Group for Blood and Marrow Transplantation (EBMT) registry, and the investigators, transplantation team members, and clinical research associates who work to maintain the updated database. We also thank Andrew Kageleiry, BS; Rachel Bhak, MS, from Analysis Group Inc. (Boston, MA); and Hui Huang, PhD, MBA, and Mo Yang, PhD, from ARIAD Pharmaceuticals for their contributions and valuable assistance to this study.
REFERENCES
- 1. Fielding AK. Current treatment of Philadelphia chromosome‐positive acute lymphoblastic leukemia. Haematologica. 2010;95:8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fielding AK. How I treat Philadelphia chromosome‐positive acute lymphoblastic leukemia. Blood. 2010;116:3409‐3417. [DOI] [PubMed] [Google Scholar]
- 3. Miller GD, Bruno BJ, Lim CS. Resistant mutations in CML and Ph(+)ALL—role of ponatinib. Biologics. 2014;8:243‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan‐BCR‐ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation‐based resistance. Cancer Cell. 2009;16:401‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Hare T. In vitro activity of Bcr‐Abl inhibitors AMN107 and BMS‐354825 against clinically relevant imatinib‐resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500‐4505. [DOI] [PubMed] [Google Scholar]
- 6. Cortes JE, Kim D‐W, Pinilla‐Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome‐positive leukemias. N Engl J Med. 2013;369:1783‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cortes JE, Kantarjian HM, Rea D, et al. Final analysis of the efficacy and safety of omacetaxine mepesuccinate in patients with chronic‐ or accelerated‐phase chronic myeloid leukemia: Results with 24 months of follow‐up. Cancer. 2015;121:1637‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicolini FE, Ibrahim AR, Soverini S, et al. The BCR‐ABLT315I mutation compromises survival in chronic phase chronic myelogenous leukemia patients resistant to tyrosine kinase inhibitors, in a matched pair analysis. Haematologica. 2013;98:1510‐1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR‐ABL T315I mutation. Blood. 2009;114:5271‐5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicolini FE, Basak GW, Soverini S, et al. Allogeneic stem cell transplantation for patients harboring T315I BCR‐ABL mutated leukemias. Blood. 2011;118:5697‐5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45‐49. [DOI] [PubMed] [Google Scholar]
- 12. Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550‐560. [DOI] [PubMed] [Google Scholar]
- 13. Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV‐positive men. Epidemiology. 2000;11:561‐570. [DOI] [PubMed] [Google Scholar]
- 14. Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper‐CVAD with ponatinib as first‐line therapy for patients with Philadelphia chromosome‐positive acute lymphoblastic leukaemia: a single‐centre, phase 2 study. Lancet Oncol. 2015;16:1547‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gratwohl A, Pfirrmann M, Zander A, et al. Long‐term outcome of patients with newly diagnosed chronic myeloid leukemia: a randomized comparison of stem cell transplantation with drug treatment. Leukemia. 2015;30:562‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]


