Abstract
Objective
Juvenile idiopathic arthritis (JIA) frequently affects the temporomandibular joints (TMJs) and is often undetected by history, examination, and plain imaging. Qualitative assessment of gadolinium‐enhanced magnetic resonance images (MRIs) is currently the standard for diagnosis of TMJ synovitis associated with JIA. The purpose of this study is to apply a quantitative analysis of synovial enhancement to MRIs of patients with and without JIA to establish a disease threshold and sensitivity and specificity for the technique.
Methods
This is a retrospective case–control study of children (age ≤16 years) who had MRIs with gadolinium including the TMJs. Subjects were divided into a JIA group and a control group. From a coronal T1‐weighted image, a ratio (enhancement ratio [ER]) of the average pixel intensity within three 0.2‐mm2 regions of interest (ROIs) in the TMJ synovium to that of a 50‐mm2 ROI of the longus capitis muscle was calculated. Receiver operating characteristic curves were used to determine the sensitivity and specificity. The inter‐ and intraexaminer reliability was evaluated with Bland‐Altman plots and 2‐way mixed, absolute agreement intraclass correlation coefficients.
Results
There were 187 and 142 TMJs included in the JIA and control groups, respectively. An ER threshold of 1.55 had a sensitivity and specificity for detecting synovitis of 91% and 96%, respectively. The inter‐ and intraexaminer reliability was excellent.
Conclusion
Calculating a ratio of pixel intensity between the TMJ synovium and the longus capitis muscle is a reliable way to quantify synovial enhancement. An ER of 1.55 differentiates normal TMJs from those affected by inflammatory arthritis.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) affects 1 in 1,000 children and adolescents worldwide, making it the most common pediatric rheumatic disease 1. Magnetic resonance imaging (MRI) studies have shown that the temporomandibular joints (TMJs) are affected in 39–75% of patients with JIA 2, 3, 4, 5. The course of TMJ disease is typically insidious and asymptomatic, so it is often unrecognized until irreversible changes have occurred 5, 6. Longstanding TMJ arthritis can result in pain, limitation in mouth opening, facial asymmetry, retrognathism, and malocclusion 7. Early diagnosis leading to treatment may minimize or prevent these secondary effects 8.
Box 1. Significance & Innovations.
A simple, reliable, and reproducible method to quantify temporomandibular joint (TMJ) synovial enhancement using magnetic resonance imaging (MRI) with gadolinium is described.
A threshold ratio of TMJ synovial uptake divided by uptake in the longus capitis muscle (standard) that reliably differentiates normal TMJ synovium from synovitis associated with inflammatory arthritis is reported. This technique could replace the usual subjective, qualitative method for evaluating TMJ MRIs in patients with inflammatory arthritis and might be applicable to other joints.
MRI with gadolinium infusion is the gold standard for the detection of TMJ inflammatory arthritis 2, 9, 10. MRI findings in JIA include synovial enhancement, condylar articular surface flattening, and alteration of the shape of the articular disc. Synovial enhancement is the most common early finding of inflammatory arthritis 11 and has been shown to correlate with clinical symptoms and predict the future development of joint erosion 12, 13, 14, 15, 16, 17, 18.
The evaluation of synovitis on an MRI is subjective and is typically reported qualitatively, resulting in interexaminer variability in the assessment of disease 6, 9. In addition, a low level of synovial enhancement is frequently seen in nonarthritic TMJs (Figure 1) 19. While synovitis is a good indicator of disease, some clinicians are hesitant to rely on this measure, because there is no standardized quantitative method to differentiate synovial enhancement that can occur in normal TMJs from synovitis due to inflammatory arthritis 10, 19, 20, 21. A reliable technique to quantify synovitis and to distinguish this pathologic finding from synovial enhancement in healthy joints would improve the assessment of disease, facilitate early intervention when indicated, and enhance the evaluation of treatment outcomes.
Figure 1.
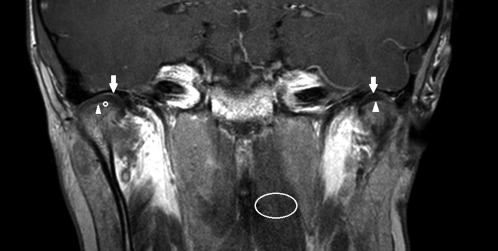
T1‐weighted gadolinium‐enhanced magnetic resonance image (coronal view) of a healthy 10‐year‐old boy without temporomandibular joint (TMJ) pathology. The inferior (arrowheads) and superior (arrows) joint spaces are shown bilaterally. Regions of interest used to calculate the enhancement ratio (ER) are indicated at the right inferior TMJ space (circle) and left longus capitis muscle (ellipse). ER = 1.33 (left) and 1.21 (right).
