Abstract
Aims
To evaluate short‐ and long‐term glycaemic control and hypoglycaemia incidence in insulin‐naïve patients ≥30 years of age with type 2 diabetes (T2DM) initiating basal insulin (BI) with or without oral anti‐hyperglycaemic drugs (OADs).
Methods
This was an observational, retrospective longitudinal analysis of electronic medical records from 5 European countries and the USA. A multivariable logistic regression model assessed baseline and short‐term (0‐3 months post BI initiation) factors associated with long‐term (3‐24 months) glycaemic control and hypoglycaemia.
Results
Overall, 40 627 patients were included; 20.9% and 27.8% achieved the general HbA1c target of ≤7% at 3 and 24 months post BI initiation, respectively. Failure to achieve HbA1c ≤7% at 3 months was associated with increased risk of failing to achieve target at 24 months (odds ratio [OR], 3.70 [95% CI, 3.41‐4.00]). Over 24 months, 8.9% of patients experienced a recorded hypoglycaemic event. Hypoglycaemia during the initial 3‐month period was associated with longer‐term risk of these events over the ensuing 3 to 24 months (OR, 5.71 [95% CI, 4.67‐6.99]).
Conclusions
Initiating BI with or without OADs is associated with short‐ and long‐term suboptimal glycaemic control; the majority of patients fail to achieve HbA1c target ≤7% in the first 3 months, or after 2 years of BI treatment. Treatment response and hypoglycaemia incidence by 3 months post BI initiation are associated with longer‐term glycaemic control and hypoglycaemic risk, respectively. These results support the need for early anti‐hyperglycaemic interventions that more effectively control blood glucose levels without increasing the risk of hypoglycaemia.
Keywords: basal insulin, glycaemic control, hypoglycaemia, type 2 diabetes
1. INTRODUCTION
The overarching goal of diabetes management is to reduce the risk of acute and chronic complications associated with the disease, in part, through appropriate glycaemic control.1, 2 The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) jointly recommend an evidence‐based general target glycated haemoglobin (HbA1c) level of ≤7.0% (≤53 mmol/mol) for people with type 2 diabetes (T2DM); this target should be further individualized, to be more or less intensive according to individual patient profiles (eg, age, comorbidities, hypoglycaemia risk) and preferences.1, 3, 4, 5, 6, 7 The ADA‐EASD position statement also recommends optimization of therapy if glycaemic targets are not achieved after 3 months.1
Effective diabetes therapy requires a delicate balance between the benefits of strict glycaemic control and the risk of hypoglycaemia and other drug‐related side effects.8 Indeed, many physicians cite hypoglycaemia as a critical barrier to insulin therapy initiation and adherence.9, 10, 11 Hypoglycaemia, or fear of such episodes, is also known to contribute to patients missing, mis‐timing or reducing insulin doses,12 which may compromise glycaemic control. Despite comprehensive treatment recommendations, many patients experience years of inadequate glycaemic control because of delays in treatment intensification (so‐called inertia), especially when transition to insulin treatment is needed.13 Once insulin therapy has been initiated, many patients still struggle to achieve or maintain glycaemic targets.14 This is a concern, given the increased risk of complications such as cardiovascular events in patients with poor glycaemic control and delayed treatment intensification.15
Therefore, despite a wide range of available treatment options, and advancements in insulin therapies offering an improved benefit/risk profile, lowering blood glucose without increasing the risk of hypoglycaemia in insulin‐treated patients remains a clinical challenge. In order to evaluate the extent of unmet medical needs of patients with T2DM initiating basal insulin (BI) in terms of glycaemic control and hypoglycaemia incidence, we conducted an analysis of an electronic medical records (EMRs) database in 5 European countries (France, Germany, Italy, Spain and the UK) and the USA. The focus of the investigation was to identify patient characteristics in the first 3 months of BI initiation that may predict longer (24‐month) achievement of glycaemic control and risk of hypoglycaemia.
2. METHODS
2.1. Study design
This was an observational, retrospective, longitudinal analysis of the Cegedim Strategic Data (CSD) patient database of general practitioner EMRs. CSD longitudinal patient databases collect daily, non‐interventional, anonymized patient‐level data from practice management software used during physician office visits worldwide; we utilized data from France, Germany, Italy, Spain, the UK and the USA in our analyses.
Patients (N = 40 627) initiating BI with or without oral anti‐hyperglycaemic drugs (OADs)/glucagon‐like peptide‐1 receptor agonists (GLP1‐RA) from January 1, 2008 to September 30, 2012 were included in the analysis if they had a diagnosis of T2DM, were aged ≥30 years at time of diagnosis, had no prior use of insulin and had used OADs at some time prior to initiating BI. Patients were excluded if they were receiving rapid‐acting or premix insulin prior to BI initiation, but use of prandial insulin was permitted in the post‐index period.
Patients initiating BI treatment were identified from a first prescription for BI (defined as the index date). Patients had to have ≥1 HbA1c measurement within 12 months pre‐index and ≥1 HbA1c measurement within 24 months post BI initiation, as well as data available for ≥1 year before and ≥2 years after the index date.
