Abstract
Objective
Cryolipolysis is a safe and effective non‐surgical procedure for reducing subcutaneous fat. It spares adjacent structures, is associated with few adverse events, and is well‐tolerated by patients. Previous studies involving one or two simultaneous treatment sites have shown no effect on serum lipid levels or liver tests. The purpose of this study was to determine whether multiple same day treatments (abdomen plus both flanks) result in changes in these blood tests, and is safe.
Study Design
Thirty‐five adult males and females underwent same‐day cryolipolysis (CoolSculpting System, ZELTIQ Aesthetics) of the lower abdomen and flanks for reduction of subcutaneous fat. Serum lipids and liver tests were measured prior to treatment and at 1, 4, and 12 weeks post‐treatment. Expected treatment side‐effects were assessed immediately post‐treatment and at the 12‐week follow‐up visit. Adverse events were also monitored.
Methods
Treatment consisted of one cycle to the lower abdomen using a large vacuum applicator and simultaneous treatment of both flanks, one cycle each, with medium vacuum applicators .Time between the abdomen and flanks procedures was not to exceed 30 minutes.
Results
The procedures were well‐tolerated by patients; expected treatment effects were in general mild or moderate, and resolved without intervention. There were no clinically meaningful changes from baseline to any subsequent time point in any serum lipid test. This was also true for all liver tests. There were no treatment‐related adverse events.
Conclusion
Multiple cycle, same day cryolipolysis treatment of the lower abdomen and both flanks is well‐tolerated and safe. It does not lead to changes in serum lipids or liver tests at any of the measured time points following the procedure. Lasers Surg. Med. 49:640–644, 2017. © 2017 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals Inc.
Keywords: non‐invasive body contouring, non‐surgical fat reduction, serum lipids, liver tests
INTRODUCTION
Cryolipolysis (CoolSculpting System, ZELTIQ Aesthetics, Pleasanton, CA) is a non‐invasive procedure for the reduction of subcutaneous fat 1, 2, 3. Cryolipolysis received FDA clearance for the reduction of fat in the flank area in 2010. FDA clearance followed for the abdomen in 2012, for the thighs in 2014, for the submental area in 2015, and for the arms, back, bra fat, and area beneath the buttocks in 2016.
The utility of cryolipolysis is based on the observation that fat tissue is uniquely sensitive to cold‐initiated damage. Thus, when energy is extracted through the skin by means of special applicators, it is possible to selectively trigger apoptosis in adipocytes while preserving adjacent structures such as skin, muscle, and nerves 3, 4. The inflammatory process associated with adipocyte apoptotic cell death peaks at about 2 weeks and is largely resolved by 3 months 1, 4. To date, over four million treatments have been administered worldwide [ZELTIQ, Inc., personal communication]. There is a high degree of patient satisfaction with the procedure itself, as well as its objective and subjective outcomes, and side‐effects are in general minor and transient 2, 5, 6, 7
A theoretical concern with this procedure is that adipocyte apoptosis could result in sufficient release of the contents of the dying cells to raise serum lipid levels and disturb liver function. An earlier study, involving cryolipolysis of two flank sites during the same treatment visit, showed that there were no clinically meaningful changes in any serum lipid levels or liver tests from baseline to any time point tested between 1 day and 3 months following the procedure 8. The aforementioned study delivered treatments at CIF 42 (−10°C) for 30 minutes, but commercial treatments are now typically delivered at −10°C for 60 minutes, depending upon the cryolipolysis applicator.
With cryolipolysis established as a safe and effective alternative to aesthetic surgery, clinicians have performed the procedure on various body regions, such as the abdomen, flanks, inner thighs, outer thighs, and submental areas. Most commonly treated are the flanks and abdomen; some practices now treat both during a single visit 6. Such multiple‐site cryolipolysis procedures presumably lead to more adipocyte death, and therefore, greater subsequent lipid release. The purpose of this study was to determine whether multiple cryolipolysis cycles performed with current commercial treatment parameters during a single visit have any effect on blood lipids or liver tests, and to determine their safety.
MATERIALS AND METHODS
This was a three‐center, prospective, single arm study involving cryolipolysis treatment of the lower abdomen, and both flanks. Male and female adults with stable weight and a BMI < 30, who agreed to refrain from major changes to their diet or exercise routine for the duration of the study were eligible. Key exclusions were prior fat reduction in the treatment areas, diabetes, or a history of cold‐induced pathology such as cryoglobulinemia. All subjects signed an IRB‐approved informed consent document agreeing to participate.