To achieve this goal, we developed a method to quantify TMJ synovial enhancement on MRIs with gadolinium 22. This technique utilizes a ratio of synovial enhancement to an internal standard. Ratios have been shown to improve the detection of small differences in signal intensity and control for variations in imaging technique 19, 23, 24, 25. The longus capitis muscle was chosen as the internal control because it is uninvolved in the TMJ pathology, it is a homogeneous muscle that enhances uniformly with the contrast agent, and it is visualized within the same coronal images as the TMJs. The use of muscle as a reference controls for small variations in the timing of postcontrast image acquisition, because muscle enhancement is relatively stable for more than 6 minutes after contrast injection 19. This quantitative assessment has been tested in a series of patients without TMJ pathology 22.
The purposes of this study were to apply this quantitative assessment to contrast‐enhanced MRIs of TMJs affected by JIA, compare synovial enhancement levels of the arthritic TMJs with nonarthritic controls, and develop a threshold enhancement ratio (TMJ synovium divided by longus capitis muscle) that consistently differentiates arthritic from nonarthritic TMJs. We hypothesized that this method would allow clinicians to quantify synovitis with high sensitivity and specificity, and with minimal intra‐ and interexaminer variability.
MATERIALS AND METHODS
Study design and subjects
This is a multicenter, retrospective case–control study including patients from Boston Children's Hospital, Massachusetts Eye and Ear Infirmary, and Massachusetts General Hospital (MGH). The institutional review boards at all 3 hospitals approved this project (MGH protocol No. 2013P000405). Subjects were divided into a JIA group and a control group.
The JIA group was comprised of children and adolescents (age ≤16 years), treated at Boston Children's Hospital from March 2007 to January 2015, who had a diagnosis of JIA made by a pediatric rheumatologist, an MRI with gadolinium that included both TMJs (from the superior aspects of the glenoid fossae to the sigmoid notches of the mandible) and the longus capitis muscles, and complete clinical records. Exclusion criteria were MRIs without gadolinium or incomplete imaging of the TMJs/longus capitis muscles, incomplete records, TMJ pain or pathology unrelated to JIA (such as myofascial pain disorder, which was diagnosed based on muscle tenderness to palpation and limited mandibular range of motion without clinical or radiographic evidence of TMJ arthritis) 26, and a history of facial trauma.
The control group included children and adolescents (age ≤16 years) treated at Massachusetts Eye and Ear Infirmary or Massachusetts General Hospital from February 2011 to December 2013, who had an MRI with gadolinium that included both TMJs and longus capitis muscles but was ordered for an indication other than TMJ pathology, the same MRI contrast agent as used for the JIA group, no symptoms or signs of TMJ or jaw pathology, complete clinical records, and no history of facial trauma. The subjects for this group were derived from the previous study demonstrating the quantitative analysis technique on normal TMJs 22, which included patients beginning in January 2006. The contrast agent utilized for MRIs at these institutions was changed in early 2011 to match the imaging protocol used at Boston Children's Hospital, so control subjects from the earlier study who had obtained MRIs prior to this change were eliminated from the current study.
Variables collected from the clinical records included, as applicable, age, sex, and current and historical systemic or local TMJ treatment for inflammatory arthritis. The predictor variables were JIA subtype, age at diagnosis, age at the time of MRI, sex, other (non‐TMJ) joints affected by arthritis, family history of autoimmune disease, and medications used for the treatment of JIA. JIA subtypes of oligoarticular, polyarticular, psoriatic, or systemic arthritis were assigned based on the assessment of the treating rheumatologist, because data obtained from the medical record were insufficient to categorize the disease by International League of Associations for Rheumatology criteria. The primary outcome variable was the ratio of signal intensity of the TMJ synovium to that of an internal standard (the longus capitis muscle).
Image acquisition
For the JIA group, MRIs were performed on one of the following 3 Tesla scanners: the Magnetom Verio 3T, the Magnetom Trio 3T, or the Magnetom Skyra 3T (all from Siemens Healthcare). Using a 32‐channel head coil, the following imaging sequences of the TMJs were acquired for each patient: axial T1‐weighted, coronal proton density–weighted, and sagittal proton density–weighted.
For the control group, MRIs were performed on a Tesla Philips 3T scanner (Philips Healthcare). Using a 16‐channel head coil, the following imaging sequences of the TMJs were acquired for each patient: axial T1‐weighted, coronal proton density–weighted, and sagittal proton density–weighted.
For both groups, sagittal and coronal T1‐weighted sequences with fat saturation were performed through the TMJs at approximately 6 minutes following the intravenous administration of gadopentetate dimeglumine (Magnevist, Bayer Healthcare Pharmaceuticals) at a dose of 0.1 mmole/kg.
