Abstract
Background
The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey data were not analysed to account for cultural and healthcare system differences across European countries (EC).
Objective
To utilize MAPP data to characterize psoriasis in Spanish patients, including severity assessment and Dermatology Life Quality Index (DLQI).
Methods
The MAPP survey was conducted between June and August 2012. This analysis included 1700 patients with self‐reported psoriasis (without psoriatic arthritis) from France (n = 349), Germany (n = 311), Italy (n = 359), Spain (n = 354) and the United Kingdom (n = 327).
Results
Patients from Spain vs. other EC self‐reported higher mean body mass index (26.9 vs. 25.6, P ≤ 0.001), lower prevalence of depression (6% vs. 12%, P = 0.002) and higher mean self‐perceived psoriasis severity at its worst (5.92 vs. 5.33, P < 0.001) despite lower estimated body‐surface‐area involvement. Overall, patients from Spain vs. other EC had lower mean global DLQI scores (4.70 vs. 6.06, P = 0.001) and lower mean scores for each DLQI dimension [all P < 0.001, except leisure (P = 0.002), treatment (P = 0.002), and work and school (P = 0.005)]. Higher DLQI values were inversely associated with age and directly correlated with perceived severity. Palmoplantar, nail and scalp psoriasis were reported less frequently in Spanish patients (P = 0.026) and were associated with higher DLQI values (P < 0.01). Spanish patients were more likely to have seen multiple healthcare providers (HCPs; P < 0.001) and achieve therapeutic goals (P < 0.001), but current treatments were similar to patients in other EC.
Conclusions
In the MAPP survey, Spanish patients differed from other EC in several characteristics, including comorbidities, extent and distribution of psoriasis lesions, perception of severity and impact on quality of life. Their perception of psoriasis severity was higher despite a lower estimated extent, and DLQI scores were significantly lower. Spanish patients had more HCP visits and a higher rate of therapeutic goal achievement. These differences might be attributed to cultural factors, phenotypical variation and differences in HCP access.
Introduction
The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) was a systematic household telephone survey (using random digit dialling to provide a probability sample) of 139 948 households in the United States, Canada, France, Germany, Italy, Spain and the United Kingdom (UK) designed to identify adults with a previous diagnosis of psoriasis and/or psoriatic arthritis.1 Interviews were conducted with 3426 patients, corresponding to approximately 1000 adults in the United States and 400 in each of the other countries. The MAPP survey lacked bias because participants were not limited to a specific clinic or geographic region and did not have to be under the medical care of a healthcare provider (HCP) or enrolled in a patient association at the time of the survey; in addition, validated quality‐of‐life assessments were used for the survey, such as the Dermatology Life Quality Index (DLQI).1 The DLQI is easy to use in clinical practice and has been employed in clinical trials of psoriasis2 and for reimbursement purposes in some health systems; however, patients with psoriasis from different countries respond differently to a substantial proportion of DLQI items despite having the same level of underlying health‐related quality‐of‐life (HRQoL) impairment.3
Differences in responses between patients in North America and Europe were primarily related to joint disease and symptoms. Almost twice as many North Americans experienced joint symptoms first vs. Europeans (24% vs. 13%).1 In addition, greater proportions of North Americans vs. Europeans with psoriasis who did not have a diagnosis of psoriatic arthritis reported joint pain (51% vs. 36%) and had more than four joints affected (49% vs. 30%).1 Thus, for the sake of homogeneity, in this subanalysis, we included only patients with psoriasis without a diagnosis of psoriatic arthritis from European countries (EC).
The aims of this exploratory, descriptive, observational and cross‐sectional study were to analyse and compare the DLQI results and assess other response variables in patients who had a diagnosis of psoriasis without psoriatic arthritis in Spain vs. other EC included in the MAPP study.