Prior to treatment, blood was drawn for measurement of serum lipids and liver tests. Measured lipids included triglycerides and VLDL, HDL, LDL, and total cholesterol. Liver tests were total bilirubin, alkaline phosphatase, ALT, and AST.
In all subjects, the cryolipolysis procedures employed a large vacuum applicator (CoolMax) for the abdomen, and medium vacuum applicators (either CoolCore or CoolCurve +) for the flanks. Two CoolSculpting System control units were used. The surface areas of the cooling plates of CoolCore and CoolCurve+ medium applicators are roughly equivalent (9.1 and 10.1 in2, respectively), while the surface area of the large CoolMax applicator is approximately twice that size (19.8 in2). The time between the abdominal and flank treatments was to be less than 30 minutes. All treatment cycles employed the commercial standard 60 minutes at CIF 42 (−10°C). Following the procedures, pain/discomfort scores were elicited from the patients, and a clinical assessment of the treatment sites was performed. Categories assessed included bruising, erythema/purpura, edema, numbness, and tingling. Since, these are all expected sequelae of the cryolipolysis procedure, they were specifically evaluated and not considered adverse events.
Blood was drawn before cryolipolysis and again at 1, 4, and 12 weeks post‐procedure after an overnight fast. All blood samples were analyzed at Quest Diagnostics. Immediately after the procedure, and again at the 12 week visit, adverse events were solicited. Co‐primary endpoints in this study were the results of the previously mentioned blood tests, and adverse events.
It was felt that 30 completed patients would provide sufficient data to meet the two primary study objectives. Thus 35 patients were enrolled to allow for dropouts. Demographic measures were characterized by calculating mean values with standard errors. To explore changes from baseline to subsequent values of each laboratory analyte, a repeated measures ANCOVA was fit. The mixed model approach was used, with change from baseline being the dependent variable and post‐treatment visit value being the independent variable. The compound symmetric correlation structure was used. The resulting least squared means from the model for each visit, with standard deviations, were calculated. P‐values were two‐sided, with a significance level of 0.05. No corrections were made for multiple testing. The safety endpoint was characterized simply by a count of qualifying events.
This study was approved by RCRC IRB, Austin, TX, on 18 February, 2013, and was registered with clinicaltrials.gov (number NCT01814007).
RESULTS
Table 1 summarizes the demographics of the patient population. A total of 35 patients were enrolled, 27 female, and 8 male, ranging in age from 20 to 67 years old (mean 45.2 years). The mean BMI was 24.7, with a range of 18.0–29.7.
Table 1.
Demographic Characteristics
| Characteristic (n = 35) | Mean | Standard error | Range |
|---|---|---|---|
| Age (years) | 45.2 | 2.2 | 20–67 |
| Weight (lbs) | 150.8 | 3.7 | 105–196 |
| Height (in) | 65.4 | 0.6 | 59–72 |
| BMI | 24.7 | 0.5 | 18.0–29.7 |
One patient was withdrawn after completing the abdominal treatment, when it was found impossible to achieve adequate tissue draw into the applicator for the flank treatments. All other patients completed all prescribed treatments and all scheduled blood tests.
As expected 1, erythema, numbness, and edema were experienced by the majority of patients immediately following the procedures. Less common were tingling and bruising (Table 2). In most cases these signs and symptoms were considered by the investigator to be minor or moderate in severity, and in all cases they resolved without treatment. At the Week 12 examination, all treatment sites appeared normal and no subjects reported any associated symptoms. Immediately following the procedures, the mean pain score was 4 (SD ± 3) on a scale of 1–10. There were no reports of late‐onset pain.
Table 2.
Clinical Assessments—All Treatment Sites Combined
| Score immediately post‐treatment | ||||
|---|---|---|---|---|
| Assessment parameter a | 0 Absent | 1 Minor | 2 Moderate | 3 Severe |
| Bruising | 64 (62%) | 31 (30%) | 7 (7%) | 1 (1%) |
| Erythema/purpura | 0 (0%) | 54 (52%) | 46 (45%) | 3 (3%) |
| Edema | 31 (30%) | 46 (45%) | 25 (24%) | 1 (1%) |
| Numbness | 14 (14%) | 30 (29%) | 47 (46%) | 12 (12%) |
| Tingling | 56 (54%) | 32 (31%) | 12 (12%) | 3 (3%) |
The abdominal site and each flank site were evaluated separately. In this Table all such evaluations are combined. One subject was treated on the abdomen only—flank treatments were not possible due to inadequate tissue draw into the applicator.