Calculation of enhancement ratio
The image visualization software Synapse (Fujifilm) and its picture archiving and communication system were used to assess each MRI for both groups (Figure 1 and Figure 2). Using images from the coronal postcontrast fat‐saturated T1‐weighted series, a 0.2‐mm2 region of interest (ROI) within the superior TMJ space was defined. The pixel intensity of the synovium was measured for this ROI. This process was repeated in order to acquire a total of 3 measurements from the superior TMJ space and 3 measurements from the inferior TMJ space. These measurements were then averaged, and the resulting value was recorded on a spreadsheet as “average TMJ synovial pixel intensity.” A 50‐mm2 ROI was then chosen within the longus capitis muscle from the same coronal series, and the pixel intensity was measured for this ROI and recorded as “longus capitis pixel intensity.” An enhancement ratio (ER) was calculated according to the following equation:
Figure 2.

T1‐weighted gadolinium‐enhanced magnetic resonance image (coronal view) of a 14‐year‐old boy with juvenile idiopathic arthritis and inflammatory temporomandibular joint (TMJ) arthritis. The superior (arrows) and inferior (arrowheads) joint spaces are shown bilaterally. Regions of interest used to calculate the enhancement ratio (ER) are indicated at the inferior right TMJ space (circle) and left longus capitis muscle (ellipse). Qualitative radiologist interpretation noted moderate to severe bilateral TMJ synovial enhancement. Quantitative analysis found ER = 2.48 (right) and 2.52 (left).
Each joint was assessed separately and all results were expressed as ERs.
Two examiners who were blinded to the clinical information scored all MRIs individually, and one of these examiners (PV) scored each image on 2 separate occasions separated by at least 24 hours. Examiners were trained in the image analysis technique by a board‐certified neuroradiologist (PC). One of the examiners is a neuroradiologist (MB) and one is a student research fellow from the Harvard School of Dental Medicine (PV).
Statistical analysis
Continuous data were compared using Student's t‐test and binary proportions using Fisher's exact test. Relationships of the predictor variables to the ER were assessed using multivariable linear regression modeling using generalized estimating equations to account for the multiple joints per patient. The Wald test was used to determine the significance of each variable. Inter‐ and intraexaminer reliability was evaluated using Bland‐Altman plots and a 2‐way mixed absolute agreement intraclass correlation coefficient (ICC).
Receiver operating characteristic (ROC) curves were constructed to identify the cutoff value for achieving the maximum combination of sensitivity and specificity by the Youden J index, using the average of the ERs. Data were also analyzed using a random‐effects mixed model logistics regression analysis, and a probability curve was derived in order to illustrate the relationship between the ER and the likelihood of JIA 27, 28. Statistical analysis was performed using SPSS, version 21.0 (IBM). Two‐tailed P values less than 0.05 were considered statistically significant.
RESULTS
Demographic data
Eighty‐nine patients with JIA had 173 gadolinium‐enhanced TMJ MRIs during the study period. Of these, 74 subjects (80% female, mean ± SD age 13.27 ± 3.81 years) with 114 MRIs, with clear visualization of 211 TMJs and longus capitis muscles, met the inclusion criteria. For the study sample, 50 subjects had 1 MRI, 13 had 2 MRIs, and 11 had 3 or more MRIs included. All included TMJs were reported by the reading radiologist to have synovitis. The remaining MRIs were excluded due to inability to visualize and assess the synovium (n = 10), inability to localize the superior and inferior synovial cavities due to image distortion (n = 7), lack of coronal series (n = 19), heterogeneous or low signal in the longus capitis muscle precluding assessment (n = 19), and artifact due to orthodontic appliances (n = 4).
The JIA subtypes in the sample were classified as oligoarticular (n = 22 subjects, 62 TMJs), polyarticular (n = 19 subjects, 55 TMJs), psoriatic (n = 27 subjects, 76 TMJs), and systemic (n = 6 subjects, 18 TMJs) (Table 1). There was a family history of an autoimmune disease in 48 subjects (138 joints). The control group included 71 subjects (54% female, mean ± SD age 11.4 ± 3.5 years) with clear visualization of 142 normal TMJs and longus capitis muscles (Table 2). Most of the MRIs were of the temporal bone and the most common indications were sensorineural hearing loss, cholesteatoma, and facial nerve palsy.
Table 1.