The 1‐year pre‐index period was used to ascertain patient characteristics and medical and medication history. Comorbidities were assessed as recorded in the EMR, which relied on a patient receiving either a primary or secondary recorded diagnosis during an office visit. The International Classification of Diseases 9th Edition (ICD‐9) was used for disease classification in Italy and the USA, and the International Classification of Diseases 10th Edition (ICD‐10) was used in Germany. In Spain, in‐country classification systems were mapped to ICD‐10. In France, in‐country classification systems were mapped to ICD‐10 and to the International Classification of Primary Care 2nd Edition (ICPC‐2). Disease classification was based on Read codes in the UK.16 Glycaemic control, hypoglycaemia incidence and medication use were evaluated during 2 years following the index date post BI initiation.
2.2. Outcomes
Glycaemic control was determined according to measured levels of HbA1c at the index date and at 3, 6, 12 and 24 months post index. Target achievement was defined as the proportion of patients achieving a threshold HbA1c value of ≤7.0% (≤53 mmol/mol). The proportion of patients experiencing hypoglycaemia was recorded at 3, 6, 12 and 24 months post index. As this was a real‐world, international study, no single standardized definition of hypoglycaemia could be used. While we expect that most clinicians used the ADA/EASD definition of hypoglycaemia, some clinicians may have used local/national guidelines alternatively to identify such events. Hypoglycaemic episodes were captured as a recorded diagnosis of any hypoglycaemia or fasting plasma glucose (FPG) of ≤3.9 mmol/L (≤70 mg/dL) during an office visit.17
2.3. Data analysis and statistics
We examined potential pre‐defined factors associated with long‐term (3‐24 months) glycaemic control and hypoglycaemia burden, based on patient characteristics, short‐term (0‐3 months) glycaemic response and hypoglycaemia experienced during the first 3 months of BI treatment. Patient characteristics included age, gender, body mass index (BMI), microvascular (nephropathy, retinopathy and neuropathy) and macrovascular comorbidities (myocardial infarction, congestive heart failure, ischemic stroke and peripheral vascular disease), pre‐index HbA1c, history of hypoglycaemia and other general comorbidities (hypertension, mental illness, dementia, chronic kidney disease [CKD]). The association between each of these factors and long‐term response was first evaluated in a univariate logistic regression analysis. The final multivariable regression model included covariates with a P value ≤0.1 identified from the univariate logistic regression analysis. The overall effect across all 6 countries was derived from a meta‐analysis using an inverse‐variance weighted method.
3. RESULTS
3.1. Study population at index date
A total of 40 627 patients were included in the analysis, with the number of patients from each country ranging from 1117 (Spain) to 30 220 (USA). Patient characteristics are shown in Table 1. In Europe, over half of the patients were older adults (>65 years of age). In the USA, the proportion of older patients was lower, with a population median age of 62 years.
Table 1.
Country‐specific patient characteristics at index date
| France | Germany | Italy | Spain | UK | USA | Total | |
|---|---|---|---|---|---|---|---|
| N = 2264 | N = 2330 | N = 1228 | N = 1117 | N = 3468 | N = 30 220 | N = 40 627 | |
| General | |||||||
| Age, years | |||||||
| Mean (SD) | 66.5 (11.8) | 67.5 (11.1) | 69.1 (11.8) | 66.9 (12.3) | 65.2 (12.0) | 62.1 (11.9) | 63.3 (8.0) |
| Median | 66 | 69 | 70 | 68 | 66 | 62 | 67 |
| BMI, kg/m2 (mean [SD]) | 29.9 (5.9) | 31.3 (6.5) | 29.5 (5.7) | 29.8 (5.5) | 31.5 (6.6) | 33.7 (6.5) | 32.9 (5.7) |
| HbA1c, % (mean [SD]) | 9.0 (1.9) | 8.5 (1.7) | 9.1 (1.9) | 9.2 (1.9) | 9.9 (1.9) | 8.9 (1.9) | 9.0 (3.0) |
| HbA1c > 7.0%, % | 87.3 | 81.9 | 87.5 | 89.4 | 96.6 | 85.5 | 86.5 |
| HbA1c > 6.5%, % | 94.5 | 93.2 | 95.0 | 93.6 | 98.4 | 93.1 | 93.7 |
| HbA1c > 9.0%, % | 43.4 | 29.8 | 43.8 | 47.4 | 62.9 | 39.5 | 41.5 |
| Prevalence of comorbidities, % | |||||||
| Hypoglycaemia | 9.0 | 2.4 | 7.7 | 1.9 | 5.1 | 3.3 | 3.8 |
| Microvascular comorbiditiesa | 5.9 | 14.4 | 7.7 | 5.0 | 25.2 | 17.5 | 16.7 |
| Macrovascular comorbiditiesb | 18.9 | 27.5 | 15.0 | 20.1 | 21.6 | 11.7 | 14.2 |
| Myocardial infarction | 4.4 | 5.6 | 5.4 | 8.6 | 7.7 | 1.7 | 2.9 |
| Ischaemic stroke | 2.4 | 4.0 | 0 | 5.1 | 5.6 | 0.3 | 1.2 |
| Congestive heart failure | 4.1 | 14.5 | 5.9 | 4.8 | 7.5 | 6.3 | 6.7 |
| Peripheral vascular disease | 10.7 | 10.3 | 5.2 | 5.1 | 6.5 | 5.0 | 5.8 |
| Chronic pulmonary disease | 11.5 | 17.8 | 15.2 | 10.8 | 19.3 | 12.6 | 13.4 |
| Renal disease | 2.6 | 8.7 | 9.3 | 8.3 | 30.8 | 11.0 | 11.9 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Microvascular comorbidities: nephropathy, retinopathy, and neuropathy.