Materials and methods
The MAPP patient survey has been published previously.1 The European subset analysed in this study included 1700 patients with psoriasis (without psoriatic arthritis) from France (n = 349), Germany (n = 311), Italy (n = 359), Spain (n = 354) and UK (n = 327). The primary response variable was the DLQI score, which is calculated by summing the score of questions designed for use in adults, resulting in a maximum score of 30 and a minimum score of 0. The questions are grouped into six subscales as follows: symptoms and feelings (questions 1 and 2), daily activities (questions 3 and 4), leisure (questions 5 and 6), work and school (question 7), personal relationships (questions 8 and 9) and treatment (question 10). To help the clinical interpretation of the DLQI scores, a banding system (consisting of five bands) has been validated.4 According to this system, a DLQI score of 0–1 indicates no effect at all on a patient's life, 2–5 indicates a small effect, 6–10 indicates a moderate effect, 11–20 indicates a very large effect and 21–30 indicates an extremely large effect. Secondary response variables were the DLQI subscales and bands. Explanatory variables included country (primary variable), gender, age, palmar and/or plantar involvement, nail involvement, scalp involvement and self‐perceived severity, which were rated on a scale from 1 to 10, with scores categorized as mild (1–3), moderate (4–7) and severe (8–10), and extent of the involved area at the time of the survey self‐assessed in palms roughly equivalent to 1% of body‐surface‐area (BSA) involvement.5
Data management and statistical analyses were performed using R version 3.1 (The R Project for Statistical Computing, The R Foundation, Vienna, Austria; http://www.r-project.org). Bivariate analyses have been performed using the chi‐square test, Fisher's exact test or likelihood ratio chi‐square test for qualitative variables, and the analysis of variance, Mann–Whitney–Wilcoxon test or Kruskal–Wallis test for quantitative variables. The compliance of application conditions was determined using the Shapiro–Wilk and Kolmogorov–Smirnov normality tests and the Levene's test for homogeneity of variances. Multivariate generalized linear models have been used, taking into account the nature of the distribution of each response variable. For all statistical tests, a nominal significance level of 5% (P < 0.05) has been applied.
Results
In the global MAPP patient survey, the self‐reported prevalence of psoriasis and/or psoriatic arthritis ranged from 1.4% in Spain to 3.3% in Canada.1 The mean age of the total study population (N = 3426) was 54.8 years, and 21% were also diagnosed with psoriatic arthritis. Among patients with psoriasis only, 27% rated their disease as severe. Self‐perceived severity correlated with BSA involvement overall; however, 22% of patients with psoriasis and BSA involvement of three or fewer palms reported a substantial disease‐related impact on daily life. The most bothersome symptoms were itching (43%), scaling (23%) and flaking (20%). Of the patients with psoriasis, nearly half (47%) had not seen an HCP in a year, and more than 80% with BSA involvement of four or more palms were not receiving systemic treatment.1
For the current analysis, the main results of demographic variables, comorbidities, symptoms and self‐reported severity and extension of psoriasis in this subpopulation (European patients with psoriasis without a diagnosis of psoriatic arthritis) are summarized in Table 1 and below.
Table 1.
Demographic data and main comorbidities, by country
| Variable | France n = 349 | Germany n = 311 | Italy n = 359 | Spain n = 354 | UK n = 327 | P value |
|---|---|---|---|---|---|---|
| Age, mean, median [IQR], years | 45.4, 43.0 [32.0; 57.0] | 49.9, 51.0 [40.0; 61.0] | 55.9, 57.0 [45.0; 69.0] | 50.9, 50.0 [39.0; 61.0] | 53.2, 54.0 [42.0; 66.0] | <0.001 |
| Age at onset, mean, median [IQR], years | 32.3, 25.0 [16.0; 40.0] | 26.9, 20.0 [14.0; 35.0] | 39.4, 35.0 [20.0; 52.0] | 30.5, 26.0 [17.0; 42.5] | 28.2, 24.0 [15.0; 39.0] | <0.001 |
| Gender, n (%) | ||||||
| Female | 187 (53.6) | 181 (58.2) | 182 (50.7) | 187 (52.8) | 200 (61.2) | 0.041 |
| Male | 162 (46.4) | 130 (41.8) | 177 (49.3) | 167 (47.2) | 127 (38.8) | |
| BMI, median [IQR] | 26.2 [23.3; 30.2] | 25.4 [22.7; 28.8] | 25.2 [23.1; 27.5] | 26.8 [23.7; 29.7] | 26.6 [24.0; 29.8] | <0.001 |
| BMI, n (%) | ||||||
| <25 | 117 (39.3) | 144 (48.2) | 174 (49.3) | 119 (35.3) | 102 (36.0) | <0.001 |
| 25–30 | 96 (32.2) | 92 (30.8) | 140 (39.7) | 140 (41.5) | 113 (39.9) | |
| 30–35 | 62 (20.8) | 49 (16.4) | 31 (8.8) | 58 (17.2) | 44 (15.5) | |
| >35 | 23 (7.7) | 14 (4.7) | 8 (2.3) | 20 (5.9) | 24 (8.5) | |
| Depression, % | 14.9 | 12.9 | 3.6 | 6.2 | 17.7 | <0.001 |
| Diabetes, % | 6.9 | 10.9 | 8.6 | 9.9 | 10.7 | 0.345 |
| Hypertension, % | 12.9 | 29.6 | 19.2 | 14.7 | 24.8 | <0.001 |
| Heart disease, % | 4.0 | 8.4 | 6.7 | 5.7 | 6.1 | 0.218 |
BMI, body mass index; IQR, interquartile range.