Sample characteristicsa
| JIA group | |
| Sample size, no. | 74 |
| Total MRIs | 114 |
| Total joints | 211 |
| Age at JIA diagnosis, mean ± SD years | 7.24 ± 4.24 |
| Age at MRI, mean ± SD years | 13.19 ± 3.78 |
| Female | 61 (82) |
| Family autoimmune disease history | 48 (65) |
| JIA subtype | |
| Psoriatic | 27 (36) |
| Oligoarticular | 22 (30) |
| Polyarticular | 19 (26) |
| Systemic | 6 (8) |
| Medication exposure | |
| Methotrexate | 25 (34) |
| Nonsteroidal antiinflammatory drugs | 14 (19) |
| Adalimumab | 10 (14) |
| Leflunomide | 7 (9) |
| Etanercept | 7 (9) |
| Sulfasalazine | 5 (7) |
| Infliximab | 4 (5) |
| Prednisone | 2 (3) |
| Control group | |
| Sample size, no. | 71 |
| Total MRIs | 71 |
| Total joints | 142 |
| Age at MRI, mean ± SD years | 11.4 ± 3.5 |
| Female | 38 (54) |
| MRI type | |
| Temporal bone | 60 (85) |
| Optic nerve | 11 (15) |
| Indication for MRI | |
| Sensorineural hearing loss | 27 (38) |
| Cholesteatoma | 13 (18) |
| Facial nerve palsy | 11 (16) |
| Otalgia | 9 (13) |
| Optic neuropathy | 8 (11) |
| Papilledema | 3 (4) |
Values are the no. (%) unless otherwise indicated. JIA = juvenile idiopathic arthritis; MRI = magnetic resonance imaging.
Table 2.
Analysis of synovial enhancement ratio to study variables for control and JIA groupsa
| Variable | Mean ± SD enhancement ratio | P |
|---|---|---|
| Control group | ||
| Age | 0.735 | |
| Sex | 0.447 | |
| Female | 1.24 ± 0.14 | |
| Male | 1.25 ± 0.15 | |
| Antiinflammatory medication | 0.183 | |
| Yes | 1.29 ± 0.19 | |
| No | 1.30 ± 0.17 | |
| MRI type | 0.403 | |
| Temporal bone | 1.27 ± 0.16 | |
| Optic nerve | 1.25 ± 0.16 | |
| MRI indication | 0.850 | |
| Sensorineural hearing loss | 1.29 ± 0.14 | |
| Cholesteatoma | 1.30 ± 0.11 | |
| Facial nerve palsy | 1.29 ± 0.13 | |
| Otalgia | 1.28 ± 0.11 | |
| Optic neuropathy | 1.27 ± 0.17 | |
| Papilledema | 1.26 ± 0.17 | |
| JIA group | ||
| Age | 0.354 | |
| Sex | 0.045b | |
| Female | ||
| Male | ||
| Family history of autoimmune disease | 0.093 | |
| Positive | 2.51 ± 0.79 | |
| Negative | 2.53 ± 0.80 | |
| JIA subtype | 0.500 | |
| Psoriatic | 2.42 ± 0.81 | |
| Oligoarticular | 2.55 ± 0.84 | |
| Polyarticular | 2.58 ± 0.79 | |
| Systemic | 2.70 ± 0.63 | |
| Medication | ||
| Methotrexate | 2.45 ± 0.8 | 0.029b |
| Nonsteroidal antiinflammatory drugs | 2.49 ± 0.75 | 0.018b |
| Adalimumab | 2.62 ± 0.82 | 0.620 |
| Leflunomide | 2.24 ± 0.71 | 0.014b |
| Etanercept | 2.59 ± 0.86 | 0.254 |
| Sulfasalazine | 3.01 ± 0.87 | 0.485 |
| Infliximab | 2.43 ± 0.70 | 0.035b |
| Prednisone | 2.03 ± 0.53 | 0.041b |
JIA = juvenile idiopathic arthritis; MRI = magnetic resonance imaging.
Statistically significant.
Assessment of synovial enhancement
The mean ER in the JIA group was 2.52 ± 0.79, and that in the control group was 1.28 ± 0.16 (P < 0.001). Males in the JIA group had a higher ER than females (P = 0.045). Neither JIA subtype nor family history of autoimmune disease had a significant effect on the ER. Within the JIA group, subjects with exposure to the following medications had significantly lower ER than those without exposure: methotrexate (P = 0.029), nonsteroidal antiinflammatory drugs (P = 0.018), infliximab (P = 0.035), prednisone (P = 0.041), and leflunomide (P = 0.014). Exposure to adalimumab (P = 0.62), etanercept (P = 0.254), and sulfasalazine (P = 0.485) was not associated with a decreased ER. For the control group, there was no effect of age, sex, MRI type, or medication (presence or absence of antiinflammatory medication) on the ER.