Macrovascular comorbidities: myocardial infarction, congestive heart failure, ischaemic stroke and peripheral vascular disease.
The prevalence of comorbid conditions reported in the database varied rather widely among countries (Table 1). The prevalence of reported microvascular comorbidities was highest in the UK (25.2% of patients), while this ranged from 5.0% (Spain) to 17.5% (USA) in the other 5 countries. The proportion of patients with reported macrovascular comorbidities was lowest in the USA (11.7%) and highest in Germany (27.5%). Renal disease prevalence ranged from 2.6% in France to 30.8% in the UK.
3.2. Glycaemic control
3.2.1. Glycaemic control prior to BI initiation
While poor glycaemic control is to be expected in patients initiating BI, almost half of the patients in France, Italy and Spain, and 62.9% of patients in the UK, initiated BI with very high HbA1c levels (>9.0% [>75 mmol/mol]); proportions of patients with HbA1c values in this category were lower in Germany and the USA (29.8% and 39.5%, respectively) (Table 1). Mean HbA1c upon BI initiation ranged from 8.5% (69 mmol/mol; Germany) to 9.9% (85 mmol/mol; UK) (Table 1).
3.2.2. Glycaemic control post BI initiation
Across all countries, mean HbA1c showed the steepest decline during the first 3 months post index date, continued to decline until 6 months post BI initiation, and then remained stable to the end of the 24‐month post‐index period (Figure 1A). The proportion of patients across all countries achieving HbA1c values ≤7.0% (53 mmol/mol) increased from 13.5% at index date to 20.9% and 27.8% at 3 and 24 months after BI initiation, respectively. Target achievement varied among countries, from 8.1% to 28.0% at 3 months, and 17.3% to 33.4% at 24 months post index (Figure 1B).
Figure 1.
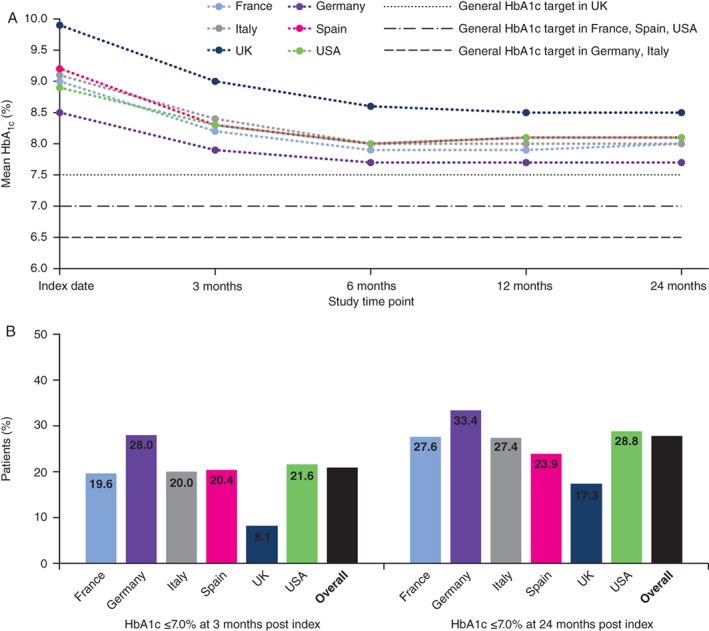
A, Mean HbA1c at index date and during 24 months post index. B, Percentage of patients at HbA1c target (≤7.0%) at 24 months post index
Compared with patients who achieved an HbA1c target ≤7.0% [≤53 mmol/mol] 3 months after BI initiation, those with suboptimal glycaemic control (HbA1c >7.0% [>53 mmol/mol]) at this time point were more likely to fail in reaching this target at 24 months (odds ratio [OR], 3.70 [95% confidence interval (CI), 3.41‐4.00]) (Figure 2A). Other factors associated with suboptimal long‐term glycaemic control were not consistently identified among countries (Figure S1A). Hypoglycaemia experience in the pre‐index period or in the first 3 months post index was not associated with failure to achieve glycaemic targets in any country.
Figure 2.