The number of adults in the households was larger in Spain vs. other EC taken as a whole [2.53 (SD 1.11) vs. 2.30 (SD 1.09), P < 0.001], as was the percentage of patients with psoriasis alone (89% vs. 83%, P = 0.009).
Mean age was similar in patients from Spain and other EC taken as a whole (52.0 years), and the same applies to the proportion of men (47% of patients from Spain vs. 44% of patients from other EC).
Regarding comorbidities, the mean body mass index was higher in patients from Spain vs. the other EC (26.9 vs. 25.6, P < 0.001). Patients from Spain vs. other EC reported a lower prevalence of depression (6% vs. 12%, P = 0.002) and hypertension (15% vs. 21%, P = 0.007), but the prevalence of diabetes was similar (10% vs. 9%, P = 0.775), as was heart disease (6% vs. 6%, P = 0.773).
The difference in age of onset of psoriasis was not statistically significant in patients from Spain vs. other EC [30.5 (SD 26.0) vs. 29.7 (SD 18.1), P = 0.765]. There were some differences in the location of psoriatic lesions by countries (Table 2). Patients from Spain vs. other EC reported a significantly lower percentage of palmar and/or plantar (16% vs. 21%, P = 0.026), nail (7% vs. 11%, P = 0.012) and scalp (33% vs. 42%, P = 0.001) involvement, but more frequent involvement of elbows (49% vs. 42%, P = 0.020), ears (7% vs. 4%, P = 0.012), fingers/fingertips (3% vs. 1%, P = 0.031), knuckles/dorsal hand (3% vs. 0.5%, P = 0.002), buttocks (5% vs. 2%, P = 0.002), legs (12% vs. 7%, P = 0.005) and generalized lesions (2% vs. 1%, P = 0.022).
Table 2.
Distribution of lesions, self‐reported severity at its worst, most troublesome symptoms and extension of lesions (palms) at the time of the telephone interview, by country
| France n = 349 | Germany n = 311 | Italy n = 359 | Spain n = 354 | UK n = 327 | P value | |
|---|---|---|---|---|---|---|
| Location, % | ||||||
| Face | 21.5 | 15.8 | 7.0 | 5.9 | 13.5 | <0.001 |
| Hands (dorsa) | 0.6 | 0.3 | 0.6 | 2.8 | 2.5 | 0.006 |
| Palms | 18.6 | 12.9 | 17.5 | 11.0 | 9.2 | 0.001 |
| Nails | 9.7 | 11.9 | 7.8 | 6.5 | 15.9 | <0.001 |
| Scalp | 45.6 | 52.1 | 22.3 | 32.8 | 52.0 | <0.001 |
| Genital areas | 6.3 | 10.6 | 4.5 | 5.4 | 7.0 | 0.019 |
| Trunk | 16.6 | 21.2 | 9.2 | 15.5 | 33.0 | <0.001 |
| Elbows | 36.7 | 49.8 | 32.9 | 48.9 | 49.5 | <0.001 |
| Arms | 1.4 | 1.3 | 4.7 | 5.9 | 8.0 | <0.001 |
| Knees | 22.1 | 32.8 | 20.3 | 28.5 | 33.3 | <0.001 |
| Legs | 3.7 | 6.1 | 7.2 | 12.1 | 12.5 | <0.001 |
| Soles | 12.9 | 13.8 | 12.8 | 9.6 | 9.2 | 0.213 |
| Generalized | 0.0 | 1.9 | 0.3 | 2.0 | 0.3 | 0.003 |
| Severity score of psoriasis at its worst, mean (SD) | 4.8 (2.2) | 5.8 (2.5) | 4.9 (2.6) | 5.9 (2.7) | 5.9 (2.7) | <0.001 |