The ROC analysis indicated a sensitivity of 91% and a specificity of 96% in detecting synovitis for an ER cutoff value of 1.55 as determined by the Youden J index. The area under the curve was 0.959 (95% confidence interval 0.937–0.980; P < 0.001) (Figure 3). The probability curve derived from logistic regression analysis revealed a high likelihood of JIA for an ER ≥1.55 (65%), 1.70 (85%), 1.90 (96%), and 2.00 (98%) (Figure 4).
Figure 3.
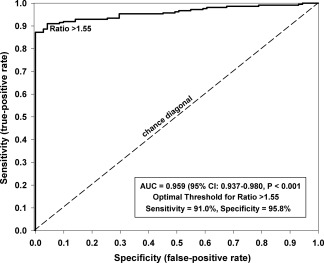
Receiver operating characteristic curve for synovial enhancement ratio (ER), indicating an area under the curve (AUC) of 0.959 (95% confidence interval [95% CI] 0.937, 0.980; P < 0.001), sensitivity of 91%, and specificity of 96%, for an optimal threshold value of ER = 1.55.
Figure 4.
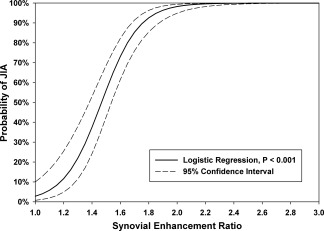
Probability curve derived from logistic regression analysis, demonstrating a high likelihood of juvenile idiopathic arthritis (JIA) for an enhancement ratio ≥1.55 (65%), 1.70 (85%), 1.90 (96%), and 2.00 (98%).
Interexaminer and intraexaminer reliability
Bland‐Altman analyses were performed for interexaminer and intraexaminer variability for both groups. For the control group, the intraexaminer analysis demonstrated a mean difference of 0.02 with 95% limits of agreement −0.10, 0.14 (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22911/abstract). Variability was constant across the observed range (Pearson's r = −0.11; P = 0.36). The interexaminer analysis for the control group found a mean difference of −0.04 with 95% limits of agreement −0.28, 0.20 (see Supplementary Figure 2, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22911/abstract). The bias is constant across the observed range (Pearson's r = −0.12; P = 0.32).
For the JIA group, the intraexaminer Bland‐Altman analysis showed a mean difference of −0.10 with 95% limits of agreement −0.50, 0.30 (see Supplementary Figure 3, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22911/abstract). Variability was constant across the observed range (Pearson's r = −0.10; P = 0.40). The interexaminer analysis for the JIA group yielded an average difference between the 2 raters of 0 with 95% limits of agreement −1.0, 1.0 (see Supplementary Figure 4, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.22911/abstract). Nonsignificant Pearson's correlation between the average of the raters and the difference between them (Pearson's r = 0.02; P = 0.74) indicates that variability between raters was similar across the range of signal intensity ratios. ICCs were considered excellent (defined as ICC >0.8) for interexaminer and intraexaminer single‐measure absolute agreement (ICC 0.830; P < 0.001, and ICC 0.956; P < 0.001, respectively).
DISCUSSION
Inflammatory arthritis of the TMJs affects the majority of patients with JIA 2, 3, 4, 5, but cannot be reliably assessed by history or physical examination 29. The utility of conventional radiographs (i.e., plain films, panoramic radiographs) for evaluation of TMJ inflammatory arthritis is limited because of poor sensitivity in identifying early condylar erosion and inability to detect synovitis 30. Conversely, MRI is sensitive for detecting both condylar erosion and synovitis, but this assessment is typically qualitative (mild, moderate, or severe) and subjective. Furthermore, the specificity of synovitis as a marker for inflammatory arthritis is unclear, as some degree of synovial enhancement is commonly seen in normal TMJs (Figure 1) 19, 22. A reliable and simple method for quantifying TMJ synovitis and differentiating it from normal background synovial enhancement would facilitate early detection and treatment 31, 32.
We developed a technique to quantify TMJ synovial enhancement on contrast‐enhanced MRIs 22 and tested this method on patients with JIA and controls without TMJ disease. This assessment is easy to use, can be applied using virtually any radiology software, and has excellent reproducibility and interexaminer consistency. The use of an internal standard controls for variations in MRI machines and technique.
Our analysis determined that an ER of 1.55 discriminates between TMJs with synovitis and unaffected controls with a sensitivity of 91% and a specificity of 96%. Logistic regression analysis demonstrated that ERs of 1.55, 1.70, 1.90, and 2.00 were associated with probabilities of having TMJ arthritis of 65%, 85%, 96%, and 98%, respectively (Figure 3). Interestingly, males had higher ERs than females in our JIA sample. Because clinical characteristics and disease progression were not evaluated in this study, this may reflect a sex‐intrinsic difference or could suggest that males in our JIA cohort had more severe TMJ disease than females. In addition, while certain medications were associated with lower ERs than others in the JIA group, the study design did not allow for assessment of causality.
