Abstract
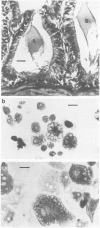
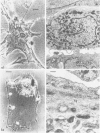
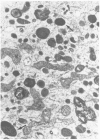
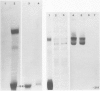
Primary cell cultures were established from explants of rat seminal vesicle. The establishment of primary cell cultures required, among other factors, the presence of testosterone. Two cell populations were detected in such primary cultures: fibroblast-like cells and epithelial-like cells; the latter encompassed a subtype of small cells and a subtype of large squamous cells (most likely the result of a degenerative process acting upon the former). Histochemical, as well as electron-microscopical observations, indicated the presence of a persistent secretory activity in the small epithelial cells; fibroblast and large squamous epithelial cells were inactive in this respect. Staining of the cells with a peroxidase-conjugated antibody and analysis of the proteins produced in the presence of labelled methionine, showed that one of the major rat seminal vesicle secretory proteins, namely RSV-IV, was also produced. Conditions which favoured the growth of epithelial cells, rather than of fibroblasts, were determined. The use of nearly homogeneous cell populations and the use of collagen-coated Petri dishes, allowed the cloning of two independently obtained permanent cell lines, namely SVC-1 and SVC-2. The in vitro growth rate of both cell lines was modulated by the amount of testosterone in the medium. Both cell lines were able to synthesize a significant amount of RSV-IV protein under testosterone control.
Full text
PDF

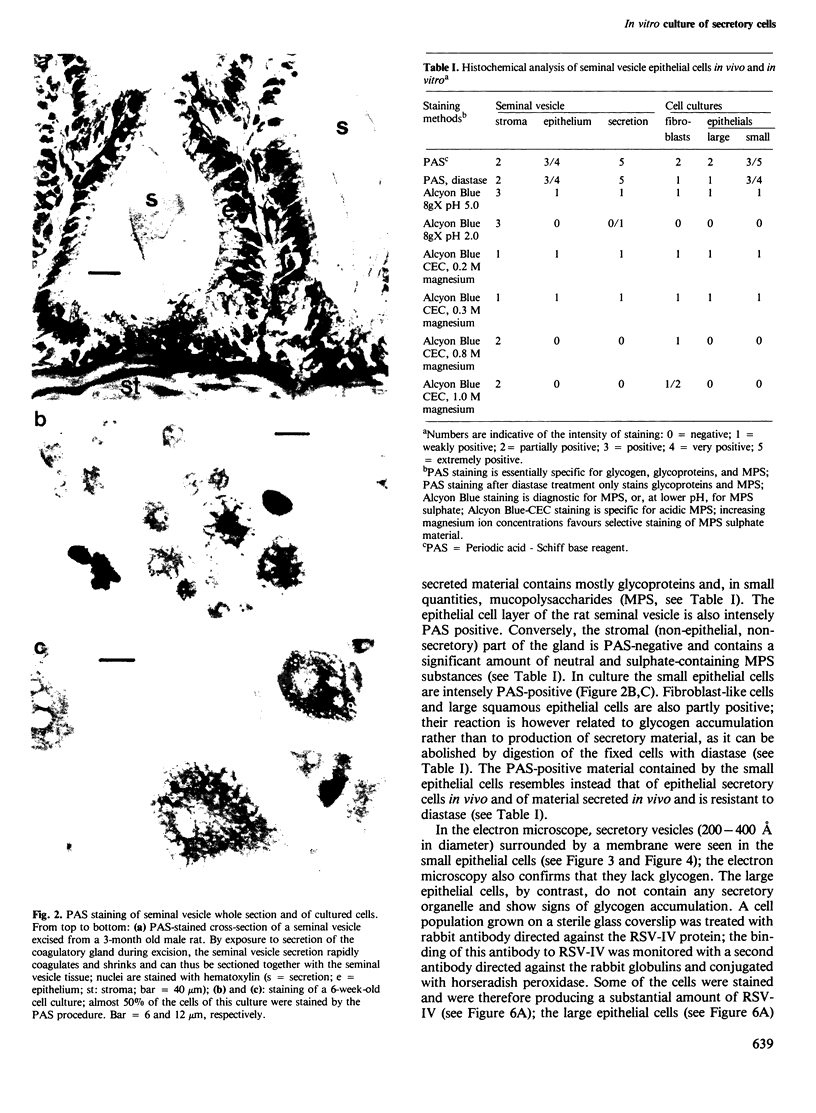
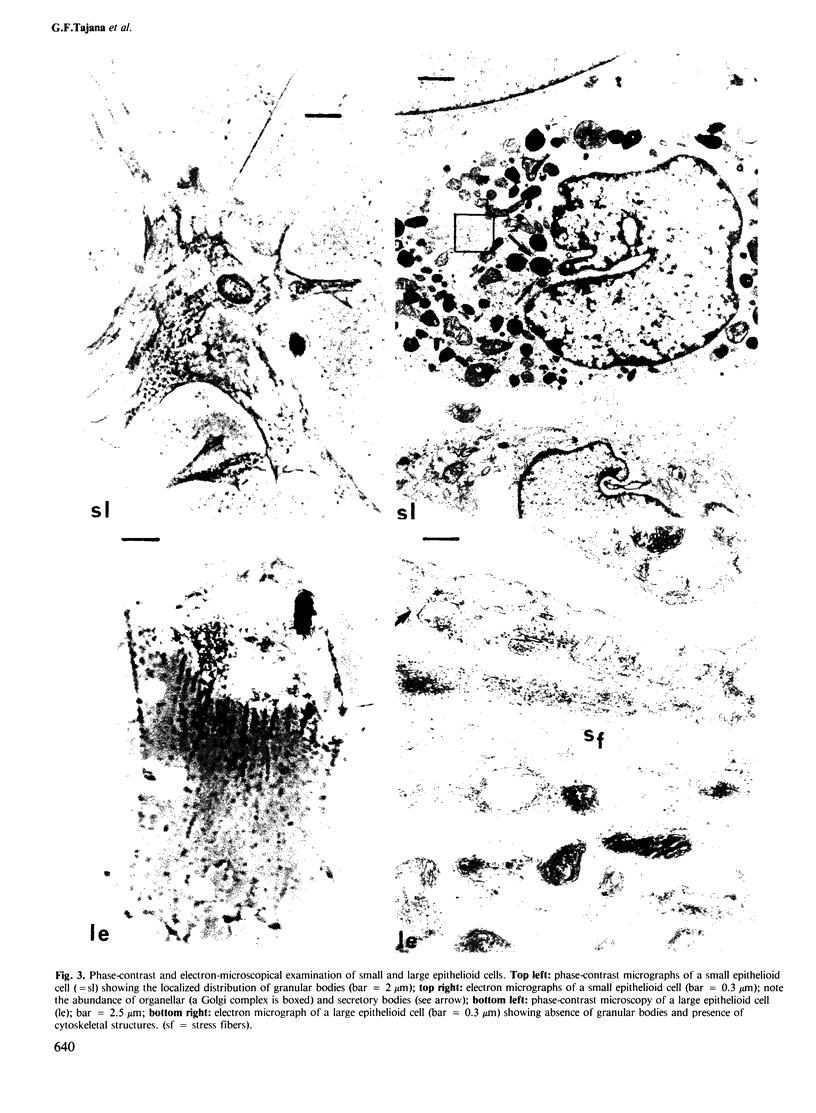