| Severity score categories, n (%) | <0.001 | |||||
| Mild (1–3) | 107 (31.7) | 68 (21.7) | 117 (33.7) | 71 (19.9) | 77 (23.4) | |
| Moderate (4–7) | 192 (56.8) | 148 (47.1) | 161 (46.4) | 176 (49.4) | 141 (42.9) | |
| Severe (8–10) | 39 (11.5) | 98 (31.2) | 69 (19.9) | 109 (30.6) | 111 (33.7) | |
| Most bothersome symptom or feature, % | <0.001 | |||||
| Itch | 43.6 | 37.3 | 48.2 | 37.9 | 35.8 | |
| Scaling | 11.2 | 26.7 | 16.2 | 10.2 | 7.7 | |
| Location or size of lesions | 20.1 | 6.4 | 2.5 | 11.6 | 24.2 | |
| Area involved, n (%) | <0.001 | |||||
| No skin lesions (<1 palm) | 91 (26.1) | 111 (35.7) | 165 (46.0) | 110 (31.1) | 105 (32.1) | |
| 1–3 palms | 165 (47.3) | 118 (37.9) | 93 (25.9) | 174 (49.2) | 145 (44.3) | |
| 4–10 palms | 62 (17.8) | 43 (13.8) | 36 (10.0) | 50 (14.1) | 52 (15.9) | |
| 11–20 palms | 10 (2.9) | 9 (2.9) | 16 (4.5) | 12 (3.4) | 15 (4.6) | |
| >20 palms | 8 (2.3) | 11 (3.5) | 24 (6.7) | 5 (1.4) | 7 (2.1) | |
| Severity score categories in patients with BSA ≤3% palms, n (%) | <0.001 | |||||
| Mild (1–3) | 100 (40.8) | 63 (27.8) | 102 (40.8) | 69 (24.5) | 74 (29.7) | |
| Moderate (4–7) | 128 (52.2) | 109 (48.0) | 109 (43.6) | 140 (49.7) | 109 (43.8) | |
| Severe (8–10) | 17 (6.9) | 55 (24.2) | 39 (15.6) | 73 (25.9) | 66 (26.5) | |
BSA, body surface area.
Itch was the most frequently reported bothersome skin symptom, with percentages ranging from 31% (UK) to 41% (Spain), but the differences were not significant. Other symptoms reported as most bothersome by patients from Spain vs. other EC were bleeding (4% vs. 2%, not significant), flaking (16% vs. 8%, P < 0.001) and stress/emotional impact (5% vs. 3%, not significant).
The self‐reported severity score of psoriasis at its worst, on a scale from 1 to 10, varied by country (Table 2). The mean self‐reported severity was higher in patients from Spain vs. other EC overall [5.92 (SD 2.71) vs. 5.33 (SD 2.57), P < 0.001]. Self‐reported severity generally correlated with the affected BSA, based on self‐reported palm counts at the time of the telephone interview, but patients from Spain classified themselves in higher categories of severity regardless of self‐reported BSA (Fig. 1). Patients from Spain vs. other EC were more likely to report involvement of one to three palms (49% vs. 39%, P < 0.001), whereas the proportions of patients reporting involvement of >10 palms or >20 palms were lower (5% vs. 7% and 1% vs. 4%, respectively, P < 0.001).
Figure 1.
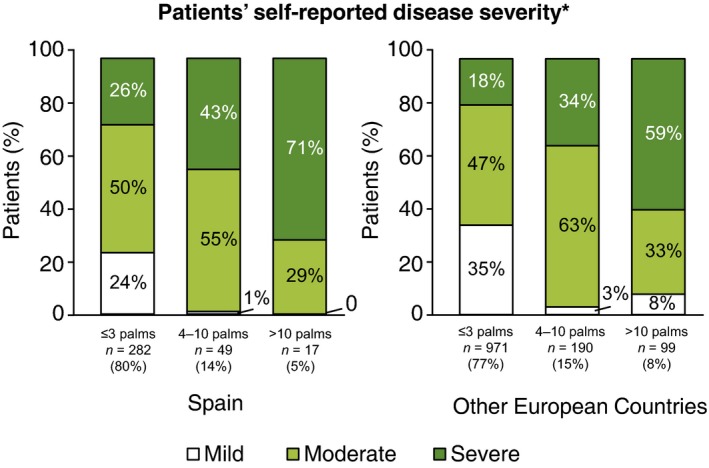
Self‐reported severity categories by self‐reported extension and country. *at its worst.