Other MRI findings besides synovial enhancement, including synovial thickening, pannus formation, joint effusion, bone marrow edema, bone erosion, condylar flattening, and disc deformity, have been used to evaluate TMJ inflammatory arthritis. Vaid et al proposed a scoring system that incorporates all of these findings 11. However, until an end‐stage deformity has developed, treatment decisions are largely based on the presence of acute disease rather than on the existence of chronic changes. Pannus formation, bone erosion, condylar flattening, and disc deformity are findings of longstanding disease and may be present even if there is no active arthritis 11. Of the indicators of acute disease, synovial enhancement correlates most closely with clinical symptoms and has been shown to be predictive for the development of bone erosion 9, 12, 13, 14, 15, 16, 17, 18. Previously, the assessment of synovial enhancement was compromised by an inability to differentiate synovitis from nonarthritic enhancement, and therefore synovial thickness was used as a surrogate for acute arthritis 21. However, measurement of synovial thickness in a small joint with 2 compartments and overlap of the disc and surrounding soft tissues is technically challenging, and the chronicity of disease implied by the presence of thickened synovium is unclear. Ma et al found that 20% of TMJs that had mild synovitis did not demonstrate hypertrophy of the synovium, and determined that synovial thickening was not a reliable marker for disease 33.
Dynamic contrast‐enhanced (DCE) MRI is a newer technique that allows for the creation of time‐intensity curves by first acquiring a noncontrast baseline image followed by a series of images over time after the injection of the contrast agent. This technique has become popular in the evaluation of cerebral microvasculature 34. Von Kalle et al recently used this technique to assess for baseline synovial enhancement in normal TMJs 19. While DCE‐MRI may further inform our understanding of contrast enhancement of arthritic TMJs in the future, the aim of this investigation was to develop and validate a simple and reliable method for quantifying synovial enhancement that can be broadly employed without the need for specialized equipment.
There were several limitations to the current study. First, the retrospective method and highly specialized patient population might have introduced some degree of selection bias toward patients with atypical clinical and radiographic findings. Second, in some subjects with severely arthritic TMJs, the analysis was difficult because of anatomic distortion. However, the method of averaging measurements from 3 areas within each joint space helps mitigate this inaccuracy, and, in patients with such severe arthritis, precise quantification of the synovitis would likely have little influence on treatment. Third, for the JIA group, some subjects had repeat studies that were included in the analysis, and this could cause bias in the results. However, this bias was minimized because each image study was treated as a separate point in time, no attempt was made to determine results of treatment or the course of disease, and the examiners were blinded to all clinical data. Fourth, the time points at which the MRIs were obtained with respect to disease and treatment course were variable, precluding direct correlation between MRI findings, disease status, and the influence of the treatment history. Finally, there were minor differences in imaging protocols between the JIA and control groups (different brands of 3 Tesla scanners and variation in the number of channels in the head coils), reflecting differences in protocols between the 2 institutions that could not be standardized in this retrospective study. Because the imaging protocols were very similar and because each measurement was internally controlled using a ratio with the longus capitis muscle from the same study, these differences should be proportionate and yield the same ratio. The internally controlled nature of this analysis allows it to be applied reliably across different MRI scanners and imaging protocols.
In conclusion, we present a reliable method to quantify TMJ synovial enhancement in JIA patients and to distinguish TMJs with pathologic synovitis from those with normal synovial enhancement using MRIs with gadolinium. This method can be used to quantify TMJ inflammatory arthritis at any point in the disease process, and may facilitate early treatment and quantification of treatment effects. In future studies we will aim to prospectively determine the utility of this method for assessing the treatment effects of systemic medications and intraarticular steroid injections. This technique may be applicable to other synovial joints.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Resnick had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Resnick, Peacock.
Acquisition of data
Resnick, Vakilian, Breen, Caruso, Peacock.
Analysis and interpretation of data
Resnick, Vakilian, Breen, Zurakowski, Henderson, Nigrovic, Kaban, Peacock.
Supporting information
Supplementary Figure 1. Bland‐Altman plot demonstrating intra‐examiner agreement for the control group.
Supplementary Figure 2. Bland‐Altman plot demonstrating agreement between the two examiners for the control group.
Supplementary Figure 3. Bland‐Altman plot demonstrating intra‐examiner agreement for the JIA group.
Supplementary Figure 4. Bland‐Altman plot demonstrating agreement between the two examiners for the JIA group.