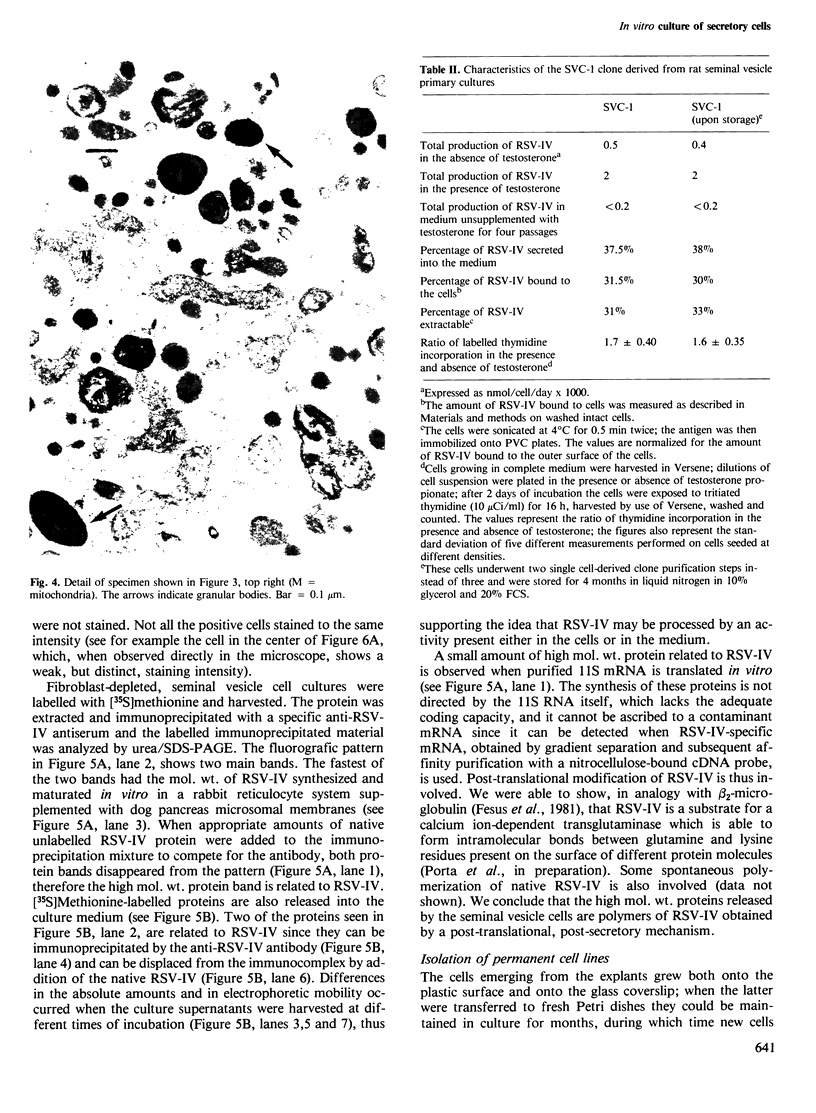

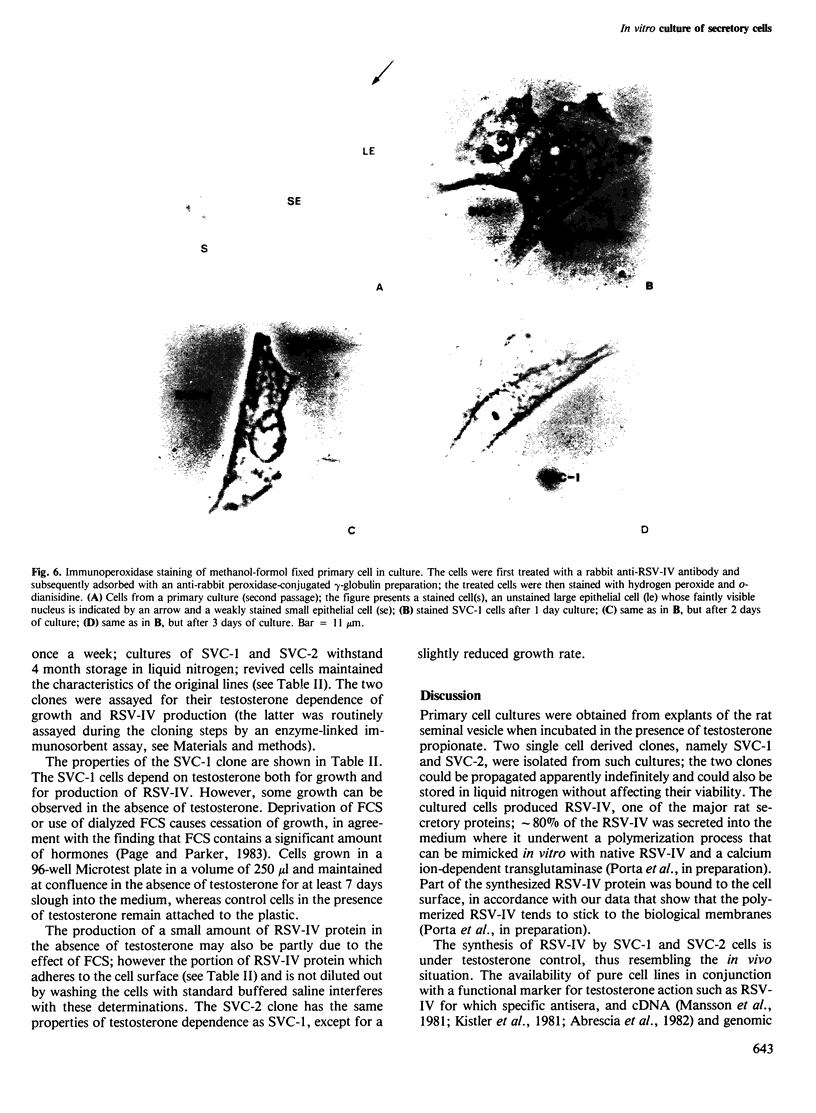

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrescia P., Guardiola J., Felsani A., Metafora S. Expression in male and genomic organization of the gene(s) coding for a major protein secreted by the rat seminal vesicle epithelium. Nucleic Acids Res. 1982 Feb 25;10(4):1159–1174. doi: 10.1093/nar/10.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso O., Gionti E., Pontarelli G., Ambesi-Impiombato F. S., Nitsch L., Tajana G., Cancedda R. The culture of chick embryo chondrocytes and the control of their differentiated functions in vitro. I. Characterization of the chondrocyte-specific phenotypes. Exp Cell Res. 1982 Nov;142(1):197–206. doi: 10.1016/0014-4827(82)90423-2. [DOI] [PubMed] [Google Scholar]
- Fésüs L., Falus A., Erdei A., Laki K. Human beta 2-microglobulin is a substrate of tissue transglutaminase: polymerization in solution and on the cell surface. J Cell Biol. 1981 Jun;89(3):706–710. doi: 10.1083/jcb.89.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Mansson P. E., Tully D. B., Burkhart B. Seminal vesicle secretion IV gene: allelic difference due to a series of 20-base-pair direct tandem repeats within an intron. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6460–6464. doi: 10.1073/pnas.80.21.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Brooks D. E., Fuller F. M. Isolation of cells from rat seminal vesicles and epididymis and their use in studying androgen action. Mol Cell Endocrinol. 1981 Aug;23(2):207–223. doi: 10.1016/0303-7207(81)90071-x. [DOI] [PubMed] [Google Scholar]