Overall, DLQI was inversely correlated with age (with a 0.5‐unit decrease for every 10 years) and directly correlated with self‐perceived severity (0.57 units of DLQI for each unit of self‐perceived severity). Nail involvement, scalp involvement or palm and/or sole involvement were associated with significantly greater impact on HRQoL, as measured by DLQI score (differences ranging from 1.1 to 2.3, P < 0.01). Treatment, daily activities and leisure were dimensions of DLQI largely affected by nail involvement, whereas daily activities, symptoms and feelings, and work and school were mainly determined by palm and/or sole involvement, and work and school were affected by scalp involvement.
The mean DLQI value in patients from Spain [4.70 (SD 4.26)] was lower than in patients from other EC [6.06 (SD 5.84), P = 0.001], mostly because of a lower proportion of those with a very large (6% vs. 12%) or extremely large (1% vs. 2%) effect on patient's life (Fig. 2). The mean scores for patients from Spain were also lower than for patients from other EC in all the dimensions of the questionnaire (symptoms and feelings, daily activities and personal relationships, P < 0.001; leisure and treatment, P = 0.002; and work and school, P = 0.005). The proportions of patients from Spain vs. other EC who scored 0 on DLQI questions were also higher in all the dimensions of the MAPP survey, respectively: symptoms and feelings (36% vs. 25%, P = 0.001, and 63% vs. 39%, P < 0.001), daily activities (75% vs. 59%, P < 0.001, and 62% vs. 48%, P < 0.001), leisure (72% vs. 60%, P = 0.006, and 70% vs. 58%, P = 0.004), work and school (69% vs. 56%, P = 0.001), personal relationships (87% vs. 67%, P < 0.001, and 84% vs. 65%, P < 0.001) and treatment (69% vs. 55%, P = 0.001).
Figure 2.
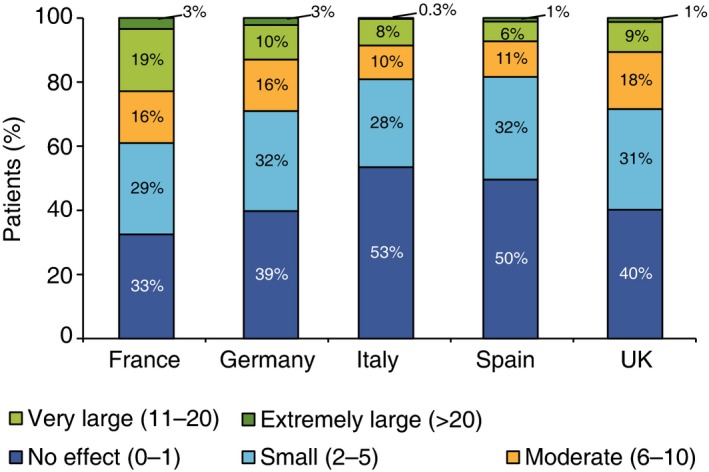
Distribution of patients’ Dermatology Life Quality Index scores according to bands, by country.
There was a significant effect and interaction of country and gender on DLQI values (P = 0.047). Only in Italy were DLQI values significantly different according to the patients’ gender (higher in women, P = 0.018), but women from Spain had significantly lower DLQI values than those from France (P = 0.001) and Germany (P = 0.026), and men from France had higher DLQI values than those from any other country (P < 0.001). Similar results were found for other DLQI dimensions with significant interactions [daily activities (P = 0.033) and leisure (P = 0.038)]; however, in the case of treatment, only women from Spain had significantly lower values than UK women (P = 0.016), and Italian men had significantly lower values than their French (P < 0.001) and UK (P < 0.001) counterparts.
When the individual components of DLQI in patients reporting some degree of impact on their emotional and social well‐being were stratified according to BSA and country, a better correlation with BSA categories was observed in patients from Spain. Nevertheless, the impact on HRQoL was lower for all dimensions except work and study; 50% of patients from Spain with extensive disease (BSA > 10%) had some degree of interference with their study or work vs. 16% from other EC.