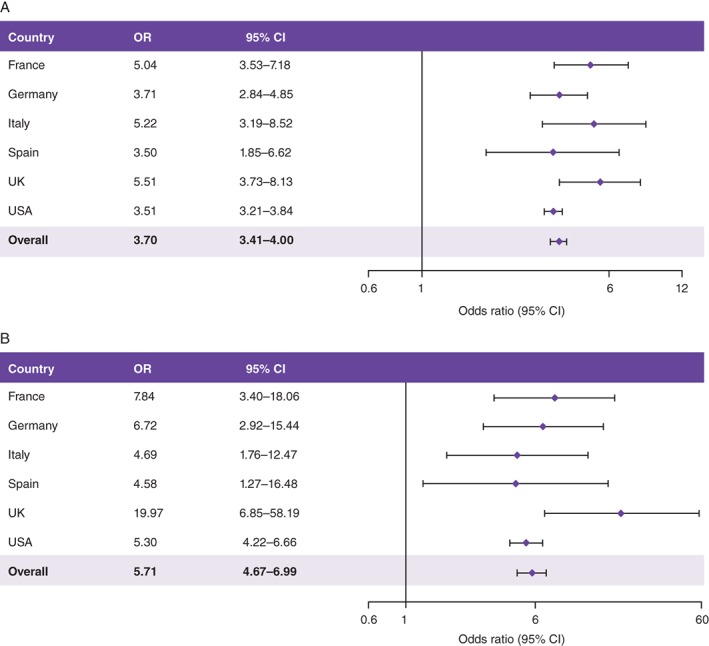
Odds ratios A, for risk of not achieving target HbA1c ≤7.0% after 24 months if target is not achieved in the first 3 months, and B, for risk of hypoglycaemia after 24 months if hypoglycaemia is experienced in the first 3 months. CI, confidence interval; OR, odds ratio. Results adjusted for A, pre‐index HbA1c levels and B, pre‐index hypoglycaemia experience. For the 3‐month time point, the closest available HbA1c value was to be used and must have been measured between 2 and 4 months after BI initiation. For other time points after the index date, the HbA1c value nearest to that time point was to be used and the measurement should not have overlapped with that used at the 3‐month time point. Overall OR was derived from a meta‐analysis of results from all 6 countries, using an inverse‐variance weighted method
3.3. Hypoglycaemia
3.3.1. Hypoglycaemia prior to BI initiation
Hypoglycaemia prevalence during the pre‐index period varied widely among countries, from 1.9% of patients in Spain to 9.0% in France (Table 1).
3.3.2. Hypoglycaemia post BI initiation
Hypoglycaemia incidence increased post BI initiation compared with the pre‐index period in Germany, Italy, Spain and the USA, but declined in France and the UK (Figure 3). During the first year after the index date, 5.1% of patients experienced a hypoglycaemic event that was recorded in the database; this increased to 8.9% of patients after 24 months (Figure 3).
Figure 3.
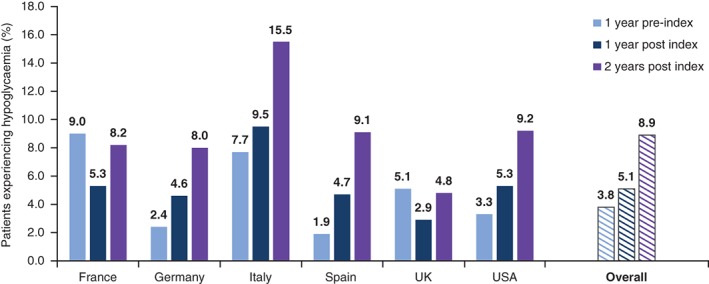
Percentage of patients experiencing hypoglycaemia during the 1‐year pre‐index period and during the 1‐year and 2‐year post‐index periods
Hypoglycaemia during the initial 3‐month period was associated with longer‐term (3‐24 months) hypoglycaemia risk (OR, 5.71 [95% CI, 4.67‐6.99]) (Figure 2B). As expected, other well‐known factors associated with long‐term risk of hypoglycaemia included comorbidities such as congestive heart failure (OR, 2.40 [95% CI, 1.04‐5.55] in France and 2.18 [95% CI, 1.03‐4.63] in the UK) and renal disease (OR, 1.83 [95% CI, 1.04‐3.21) in Germany, 2.61 [95% CI, 1.15‐5.92] in Italy and 1.70 [95% CI, 1.46‐1.97] in the USA), but these were not consistently identified across all countries (Figure S1B).
3.4. Medication use
3.4.1. Medication use prior to BI initiation
In the pre‐index period, biguanides and sulphonylureas (SUs) were the most recorded concomitant medications (70.3% and 67.9% of patients, respectively), while prescriptions of meglitinides, dipeptidyl peptidase‐4 (DPP‐4) inhibitors and GLP1‐RA were less common (recorded for 6.3%, 27.6% and 12.3% of patients, respectively) (Figure 3A and Table S1). DPP‐4 inhibitors were prescribed in more than one‐quarter of patients in France, the UK and the USA, but these prescriptions were only recorded in a small proportion of patients in Italy (4.4%). Thiazolidinediones and GLP1‐RA were most commonly prescribed in the UK and USA. Conversely, meglitinide prescriptions were lowest in the UK and USA, and highest in Italy.
3.4.2. Medication use post BI initiation
There was a decline in OAD/GLP1‐RA prescriptions during the 12‐month post‐index period compared with the pre‐index period (Figure 4A and Table S1). The exception to this was a small rise in the proportion of patients receiving meglitinide treatment in Italy (increasing from 32.7% of patients pre‐index to 34.4% post index). Substantial reductions in the proportion of patients treated with SU were observed in all countries post index; however, SU use still remained high in the post‐index period (55.1%), particularly in the UK and USA.
Figure 4.