- Kandala J. C., Kistler M. K., Lawther R. P., Kistler W. S. Characterization of a genomic clone for rat seminal vesicle secretory protein IV. Nucleic Acids Res. 1983 May 25;11(10):3169–3186. doi: 10.1093/nar/11.10.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum A. L., DePhilip R. M., Spruill W. A., Takenaka I. Isolation and culture of rat seminal vesicle epithelial cells. The use of the secretory protein SVS IV as a functional probe. Exp Cell Res. 1983 May;145(2):293–304. doi: 10.1016/0014-4827(83)90008-3. [DOI] [PubMed] [Google Scholar]
- Kirk D., Irwin J. C. Normal human endometrium in cell culture. Methods Cell Biol. 1980;21B:51–77. doi: 10.1016/s0091-679x(08)60678-0. [DOI] [PubMed] [Google Scholar]
- Kistler M. K., Ostrowski M. C., Kistler W. S. Developmental regulation of secretory protein synthesis in rat seminal vesicle. Proc Natl Acad Sci U S A. 1981 Feb;78(2):737–741. doi: 10.1073/pnas.78.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler M. K., Taylor R. E., Jr, Kandala J. C., Kistler W. S. Isolation of recombinant plasmids containing structural gene sequences for rat seminal vesicle secretory proteins IV and V. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1161–1166. doi: 10.1016/0006-291x(81)90740-3. [DOI] [PubMed] [Google Scholar]
- Mansson P. E., Sugino A., Harris S. E. Use of a cloned double stranded cDNA coding for a major androgen dependent protein in rat seminal vesicle secretion: the effect of testosterone in gene expression. Nucleic Acids Res. 1981 Feb 25;9(4):935–946. doi: 10.1093/nar/9.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D., Schäfer B. Biochemical and morphological characterization of glycogen-storing epithelial liver cell lines. Exp Cell Res. 1982 Mar;138(1):1–14. doi: 10.1016/0014-4827(82)90085-4. [DOI] [PubMed] [Google Scholar]