Table 3 displays the mean serum lipid tests. Values were all well within the reference range, and varied little from baseline to subsequent time points. Repeated measures ANCOVA (uncorrected for multiple testing) showed no statistically significant changes from baseline in total, HDL, LDL, or VLDL cholesterol. The mean values for total cholesterol at baseline and 1, 4, and 12 weeks post‐treatment are shown in Figure 1 and mean values for triglycerides are shown in Figure 2. The mean triglyceride value at Week 12, 83.4 mg/dl, was slightly but statistically significantly different from the baseline value of 77.0 mg/dl (P = 0.04), though still well below the upper limit of the reference range (150 mg/dl).
Table 3.
Serum Lipid Values Over Time
| Analyte (units) [Reference range] | Time point | Mean | SD | P‐value* |
|---|---|---|---|---|
| Cholesterol (mg/dl) [125–200] | Baseline | 186.5 | 39.1 | – |
| Week 1 | 185.6 | 36.9 | 0.57 | |
| Week 4 | 188.4 | 37.6 | 0.85 | |
| Week 12 | 189.2 | 39.8 | 0.69 | |
| Triglycerides (mg/dl) [<150] | Baseline | 77.0 | 32.5 | – |
| Week 1 | 80.5 | 33.2 | 0.18 | |
| Week 4 | 77.7 | 33.2 | 0.53 | |
| Week 12 | 83.4 | 38.6 | 0.04 | |
| HDL Cholesterol (mg/dl) [>45 (F), >40 (M)] | Baseline | 71.0 | 26.8 | – |
| Week 1 | 68.6 | 26.4 | 0.13 | |
| Week 4 | 70.6 | 27.4 | 0.50 | |
| Week 12 | 73.8 | 31.7 | 0.50 | |
| LDL Cholesterol (mg/dl) [<130] | Baseline | 100.0 | 23.1 | – |
| Week 1 | 100.9 | 26.8 | 0.84 | |
| Week 4 | 102.7 | 21.7 | 0.46 | |
| Week 12 | 102.5 | 28.7 | 0.49 | |
| VLDL Cholesterol (mg/dl) [<30] | Baseline | 15.7 | 6.8 | – |
| Week 1 | 16.1 | 6.5 | 0.32 | |
| Week 4 | 15.5 | 6.6 | 0.89 | |
| Week 12 | 16.8 | 7.9 | 0.06 |
A P‐value <0.05 is considered statistically significant.
Figure 1.
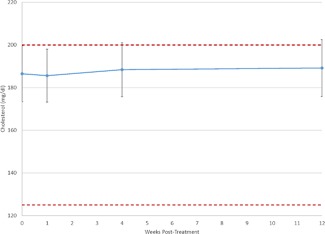
Plot of mean cholesterol values reveals no significant change over time. Further, mean values ± their 95% confidence interval (denoted by the error bars) remain near the reference range (shown as dashed lines).
Figure 2.
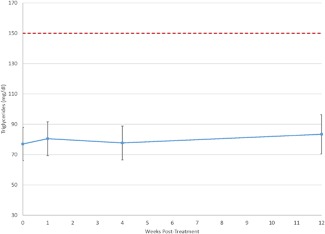
Plot of mean triglyceride values reveals no clinically meaningful change over time. Further, mean values ± their 95% confidence interval (denoted by the error bars) remain well below the upper limit of the reference range (shown as a dashed line).
Table 4 displays mean liver test values at each time point. As with the lipid tests, all mean values were well within the reference range, and post‐treatment values varied little from baseline. The mean values for AST/SGOT at each time point are shown in Figure 3 and mean values for ALT/SGPT are shown in Figure 4. Repeated measures ANCOVA (uncorrected) showed that mean bilirubin levels at weeks 1 (0.53 mg/dl) and 12 (0.58 mg/dl) were slightly, but statistically significantly lower than the baseline value of 0.65 mg/dl.
Table 4.