The probability of having seen an HCP was dependent on BSA involvement; 61% of patients from other EC with involvement of less than one palm had not seen an HCP in the past 12 months compared with 42% with one to three palms, 35% with four to 10 palms and 41% with >10 palms. The corresponding percentages in patients from Spain were 68%, 47%, 44% and 29%. In approximately 50% of patients from Spain with BSA involvement >3% low expectations were the main reason for not having seen an HCP, but differences in healthcare system or personal attitudes might explain the lower rate of non‐frequentation of patients from Spain with the most extensive disease in comparison with their EC counterparts.
Survey patients from Spain also differed from their EC counterparts in frequentation of HCPs (mostly dermatologists): 17% of patients from Spain had seen more than four HCPs in the last year vs. 10% of patients from other EC (P < 0.001). For patients from Spain vs. other EC, the top goal of treatment was keeping symptoms at bay (33% vs. 38%, P < 0.001) and reducing flaking (24% vs. 13%, P < 0.001), and Spanish patients were more likely to achieve their main therapeutic goal (73% vs. 61%, P < 0.001).
Psoriatic arthritis may be underdiagnosed among patients in Spain with a diagnosis of ‘psoriasis only’, as 34% of patients reported joint pain or soreness, and sausage digits or heel pain and/or swelling were reported to have occurred upon specific questioning by 29% and 24% of patients, respectively.
The vast majority (84%) of survey patients from Spain with psoriasis only were receiving no treatment whatsoever or just topical treatment, regardless of the self‐reported extension of disease (Fig. 3), but 31% had discussed the possibility of systemic treatment with their HCP, 13% had discussed biologic treatment and 4% were actually receiving biologic treatment (compared with 3% of EC patients).
Figure 3.
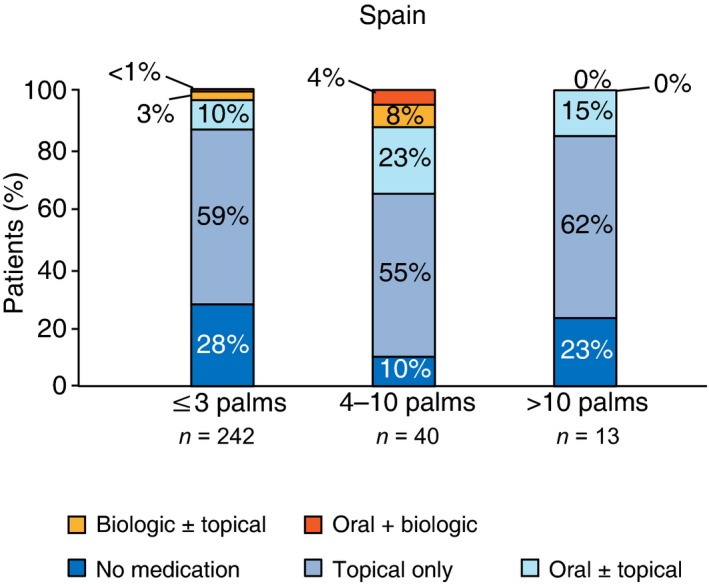
Treatment categories by self‐reported involvement in Spanish patients.
Discussion
Multinational Assessment of Psoriasis and Psoriatic Arthritis, the largest multinational probability survey of patients with psoriasis and/or psoriatic arthritis, was not biased by a specific clinic or geographic region, and patients did not have to be under medical care or enrolled in a patient association to participate.1 Questions for the MAPP survey were designed to determine why patients find their psoriatic condition and/or treatment burdensome and were based on validated HRQoL assessments, such as the DLQI, as well as and qualitative factors related to medical care.1 Results of the global MAPP patient survey revealed that there is a high rate of undertreatment of psoriasis and a disconnect between patient‐perceived disease severity and medical assessment of disease severity.1 This report focuses on the findings from the survey of European patients with psoriasis only, and specifically in patients from Spain. The results demonstrate the need for a local approach in defining therapeutic goals based on patient‐reported outcomes.
Some demographic differences may have arisen from sampling; even though female employment rates are high in Europe, a fixed‐line residential telephone survey is more likely to be answered by women. On the other hand, only one family member could be included in the survey, even in households with multiple cases of psoriasis.