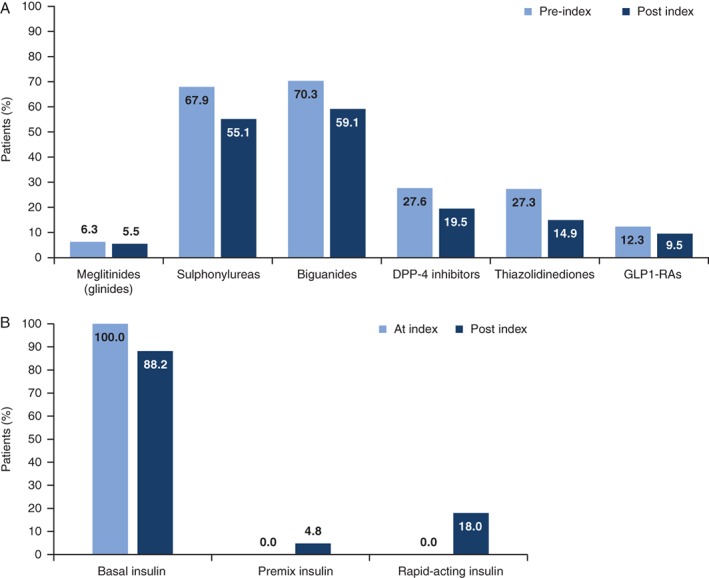
Concomitant medication use (proportion of patients who filed for a prescription at least once) during the 1‐year pre‐index period/at index date and at 1‐year post index. A, Concomitant non‐insulin antihyperglycaemic medications; B, basal insulin, premix and rapid‐acting insulin (post index only). DPP‐4, dipeptidyl peptidas‐4; GLP1‐RA, glucagon‐like peptide‐1 receptor agonists
The majority of patients (88.2%) remained on BI therapy 1 year after the index date (Figure 4B and Table S1). The proportion of patients receiving premix insulin remained <10% post index in all countries, with the exception of the UK, where 14.9% of patients had received premix insulin at least once within 12 months after BI initiation. Proportions of patients initiating rapid‐acting insulin within the first year post index varied relatively widely among countries, ranging from approximately 5% in Spain to approximately 20% in Italy and the USA.
3.5. Body weight
No clinically meaningful changes in mean body weight were observed following BI initiation; mean increases ranged from 0.1 kg in Germany and the USA to 1.5 kg in France and the UK (Table S2).
4. DISCUSSION
In this study of patients with T2DM initiating BI in 5 European countries and the USA, a consistent pattern of short‐ and long‐term suboptimal glycaemic control emerged. The majority of patients failed to reach HbA1c target (≤7.0%) at 3 months or 2 years post index. In addition, glycaemic control and hypoglycaemia incidence at 3 months were associated with long‐term glycaemic control and hypoglycaemia incidence, respectively.
As seen in previous studies in populations initiating BI,13, 18, 19 these patients were characterized by elevated HbA1c levels at baseline; a high proportion of patients initiated BI with HbA1c > 9.0%, highlighting a disconnect between real‐world clinical practice and recommendations in clinical guidelines, and indicating a delay in initiating insulin far beyond the point of need. This is of critical concern given the need for tight glycaemic control and/or appropriate treatment intensification to reduce the risk of diabetes‐related complications,15, 20, 21, 22 to help preserve β‐cell function23, 24 and to achieve long‐term glycaemic goals.25, 26, 27 As such, the success of BI‐supported oral therapy is dependent on timely initiation during the natural history of T2DM in the individual patient. In support of this, across the 6 countries in this study, patients in Germany initiated BI at the lowest HbA1c level, and had the highest proportion of patients achieving HbA1c ≤7.0% post index. Given the large proportion of the study population who did not reach glycaemic targets using BI and OADs, considering addition of prandial coverage through premix or basal‐bolus insulin regimens might have been a suitable option for some patients.
The broader literature supports a widespread existence of clinical or therapeutic inertia,13, 28, 29, 30, 31, 32, 33 with some patients in the UK experiencing more than 7 years of poor glycaemic control before initiating insulin.13, 32 Future research priorities should address how delays in insulin therapy initiation and how barriers to effective titration could be overcome.
A high proportion of patients in this analysis did not achieve the HbA1c target of ≤7.0% in the 2 years following BI initiation, similar to findings from other database studies performed in the UK and Germany,34, 35 and in the Study of Once‐Daily Levemir (SOLVE), conducted in 10 countries.36 In our study, many patients had comorbid conditions and those from Europe had a median age >65 years; thus, it is possible that relaxed HbA1c targets were set for these patients, in line with current guidelines.3, 37 For example, it may be appropriate to set less stringent glycaemic goals in older adults with comorbidities, who may have previously experienced hypoglycaemia or be at increased risk of hypoglycaemia and more vulnerable to the adverse effects associated with these events.8 However, data on individual patient targets were not available in this retrospective study. It is important to note that, although many patients do not reach recommended HbA1c targets, they may still experience a clinical benefit from the reduction they do achieve.38
Though HbA1c varied by country, failure to achieve a target of ≤7.0% by 3 months post BI initiation was associated consistently with suboptimal long‐term blood glucose control. Short‐term failure to achieve HbA1c target with BI alone may predict the need for early treatment intensification, in line with ADA‐EASD recommendations.1 Between‐country differences in HbA1c values may reflect varying approaches to diabetes management, target setting and treatment intensification.
An unexpected finding was that experience of hypoglycaemia in the pre‐index period or in the first 3 months post index was not associated with HbA1c target achievement. However, with only 5.1% of patients experiencing ≥1 hypoglycaemic event during the first year after BI initiation, the reported incidence of hypoglycaemia was lower than that observed in randomised controlled trial settings 39; this may be explained by the underreporting of such events in database studies (discussed below). The low incidence of hypoglycaemia may have masked any potential association between experience of hypoglycaemia and failure to achieve glycaemic targets.