Liver‐Related Test Values Over Time
| Analyte (units)[Reference range] | Time point | Mean | SD | P‐value* |
|---|---|---|---|---|
| Total Bilirubin (mg/dl) [0.2–1.2] | Baseline | 0.65 | 0.4 | – |
| Week 1 | 0.53 | 0.2 | >0.01 | |
| Week 4 | 0.61 | 0.3 | 0.25 | |
| Week 12 | 0.58 | 0.3 | 0.02 | |
| Alkaline Phosphatase (U/L) (40–115 [≥20 M], 33–115[20‐49F], 33–130[≥50 F]) | Baseline | 62.1 | 15.7 | – |
| Week 1 | 63.9 | 14.7 | 0.4 | |
| Week 4 | 63.7 | 15.6 | 0.43 | |
| Week 12 | 62.1 | 15.4 | 0.89 | |
| AST‐SGOT (U/L) (10–40 [20–49 M], 10–35 [≥50 M], 10–30 [20‐44 F], 10–35 [≥45 F]) | Baseline | 19.7 | 5.2 | – |
| Week 1 | 19.8 | 6.9 | 0.99 | |
| Week 4 | 22.2 | 8.8 | 0.20 | |
| Week 12 | 23.0 | 16.3 | 0.09 | |
| ALT‐SGPT (U/L) (9‐46 [M], 6‐29[F]) | Baseline | 17.7 | 7.5 | – |
| Week 1 | 20.4 | 12.4 | 0.13 | |
| Week 4 | 20.0 | 10.6 | 0.21 | |
| Week 12 | 19.4 | 15.3 | 0.32 |
A P‐value <0.05 is considered statistically significant.
Figure 3.
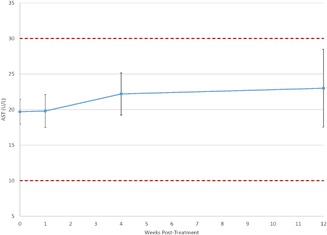
Plot of mean AST values reveals no significant change over time. Further, mean values ± their 95% confidence interval (denoted by the error bars) remain well within the reference range (shown as dashed lines).
Figure 4.
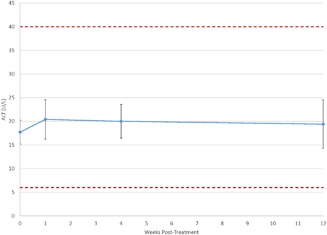
Plot of mean ALT values reveals no significant change over time. Further, mean values ± their 95% confidence interval (denoted by the error bars) remain well within the reference range (shown as dashed lines).
Three patients experienced adverse events, all judged by the investigator to be unrelated to the procedures. One had transaminases above the upper limit of the reference range for the first time at Week 12 (AST = 104, ALT = 51). These were associated with alcohol use the evening before the blood draw; they subsequently returned to within the normal range. Another patient had bronchitis, which resolved without specific treatment, and a third was diagnosed with an inguinal hernia 36 days after treatment. The hernia site was unassociated with any of the treatment areas, and was successfully repaired.
DISCUSSION
Mean values of all measured liver and lipid tests were well within the reference range at every tested time point, and were never clinically meaningfully different from the baseline values. Since, each of the nine analytes tested was measured at three time points after baseline, 27 P‐values were calculated, without correction for multiple testing. In only three cases were P‐values <0.05, two involving total bilirubin, and one triglycerides. Mean total bilirubin was statistically significantly lower than the baseline value at weeks 1 and 12. The numerical differences were quite small, and there is no clinical importance to a lower bilirubin—these small changes were almost certainly due to random variation.
Of the five lipids measured, only triglycerides showed a statistically significant change from baseline, and this occurred only at Week 12. That mean increase was less than 7 mg/dl, (from 77.0 to 83.4), a clinically trivial difference. Moreover, the Week 12 value was far below the upper limit of the reference range of 150. The slightly increased Week 12 mean was completely driven by the results from a single patient. That patient's baseline triglyceride level was 169, at Week 1 it had decreased to 142, at Week 4 was 202, and at Week 12 was 223. Such variation is quite typical of serum triglycerides 9. These laboratory data strongly support the conclusion that same‐day cryolipolysis at multiple sites has no effect on liver tests or serum lipids.
It is not surprising that cryolipolysis, even when performed on the same treatment visit at the equivalent of four flank treatment sites, has no effect on serum lipids. Treatment of one flank leads to the loss of about 40 g of fat over 3 months 10. Thus, treating the abdomen and bilateral flanks will cause about 160 g of fat to be lost during this same time period. This would average about 1.8 g of fat release per day. To put this figure in perspective, the typical American diet contains at least 75 g fat/day 11. Moreover, it has been shown that consuming as much as 261 g of fat per day causes no ill effects or laboratory abnormalities 12. With intravenous total parenteral nutrition, patients can safely metabolize ∼250 g of fat/day 13, and with the triglyceride clamp technique it has been shown that subjects can clear 300–450 g of triglycerides per day without ill effects 14. Thus, even if the treated adipocytes released their fat stores much more quickly, there would seem to be little chance that this would lead to any harmful effects.