The sample population from Spain was characterized by a relatively high body mass index and a lower prevalence of diagnosed depression compared with the other EC. The prevalence of cardiovascular comorbidities seems to be relatively low for a psoriasis population, but the severity distribution is likely to be more representative of the general population than general practitioners and insurance claims databases. Differences in distribution may partially account for differences in DLQI, as palmoplantar, nail and scalp psoriasis, which occurred less frequently in the population from Spain, are associated with higher DLQI values. The self‐perceived severity of psoriasis at its worst was slightly but significantly higher in patients from Spain than from other EC, and there were also significant differences in the symptoms perceived as most bothersome, even though itch was predominant (41%), irrespective of country.
Self‐reported severity of psoriasis at its worst generally correlated with self‐reported BSA involvement at the time of the survey, but the trend reversed in patients from Spain, who scored high in self‐reported severity and had relatively low BSA estimations; 26% of these patients with a BSA ≤3% palms rated their disease as severe.
Several variables have been found to affect DLQI and their dimensions, including BSA, age, gender and specific locations characterized by visibility, pruritus or dexterity impairment, but country‐specific variability is the most remarkable finding, and the results of the present study are consistent with the acknowledged limitations of DLQI as regards cross‐cultural equivalence.3, 6 Although the DLQI is designed to help determine the level of HRQoL impairment, the majority of the questions are influenced by external factors, including age, gender, diagnosis and nationality.7
Our results show some similarities to those of prior observational studies of Spanish patients with psoriasis.8, 9, 10 In an observational study evaluating HRQoL in 395 patients with psoriasis, factors associated with impaired HRQoL included age (with greater impact in younger patients), female gender and greater BSA involvement.8 In addition, among the DLQI domains, cutaneous symptoms (i.e. itching and pain) had the greatest effect on HRQoL. ARIZONA, a large, cross‐sectional, observational study conducted in Spain, evaluated comorbidities in 1022 patients with moderate‐to‐severe psoriasis.9 The most common comorbidities associated with psoriasis were dyslipidemia (28.1% of patients), obesity (26.0%), psoriatic arthritis (21.8%) and hypertension (18.3%). Another analysis of the ARIZONA study data found that the mean DLQI score significantly varied among age groups, with greater adverse effects on HRQoL observed in younger patients vs. older patients.10
Overall, patients from Spain had lower mean and median DLQI scores, global and in all dimensions, than their counterparts from other EC. These patients, regardless of BSA involvement, were less likely to report an impact on DLQI vs. patients from other EC for all dimensions except work and study; 50% vs. 16% with BSA involvement >10% had some degree of interference with their study or work, as measured by DLQI.
In a previous study, patients from some EC, including Italians and Belgians, experienced significantly less ‘problems with loved ones’ (item 8 of DLQI) and ‘sexual difficulties’ (item 9) because of their psoriasis compared with patients from other countries who had similar levels of disability measured by the DLQI.6 These patients may be less self‐conscious during physical intimacy because of psychological differences, such as identification, coping and negation, between different cultures.6 Likewise, observed differences across countries may be due to different interpretation of scores and/or response patterns.6 In the present study, the lowest mean DLQI values corresponded to Spain and Italy, and the highest to France. This was mainly due to differences in the percentages of patients scoring in the extreme bands of DLQI, and especially those reporting ‘no effect’ of psoriasis on their HRQoL.
Finally, the present study suggests that patients from Spain may differ from those in other EC included in this survey with regard to the frequency of HCP visits, their described therapeutic goals and the degree to which these goals were achieved. Based on their reported signs and symptoms, some patients in the Spanish and the other EC cohorts may have undiagnosed psoriatic arthritis. In addition, very small percentages of both cohorts reported receiving biologic treatments (3–4%) for their psoriasis.
In conclusion, the Spanish cohort in the MAPP survey, although sharing many similarities with the other EC cohort, have a number of intriguing differences which may require further exploration.
Acknowledgements
The authors received editorial support in the preparation of the report from Peloton Advantage, LLC, funded by Celgene Corporation. The authors, however, directed and are fully responsible for all content and editorial decisions for this report.