Given the established association between insulin use and increased risk of hypoglycaemia, the incidence of hypoglycaemia was expected to increase following BI initiation.40 This was observed in Germany, Italy, Spain and the USA; however, there was a decline in recorded hypoglycaemia in France and the UK during the 1‐year post‐index period. The use of relaxed glycaemic targets in some patients, and differences in patient education or concomitant medication usage may have affected hypoglycaemia incidence. A similar observation was made during the SOLVE study, during which severe hypoglycaemia rates declined after BI initiation, despite the fact that the majority of patients continued to have HbA1c levels above target, indicating a general lack of aggressiveness in titration practices.36 Certain high‐risk OADs, such as SUs, are known to increase the incidence of hypoglycaemia compared with other agents, such as metformin and DPP‐4 inhibitors.1, 41, 42 In the SOLVE study, patients who continued SUs after BI initiation experienced higher rates of hypoglycaemia than those who discontinued this treatment.43 In our study, use of concomitant medication varied by country, which may reflect differing treatment practices; however, the UK had the highest proportion of patients using SUs and undergoing further treatment intensification with prandial or rapid‐acting insulin in the post‐index period. It is difficult to explain the decline in hypoglycaemia after BI initiation in France and the UK as dosing data were not available, although reporting bias may have played a role. This merits further investigation, potentially with other data sources.
Independent of baseline hypoglycaemia experience, hypoglycaemia during the first 3 months after BI initiation was associated consistently with hypoglycaemia risk over the ensuing 3 to 24 months. Our analysis also suggested that some comorbidities known to be associated with high hypoglycaemic risk (eg, renal disease, congestive heart failure) were predictive of post‐index hypoglycaemia in some countries. Broad strategies to address some of the unmet needs associated with insulin therapy may include next‐generation blood glucose‐lowering agents, both oral therapies and BIs.44 For example, a BI that is able to control HbA1c without increasing hypoglycaemic risk may help to overcome patient and physician fears surrounding insulin initiation. Combining BIs with anti‐hyperglycaemic drugs associated with a low risk of hypoglycaemia, and introduction of GLP1‐RA to treatment regimens rather than prandial insulin, may further reduce hypoglycaemic risk.45, 46
A key study strength is that the results reflect real‐life clinical practice in a diverse range of countries, and there is currently a regrettable paucity of such clinically relevant data in this setting. Data were included from a large number of patients from multiple countries, allowing a substantial and representative cohort of the European and US patient population to be analysed; indeed, the results may be judiciously extrapolated to other countries.
As an observational study, this analysis is necessarily limited by the information captured in patient records. Some findings, such as between‐country differences in comorbidity prevalence, particularly renal disease, may be explained by reporting bias. The lower rates observed in some countries may relate to differing definitions of renal disease, and also to under‐reporting of the condition. Evidence from the European Society of Cardiology and the EASD suggests that approximately 25% of people with T2DM have CKD (stage 3‐4).47 However, other data indicate that CKD rates may be under‐reported,48 possibly because of lack of awareness and screening for the condition.49 A study in the USA indicated that 54% of patients with T2DM had CKD (stage 1‐5), but this was diagnosed in only 12% of cases.48
Complications, particularly hypoglycaemia, may also have been under‐reported; it is of note that the true burden of hypoglycaemia in this population is likely to be underestimated because only EMR‐captured episodes were analysed. Identification of hypoglycaemia relied on accurate documentation, which may itself depend on patient recall that can be subject to memory bias50; asymptomatic events would not have been recorded unless they occurred during an office visit. As discussed above, the incidence of hypoglycaemia is likely to be influenced by the use of certain concomitant medications. However, as medication usage was based on written prescriptions, and treatment switching, adherence and persistence were not evaluated, the incidence of hypoglycaemia according to concomitant medication should be interpreted with caution.
As might be expected from such a study, a proportion of the total patient population would have been excluded because of the lack of available data. However, we would expect the large sample of patients included to be generally representative of the total population, noting that there may have been a selection bias towards patients who were more likely to undergo HbA1c testing and to complete the test in a timely manner. In comparison to the SOLVE study of patients initiating insulin (detemir) treatment, the patients in our study were of a similar age and had a similar level of glycaemic control at baseline (including high numbers of patients with HbA1c >9.0%), but had lower levels of micro‐ and macro‐vascular comorbidities.36 Not all patients were included at each post‐index time point, and patients may have received care from physicians who did not contribute to the database. Individualized HbA1c targets could not be captured and, thus, were not considered when determining glycaemic target achievement. Furthermore, insulin dosage information was not available; therefore, we can only speculate that potential inadequate titration may also have contributed to failure to achieve HbA1c target.
The limitations of this retrospective analysis may, in part, be overcome in future studies using a prospective design. Following on from the current study, results from the ongoing, large, prospective Diabetes Unmet Needs with basal insulin Evaluation (DUNE) study may provide a more robust evaluation of glycaemic target achievement and hypoglycaemia incidence in patients initiating BI, and also in BI users requiring treatment optimization.51
In conclusion, these results highlight hypoglycaemia incidence and inadequate treatment responses in patients with T2DM initiating BI within a diverse range of countries and healthcare systems. Many patients had high HbA1c levels prior to BI initiation, indicating a delay in treatment intensification. Despite the availability of multiple treatment options, the majority of patients did not achieve target HbA1c at 3 or 24 months. The study has implications for clinical practice, as early failure to reach glycaemic targets or experience of a hypoglycaemic episode was indicative of longer‐term blood glucose control and future hypoglycaemia risk, respectively.