Treatment of four sites on the same day with the cryolipolysis procedure did not appear to increase the frequency or intensity of the expected sequelae immediately post‐procedure. As in other studies involving the treatment of fewer sites, erythema, numbness, and edema were most common, followed by a lesser incidence of tingling, and bruising 1, 2. In all cases, these signs and symptoms resolved without treatment. No other adverse events related to the procedure or the device were identified.
CONCLUSION
In conclusion, same day treatment of the abdomen and two flanks with the cryolipolysis procedure has no effect on serum lipid levels or liver tests at any time point following the procedures. Such multiple site treatment appears safe and well‐tolerated.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Dr Klein is a consultant to Zeltiq Aesthetics, Inc. Drs Bachelor, Becker, and Bowes were paid investigators for this study. Dr Bachelor is on the Zeltiq speaker's bureau.
REFERENCES
- 1. Jalian HR, Avram MM. Cryolipolysis: A historical perspective and current clinical practice. Semin Cutan Med Surg 2013; 32:31–34. [PubMed] [Google Scholar]
- 2. Ingargiola MJ, Motakef S, Chung MT, Vasconez HC, Sasaki GH. Cryolipolysis for fat reduction and body contouring: Safety and efficacy of current treatment paradigms. Plast Reconstr Surg 2015; 135:1581–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derrick CD, Shridharani SM, Broyles JM. The Safety and efficacy of cryolipolysis: A systematic review of available literature. Aesthet Surg J 2015; 35:830–836. [DOI] [PubMed] [Google Scholar]
- 4. Avram MM, Harry RS. Cryolipolysis for subcutaneous fat layer reduction. Lasers Surg Med 2009; 41:703–708. [DOI] [PubMed] [Google Scholar]
- 5. Dierickx CC, Mazer JM, Sand M, Koenig S, Arigon V. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg 2013; 39:1209–1216. [DOI] [PubMed] [Google Scholar]
- 6. Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J 2013; 33:835–846. [DOI] [PubMed] [Google Scholar]
- 7. Krueger N, Mai SV, Luebberding S, Sadick NS. Cryolipolysis for noninvasive body contouring: Clinical efficacy and patient satisfaction. Clin Cosmet Investig Dermatol 2014; 7:201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein KB, Zelickson B, Riopelle JG, Okamoto E, Bachelor EP, Harry RS. Non‐invasive cryolipolysis for subcutaneous fat reduction does not affect serum lipid levels or liver function tests. Lasers Surg Med 2009; 41:785–790. [DOI] [PubMed] [Google Scholar]
- 9. Bachorik PS, Myers GL, Ross JW, Stein EA, Warnick GR, Wood PD. Part Three: Recommendations for triglyceride measurement, National Cholesterol Education Program Working Group on lipoprotein measurement. National Heart, Lung and Blood Institute. NHI Publication No. 95–3044 1995;125–165.
- 10. Garibyan L, Sipprell WH, Jalian HR, Sakamoto FH, Avram M, Anderson RR. Three‐dimensional volumetric quantification of fat loss following cryolipolysis. Lasers Surg Med 2014; 46:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin B‐H, Guthrie J. Nutritional quality of food prepared at home and away from home, 1977–2008. Economic Research Service, USDA 2012. Available from: http://www.ers.usda.gov/media/977765/summaryeib105.pdf
- 12. Kechagias S, Ernersson A, Dahlqvist O, Lundberg P, Lindstrom T, Nystrom FH. Fast‐food‐based hyper‐alimentation can induce rapid and profound elevation of serum alanine aminotransferase in healthy subjects. Gut 2008; 57:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crook MA. Lipid clearance and total parenteral nutrition: The importance of monitoring plasma lipids. Nutrition 2000; 16:774–775. [DOI] [PubMed] [Google Scholar]
- 14. Nordenström J, Thörne A, Aberg W, Carneheim C, Olivecrona T. The hypertriglyceridemic clamp technique. Studies using long‐chain and structured triglyceride emulsions in healthy subjects. Metab Clin Exp 2006; 55:1443–1450. [DOI] [PubMed] [Google Scholar]