- McDonald C., Williams L., McTurk P., Fuller F., McIntosh E., Higgins S. Isolation and characterisation of genes for androgen-responsive secretory proteins of rat seminal vesicles. Nucleic Acids Res. 1983 Feb 25;11(4):917–930. doi: 10.1093/nar/11.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler M. K., Kistler W. S. Purification and cell-free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem. 1979 Jan 25;254(2):383–390. [PubMed] [Google Scholar]
- Page M. J., Parker M. G. Androgen-regulated expression of a cloned rat prostatic c3 gene transfected into mouse mammary tumor cells. Cell. 1983 Feb;32(2):495–502. doi: 10.1016/0092-8674(83)90469-5. [DOI] [PubMed] [Google Scholar]
- Pan Y. C., Silverberg A. B., Harris S. E., Li S. S. Complete amino acid sequence of a major secretory protein from rat seminal vesicle. Int J Pept Protein Res. 1980 Aug;16(2):143–146. doi: 10.1111/j.1399-3011.1980.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Stoker M., Perryman M. Studies on differentiation of human mammary epithelial cells in culture: distinctive specificities of conditioned media. Mol Biol Med. 1983 Jul;1(1):117–122. [PubMed] [Google Scholar]
- Veneziale C. M., Burns J. M., Lewis J. C., Büchi K. A. Specific protein synthesis in isolated epithelium of guinea-pig seminal vesicle. Effects of castration and androgen replacement. Biochem J. 1977 Aug 15;166(2):167–173. doi: 10.1042/bj1660167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks G. D., Leipus E., Tsurumoto D. Tissue culture of the human uterine cervix. Methods Cell Biol. 1980;21B:29–50. doi: 10.1016/s0091-679x(08)60677-9. [DOI] [PubMed] [Google Scholar]