Supporting information
Table S1. Concomitant medication use (proportion of patients who filed for a prescription at least once) during the 1‐year pre‐index period/at index date and at 12 months post index.
Table S2. Body weight at index date and at 24 months post index.
Figure S1. Additional factors associated with A, HbA1c > 7.0% and B, hypoglycaemia during 3 to 24 months post index.
ACKNOWLEDGEMENTS
The authors received editorial support for preparation of this manuscript from Julianna Solomons and Leanne Regan of Fishawack Communications, funded by Sanofi. K. K. acknowledges support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM) and the NIHR Leicester‐Loughborough Diet, Lifestyle and Physical Activity Biomedical Research Unit.
Aspects of this study were previously presented as a poster presentation at the 51st Annual Meeting of the European Association for the Study of Diabetes, September 14 to 18, 2015, Stockholm, Sweden and at the IDF World Diabetes Congress, November 30 to December 4, 2015, Vancouver, Canada.
Conflicts of interest
D. M. has received honoraria for consulting and/or speaking for Abbott, AstraZeneca, Boehringer Ingelheim, Ferrer, GlaxoSmithKline, Eli Lilly, Janssen, Medtronic, Menarini, Merck Sharp & Dohme, Novartis, Novo Nordisk, Praxis Pharmaceutical and Sanofi. He is also a principal investigator and research group leader of CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM), which is an initiative from Instituto de Salud Carlos III (Spain). L. M. has been a consultant and advisory board member for Novo Nordisk and Sanofi. J. S. has attended advisory boards and/or speaker's bureaus for Takeda, Bayer, Novartis, Merck Sharp & Dohme, Amgen, AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Sanofi, Berlin‐Chemie, Eli Lilly, Boehringer Ingelheim, Merck, Roche, Ipsen, Pfizer, Janssen and LifeScan, and has received research support from Takeda, Novartis, Merck Sharp & Dohme, Amgen, GlaxoSmithKline, Novo Nordisk, Sanofi, Ipsen, Pfizer, Janssen, Servier, Eli Lilly, Apitope, Intarcia and Roche. K. K. has received honoraria for consulting and/or speaking for Amgen, Novo Nordisk, Eli Lilly, Merck Sharp & Dohme, Bristol‐Myers Squibb, AstraZeneca, Sanofi, Boehringer Ingelheim, Roche and Servier. H. W., A. C., P. S. and P. C. are employees of Sanofi. L. L. was an employee of Sanofi at the time of the study. L. T. was a consultant to Sanofi. A. C. and P. S. are Sanofi shareholders.
Author contributions
D. M., J. S., K. K., P. C., H. W. and L. L. were involved in designing the study. H. W. and L. L. collected the data. All authors contributed to the analysis of study data and writing the manuscript and all authors reviewed the final manuscript.
Mauricio D, Meneghini L, Seufert J, Liao L, Wang H, Tong L, Cali A, Stella P, Carita P and Khunti K. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA, Diabetes Obes Metab. 2017;19:1155–1164. https://doi.org/10.1111/dom.12927
Funding Information This study was funded by Sanofi.
REFERENCES
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Clinical Excellence . NICE guideline: type 2 diabetes in adults: management. 2015. http://www.nice.org.uk/guidance/ng28. Accessed February 14, 2017.
- 4. Working Group of the CPG on type 2 Diabetes 2 . Clinical practice guideline for type 2 diabetes [Guía de Práctica Clínica sobre Diabetes tipo 2]. 2010. http://www.guiasalud.es/egpc/traduccion/ingles/diabetes/completa/index.html. Accessed December 11, 2015.
- 5. Ceriello A, Gallo M, Candido R, et al. Personalized therapy algorithms for type 2 diabetes: a phenotype‐based approach. Pharmgenomics Pers Med. 2014;7:129‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haut autorité de la Santé . Stratégie médicamenteuse du contrôle glycémique du diabète de type 2. 2013. http://www.has‐sante.fr/portail/upload/docs/application/pdf/2013‐02/reco2clics__diabete_type_2.pdf. Accessed February 14, 2017.
- 7. Programm für Nationale VersorgungsLeitlinien . Therapie des Typ‐2‐Diabetes. 2014. http://www.leitlinien.de/mdb/downloads/nvl/diabetes‐mellitus/dm‐therapie‐1aufl‐vers4‐lang.pdf. Accessed February 14, 2017.
- 8. ADA . Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(suppl 1):S1‐S112. [DOI] [PubMed] [Google Scholar]
- 9. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polonsky WH, Fisher L, Guzman S, Villa‐Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care. 2005;28:2543‐2545. [DOI] [PubMed] [Google Scholar]
- 11. Walz L, Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wandell P. Impact of symptomatic hypoglycemia on medication adherence, patient satisfaction with treatment, and glycemic control in patients with type 2 diabetes. Patient Prefer Adherence. 2014;8:593‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leiter LA, Boras D, Woo VC. Dosing irregularities and self‐treated hypoglycemia in type 2 diabetes: results from the Canadian cohort of an international survey of patients and healthcare professionals. Can J Diabetes. 2014;38:38‐44. [DOI] [PubMed] [Google Scholar]
- 13. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28:1647‐1656. [DOI] [PubMed] [Google Scholar]
- 15. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. UK Terminology Centre . Read codes. 2016. http://systems.digital.nhs.uk/data/uktc/readcodes. Accessed November 10, 2016.