Supplementary Figures Legends
REFERENCES
- 1. Ringold S, Thapa M, Shaw EA, Wallace CA. Heterotopic ossification of the temporomandibular joint in juvenile idiopathic arthritis. J Rheumatol 2011;38:1423–8. [DOI] [PubMed] [Google Scholar]
- 2. Cannizzaro E, Schroeder S, Muller LM, Kellenberger CJ, Saurenmann RK. Temporomandibular joint involvement in children with juvenile idiopathic arthritis. J Rheumatol 2011;38:510–5. [DOI] [PubMed] [Google Scholar]
- 3. Ringold S, Cron RQ. The temporomandibular joint in juvenile idiopathic arthritis: frequently used and frequently arthritic. Pediatr Rheumatol Online J 2009;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoll ML, Sharpe T, Beukelman T, Good J, Young D, Cron RQ. Risk factors for temporomandibular joint arthritis in children with juvenile idiopathic arthritis. J Rheumatol 2012;39:1880–7. [DOI] [PubMed] [Google Scholar]
- 5. Twilt M, Mobers SM, Arends LR, ten Cate R, van Suijlekom‐Smit L. Temporomandibular involvement in juvenile idiopathic arthritis. J Rheumatol 2004;31:1418–22. [PubMed] [Google Scholar]
- 6. Arabshahi B, Cron RQ. Temporomandibular joint arthritis in juvenile idiopathic arthritis: the forgotten joint. Curr Opin Rheumatol 2006;18:490–5. [DOI] [PubMed] [Google Scholar]
- 7. Carvalho RT, Braga FS, Brito F, Capelli J Jr, Figueredo CM, Sztajnbok FR. Temporomandibular joint alterations and their orofacial complications in patients with juvenile idiopathic arthritis. Rev Bras Reumatol 2012;52:907–11. [PubMed] [Google Scholar]
- 8. Pedersen TK. Clinical aspects of orthodontic treatment for children with juvenile chronic arthritis. Acta Odontol Scand 1998;56:366–8. [DOI] [PubMed] [Google Scholar]
- 9. Abramowicz S, Cheon JE, Kim S, Bacic J, Lee EY. Magnetic resonance imaging of temporomandibular joints in children with arthritis. J Oral Maxillofac Surg 2011;69:2321–8. [DOI] [PubMed] [Google Scholar]
- 10. Muller L, Kellenberger CJ, Cannizzaro E, Ettlin D, Schraner T, Bolt IB, et al. Early diagnosis of temporomandibular joint involvement in juvenile idiopathic arthritis: a pilot study comparing clinical examination and ultrasound to magnetic resonance imaging. Rheumatology (Oxford) 2009;48:680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaid YN, Dunnavant FD, Royal SA, Beukelman T, Stoll ML, Cron RQ. Imaging of the temporomandibular joint in juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2014;66:47–54. [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Stewart N, Crabbe J, Robinson E, McLean L, Yeoman S, et al. A 1‐year follow‐up study of dynamic magnetic resonance imaging in early rheumatoid arthritis reveals synovitis to be increased in shared epitope‐positive patients and predictive of erosions at 1 year. Rheumatology (Oxford) 2000;39:407–16. [DOI] [PubMed] [Google Scholar]
- 13. Jee WH, McCauley TR, Lee SH, Kim SH, Im SA, Ha KY. Sacroiliitis in patients with ankylosing spondylitis: association of MR findings with disease activity. Magn Reson Imaging 2004;22:245–50. [DOI] [PubMed] [Google Scholar]
- 14. McQueen FM, Stewart N, Crabbe J, Robinson E, Yeoman S, Tan PL, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis 1999;58:156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging–determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum 1999;42:918–29. [DOI] [PubMed] [Google Scholar]
- 16. Ostergaard M, Stoltenberg M, Lovgreen‐Nielsen P, Volck B, Jensen CH, Lorenzen I. Magnetic resonance imaging–determined synovial membrane and joint effusion volumes in rheumatoid arthritis and osteoarthritis: comparison with the macroscopic and microscopic appearance of the synovium. Arthritis Rheum 1997;40:1856–67. [DOI] [PubMed] [Google Scholar]
- 17. Peterfy CG. MRI of the wrist in early rheumatoid arthritis. Ann Rheum Dis 2004;63:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Savnik A, Malmskov H, Thomsen HS, Graff LB, Nielsen H, Danneskiold‐Samsoe B, et al. MRI of the wrist and finger joints in inflammatory joint diseases at 1‐year interval: MRI features to predict bone erosions. Eur Radiol 2002;12:1203–10. [DOI] [PubMed] [Google Scholar]
- 19. Von Kalle T, Winkler P, Stuber T. Contrast‐enhanced MRI of normal temporomandibular joints in children: is there enhancement or not? Rheumatology (Oxford) 2013;52:363–7. [DOI] [PubMed] [Google Scholar]