Conflicts of interest
L. Puig has served as a consultant and/or paid speaker for and/or participated in clinical trials for AbbVie, Amgen, Celgene Corporation, Centocor, Janssen‐Cilag, LEO Pharma, Merck, Merck Sharpe & Dohme, Novartis and Pfizer. P.C.M. van de Kerkhof has served as a consultant for Abbott, AbbVie, Almirall, Amgen, Celgene Corporation, Centocor, Eli Lilly, Galderma, Jansen‐Cilag, LEO Pharma, Mitsubishi, Novartis, Pfizer, Philips and Sandoz and has carried out clinical trials for AbbVie, Amgen, Basilea Pharmaceutica, Eli Lilly, Jansen‐Cilag, LEO Pharma, Pfizer and Philips Lighting. K. Reich has served on the advisory board for AbbVie, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Forward Pharma, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer, Regeneron, Takeda, UCB Pharma and Xenoport; has served as a speaker for AbbVie, Celgene Corporation, Covagen, Eli Lilly, Janssen‐Cilag, LEO Pharma, Medac, Merck Sharp & Dohme and Novartis; has received grant/research support from AbbVie, Amgen, Biogen Idec, Boehringer Ingelheim, Celgene Corporation, Covagen, Eli Lilly, Forward Pharma, GlaxoSmithKline, Janssen‐Cilag, LEO Pharma, Medac, Merck Sharp & Dohme, Novartis, Regeneron, Takeda and UCB Pharma; has a patent for and is a stockholder in Forward Pharma; and has served as a consultant for AbbVie, Boehringer Ingelheim, Covagen, Eli Lilly, Forward Pharma, Janssen‐Cilag, LEO Pharma, UCB Pharma and Xenoport. H. Bachelez has served as a consultant, advisor, speaker or investigator for AbbVie, Amgen, Baxalta, Celgene Corporation, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Pierre Fabre and Sun Pharmaceutical. J. Barker has served on the advisory board or as a speaker for AbbVie, Celgene Corporation, Eli Lilly, Janssen and Sun Pharmaceutical. G. Girolomoni has served as a principal investigator for AbbVie, Abiogen Pharma, Almirall, Amgen, Bayer, Biogen Idec, Boehringer Ingelheim, Celgene Corporation, Eli Lilly, Galderma, Hospira, Janssen, LEO Pharma, Merck Sharpe & Dohme, Mundipharma, Novartis, Pfizer, Pierre Fabre, Regeneron, Sandoz, Sanofi, Serono and Sun Pharmaceutical. C. Paul has served as an investigator or consultant for AbbVie, Amgen, Astellas, Boehringer Ingelheim, Celgene Corporation, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer and Pierre Fabre.
Funding source
The MAPP survey was sponsored by Celgene Corporation.
References
- 1. Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Am Acad Dermatol 2014; 70: 871–881. [DOI] [PubMed] [Google Scholar]
- 2. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 3. Bronsard V, Paul C, Prey S et al What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. J Eur Acad Dermatol Venereol 2010; 24(Suppl 2): 17–22. [DOI] [PubMed] [Google Scholar]
- 4. Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol 2005; 125: 659–664. [DOI] [PubMed] [Google Scholar]
- 5. Pariser DM, Bagel J, Gelfand JM et al National Psoriasis Foundation clinical consensus on disease severity. Arch Dermatol 2007; 143: 239–242. [DOI] [PubMed] [Google Scholar]
- 6. Nijsten T, Meads DM, de Korte J et al Cross‐cultural inequivalence of dermatology‐specific health‐related quality of life instruments in psoriasis patients. J Invest Dermatol 2007; 127: 2315–2322. [DOI] [PubMed] [Google Scholar]
- 7. Nijsten T. Dermatology life quality index: time to move forward. J Invest Dermatol 2012; 132: 11–13. [DOI] [PubMed] [Google Scholar]
- 8. Fernández‐Torres RM, Pita‐Fernández S, Fonseca E. Quality of life and related factors in a cohort of plaque‐type psoriasis patients in La Coruna, Spain. Int J Dermatol 2014; 53: e507–e511. [DOI] [PubMed] [Google Scholar]
- 9. Sánchez‐Carazo JL, López‐Estebaranz JL, Guisado C. Comorbidities and health‐related quality of life in Spanish patients with moderate to severe psoriasis: a cross‐sectional study (Arizona study). J Dermatol 2014; 41: 673–678. [DOI] [PubMed] [Google Scholar]
- 10. López‐Estebaranz JL, Sánchez‐Carazo JL, Sulleiro S. Effect of a family history of psoriasis and age on comorbidities and quality of life in patients with moderate to severe psoriasis: results from the ARIZONA study. J Dermatol 2016; 43: 395–401. [DOI] [PubMed] [Google Scholar]