- 17. Workgroup on Hypoglycemia ADA . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 18. Khunti K, Damci T, Meneghini L, Pan CY, Yale JF. Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654‐661. [DOI] [PubMed] [Google Scholar]
- 19. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18:401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 21. Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517‐523. [DOI] [PubMed] [Google Scholar]
- 22. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 23. Harrison LB, Adams‐Huet B, Raskin P, Lingvay I. Beta‐cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. 2012;35:1406‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kramer CK, Zinman B, Retnakaran R. Short‐term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2013;1:28‐34. [DOI] [PubMed] [Google Scholar]
- 25. Watson L, Das R, Farquhar R, Langerman H, Barnett AH. Consequences of delaying treatment intensification in type 2 diabetes: evidence from a UK database. Curr Med Res Opin. 2016;32:1465‐1475. [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharya R, Zhou S, Wei W, Ajmera M, Sambamoorthi U. A real‐world study of the effect of timing of insulin initiation on outcomes in older medicare beneficiaries with type 2 diabetes mellitus. J Am Geriatr Soc. 2015;63:893‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Punthakee Z, Miller ME, Simmons DL, et al. Durable change in glycaemic control following intensive management of type 2 diabetes in the ACCORD clinical trial. Diabetologia. 2014;57:2030‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nichols GA, Koo YH, Shah SN. Delay of insulin addition to oral combination therapy despite inadequate glycemic control: delay of insulin therapy. J Gen Intern Med. 2007;22:453‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535‐1540. [DOI] [PubMed] [Google Scholar]
- 30. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28:600‐606. [DOI] [PubMed] [Google Scholar]
- 31. Harris SB, Kapor J, Lank CN, Willan AR, Houston T. Clinical inertia in patients with T2DM requiring insulin in family practice. Can Fam Physician. 2010;56:e418‐e424. [PMC free article] [PubMed] [Google Scholar]
- 32. Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57:455‐460. [PMC free article] [PubMed] [Google Scholar]
- 33. Khunti K, Millar‐Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11:3‐12. [DOI] [PubMed] [Google Scholar]
- 34. Bennett H, McEwan P, Bergenheim K, Gordon J. Assessment of unmet clinical need in type 2 diabetic patients on conventional therapy in the UK. Diabetes Ther. 2014;5:567‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kostev K, Dippel FW, Rathmann W. Glycemic control after initiating basal insulin therapy in patients with type 2 diabetes: a primary care database analysis. Diabetes Metab Syndr Obes. 2015;8:45‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khunti K, Caputo S, Damci T, et al. The safety and efficacy of adding once‐daily insulin detemir to oral hypoglycaemic agents in patients with type 2 diabetes in a clinical practice setting in 10 countries. Diabetes Obes Metab. 2012;14:1129‐1136. [DOI] [PubMed] [Google Scholar]
- 37. American Diabetes Association . Standards of medical care in diabetes – 2015. Diabetes Care. 2015;38(suppl 1):S1‐S2. [PubMed] [Google Scholar]
- 38. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin‐naive people with type 2 diabetes on oral glucose‐lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tschope D, Bramlage P, Binz C, Krekler M, Deeg E, Gitt AK. Incidence and predictors of hypoglycaemia in type 2 diabetes – an analysis of the prospective DiaRegis registry. BMC Endocr Disord. 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mogensen UM, Andersson C, Fosbol EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50‐58. [DOI] [PubMed] [Google Scholar]
- 42. Shankar RR, Xu L, Golm GT, et al. A comparison of glycaemic effects of sitagliptin and sulfonylureas in elderly patients with type 2 diabetes mellitus. Int J Clin Pract. 2015;69:626‐631. [DOI] [PubMed] [Google Scholar]
- 43. Vora J, Caputo S, Damci T, et al. Effect of once‐daily insulin detemir on oral antidiabetic drug (OAD) use in patients with type 2 diabetes. J Clin Pharm Ther. 2014;39:136‐143. [DOI] [PubMed] [Google Scholar]
- 44. Sorli C. New developments in insulin therapy for type 2 diabetes. Am J Med. 2014;127(suppl):S39‐S48. [DOI] [PubMed] [Google Scholar]
- 45. Raccah D, Lin J, Wang E, et al. Once‐daily prandial lixisenatide versus once‐daily rapid‐acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications. 2014;28:40‐44. [DOI] [PubMed] [Google Scholar]
- 46. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care. 2014;37:2317‐2325. [DOI] [PubMed] [Google Scholar]
- 47.Authors/Task Force Members, Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035‐3087. [DOI] [PubMed] [Google Scholar]
- 48. Szczech LA, Stewart RC, Su HL, et al. Primary care detection of chronic kidney disease in adults with type‐2 diabetes: the ADD‐CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease). PLoS One. 2014;9:e110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saran R, Li Y, Robinson B, et al. US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67(suppl 1):A7‐A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18:907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. NHS . Diabetes unmet need with basal insulin evaluation. 2015. http://www.hra.nhs.uk/news/research‐summaries/diabetes‐unmet‐need‐with‐basal‐insulin‐evaluation/. Accessed 1 August 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Concomitant medication use (proportion of patients who filed for a prescription at least once) during the 1‐year pre‐index period/at index date and at 12 months post index.
Table S2. Body weight at index date and at 24 months post index.
Figure S1. Additional factors associated with A, HbA1c > 7.0% and B, hypoglycaemia during 3 to 24 months post index.