- 20. Kottke R, Saurenmann RK, Schneider MM, Muller L, Grotzer MA, Kellenberger CJ. Contrast‐enhanced MRI of the temporomandibular joint: findings in children without juvenile idiopathic arthritis. Acta Radiol 2015;56:1145–52. [DOI] [PubMed] [Google Scholar]
- 21. Weiss PF, Arabshahi B, Johnson A, Bilaniuk LT, Zarnow D, Cahill AM, et al. High prevalence of temporomandibular joint arthritis at disease onset in children with juvenile idiopathic arthritis, as detected by magnetic resonance imaging but not by ultrasound. Arthritis Rheum 2008;58:1189–96. [DOI] [PubMed] [Google Scholar]
- 22. Peacock ZS, Vakilian P, Caruso P, Resnick CM, Vangel M, Kaban LB. Quantifying synovial enhancement of the pediatric temporomandibular joint. J Oral Maxillofac Surg 2016;74:1937–45. [DOI] [PubMed] [Google Scholar]
- 23. Cimmino MA, Barbieri F, Boesen M, Paparo F, Parodi M, Kubassova O, et al. Dynamic contrast‐enhanced magnetic resonance imaging of articular and extraarticular synovial structures of the hands in patients with psoriatic arthritis. J Rheumatol 2012;Suppl 89:44–8. [DOI] [PubMed] [Google Scholar]
- 24. Pogrel MA, Kopf J, Dodson TB, Hattner R, Kaban LB. A comparison of single‐photon emission computed tomography and planar imaging for quantitative skeletal scintigraphy of the mandibular condyle. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995;80:226–31. [DOI] [PubMed] [Google Scholar]
- 25. Schueller‐Weidekamm C, Lodemann KP, Grisar J, Schueller G, Weber M, Kainberger F, et al. Contrast‐enhanced MR imaging of hand and finger joints in patients with early rheumatoid arthritis: do we really need a full dose of gadobenate dimeglumine for assessing synovial enhancement at 3 T? Radiology 2013;268:161–9. [DOI] [PubMed] [Google Scholar]
- 26. Abramowicz S, Kim S, Susarla HK, Kaban LB. Differentiating arthritic from myofascial pain in children with juvenile idiopathic arthritis: preliminary report. J Oral Maxillofac Surg 2013;71:493–6. [DOI] [PubMed] [Google Scholar]
- 27. Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis In: Springer series in statistics. New York: Springer; 2001: pp. 215–48. [Google Scholar]
- 28. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 29. Guzman J, Burgos‐Vargas R, Duarte‐Salazar C, Gomez‐Mora P. Reliability of the articular examination in children with juvenile rheumatoid arthritis: interobserver agreement and sources of disagreement. J Rheumatol 1995;22:2331–6. [PubMed] [Google Scholar]
- 30. Doria AS, de Castro CC, Kiss MH, Sernik RA, Vitule LF, Silva CH, et al. Inter‐ and intrareader variability in the interpretation of two radiographic classification systems for juvenile rheumatoid arthritis. Pediatr Radiol 2003;33:673–81. [DOI] [PubMed] [Google Scholar]
- 31. Albers HM, Wessels JA, van der Straaten RJ, Brinkman DM, Suijlekom‐Smit LW, Kamphuis SS, et al. Time to treatment as an important factor for the response to methotrexate in juvenile idiopathic arthritis. Arthritis Rheum 2009;61:46–51. [DOI] [PubMed] [Google Scholar]
- 32. Vilca I, Munitis PG, Pistorio A, Ravelli A, Buoncompagni A, Bica B, et al. Predictors of poor response to methotrexate in polyarticular‐course juvenile idiopathic arthritis: analysis of the PRINTO methotrexate trial. Ann Rheum Dis 2010;69:1479–83. [DOI] [PubMed] [Google Scholar]
- 33. Ma GM, Amirabadi A, Inarejos E, Tolend M, Stimec J, Moineddin R, et al. MRI thresholds for discrimination between normal and mild temporomandibular joint involvement in juvenile idiopathic arthritis. Pediatr Rheumatol Online J 2015;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Connor JP, Tofts PS, Miles KA, Parkes LM, Thompson G, Jackson A. Dynamic contrast‐enhanced imaging techniques: CT and MRI. Br J Radiol 2011;84:S112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Bland‐Altman plot demonstrating intra‐examiner agreement for the control group.
Supplementary Figure 2. Bland‐Altman plot demonstrating agreement between the two examiners for the control group.
Supplementary Figure 3. Bland‐Altman plot demonstrating intra‐examiner agreement for the JIA group.
Supplementary Figure 4. Bland‐Altman plot demonstrating agreement between the two examiners for the JIA group.
Supplementary Figures Legends


