Abstract
Preclinical studies have demonstrated a return to methamphetamine (meth)-seeking behavior (reinstatement) induced by injections of meth administered by the experimenter (drug-prime). Notably, females tend to be more sensitive to drug-prime; often displaying more reinstatement behavior when compared to males. While meth-primed reinstatement of meth-seeking behavior has been established, little is known about the ability of other drugs of abuse to substitute for meth during drug-primed reinstatement; nicotine and cocaine were the focus of the present work. We also examined if self-administration and/or reinstated meth-seeking behavior was affected by repeated nicotine administration. Male and female Sprague-Dawley rats were trained to self-administer meth during daily sessions. During this self-administration phase, rats were placed into 1 of 2 groups: saline or repeated nicotine exposure. Rats in the repeated nicotine group received nicotine injections 4h after meth self-administration sessions, whereas the remaining rats received saline. Following self-administration was extinction in which meth was no longer available and nicotine was no longer administered. After extinction, rats were tested to determine if 0 (saline), 0.2, and 0.4 mg/kg nicotine reinstated meth-seeking behavior. Three days of re-extinction followed nicotine testing. Finally, rats received reinstatement tests with 0 (saline), 5, and 10 mg/kg cocaine. Nicotine and cocaine reinstated meth-seeking behavior in male and female rats with no difference between the sexes. Repeated nicotine administration potentiated meth reinstatement following the 0.4 mg/kg nicotine-prime. While females may be more sensitive to reinstatement triggered with the original self-administration drug, this effect may not generalize to priming with other drugs of abuse.
Keywords: Relapse, Rat, Poly Drug Use, Sprague-Dawley, Drug Substitution
1. Introduction
The majority of meth-dependent individuals that seek drug treatment return to meth use within 6 months of treatment (Brackins et al., 2011; Brecht et al., 2004). The inability to maintain meth cessation following treatment highlights the insufficiency of current behavioral and pharmacological interventions. Understanding the factors that may influence a return to drug use following cessation (i.e., relapse) is crucial to increasing the efficacy of current interventions. Clinical studies have shown that an intense urge or desire to use a drug can be precipitated by drug-associated cues, by stress, and by a priming injection of the drug itself (Blum et al., 2009; Carter and Tiffany, 1999; Chornock et al., 1992; Jaffe et al., 1989; Kaplan et al., 1985; Katz and Higgins, 2003; Preston et al., 1992; Self, 1998; Self and Nestler, 1998; Stockwell et al., 1982; Walsh et al., 2000). Pre-clinical models of relapse (termed reinstatement) have also reliable demonstrated that drug-seeking behavior following extinction can be induced by drug-associated cues, stress, or a priming injection of the drug (Beardsley et al., 2010, Epstein et al., 2006; Hofford et al., 2014, Katz and Higgins, 2003; Kufahl and Olive, 2011; Pittenger et al., 2016; Shaham et al., 2003, Shepard et al., 2004). Notably, while the drug-primed model of reinstatement does serve as a useful tool for the evaluation of behavioral and pharmacological intervention, as well identification of the neurobiology underlying relapse, it does have a limitation. If an individual administers a small amount of meth, it can be argued that relapse has already occurred. Therefore we would be studying the behavior and mechanisms following a relapse and not necessarily precipitating it. This notion makes the investigation of other drugs that may facilitate relapse to meth very important.
Nicotine is of interest given its legality, availability, and the finding that 97% of meth users also smoke cigarettes (Brecht et al., 2004). Research does suggest that under certain parameters nicotine can indeed trigger meth reinstatement. In a study conducted by Neugebauer et al. (2010), male rats were repeatedly injected with nicotine or saline, unpaired (temporally separate) from meth self-administration sessions. Rats then underwent extinction of meth-maintained lever pressing followed by reinstatement testing. Rats were administered nicotine before the reinstatement session (no meth available). Interestingly, rats that had a prior history with repeated nicotine administration demonstrated meth-seeking behavior; increase in lever pressing on the previously meth-maintained lever. Reinstatement was not triggered in male rats that had no previous experience with nicotine (Neugebauer et al., 2010). However, others have found acute nicotine can induce meth reinstatement (Hiranita et al., 2006). These findings demonstrate that under some circumstances nicotine can serve as a trigger for meth reinstatement in male rats.
The effect of acute and repeated nicotine on reinstatement of meth-seeking in female rats has not been studied. This is quite surprising given the high comorbidity of meth and nicotine dependence, large number of female meth users, and research suggesting females may be at increased risk for relapse (Brecht et al., 2004; Cox et al., 2013; Holtz et al., 2012; Reichel et al., 2012). In preclinical research, previous work has found that female rats display more meth-seeking reinstatement behavior than their male counterparts (Cox et al., 2013; Holtz et al., 2012; Reichel et al., 2012). Further, the partial α4β2 and full α7 nicotinic acetylcholine receptor (nAChR) agonist varenicline potentiated reinstatement in female, but not male rats (Coe et al., 2005a; Coe et al., 2005b; Gonzales et al., 2006; Mihalak et al., 2006; Smith et al., 2007). Determining if nicotine could also differentially reinstate meth drug-seeking in males and females was one of the goals of the study reported herein. Additionally, we examined the generality of drug-primed meth reinstatement by examining cocaine as an alternative drug trigger. Cocaine, in particular, was interesting in that it activates similar brain regions during drug-primed reinstatement (Kufahl and Olive, 2011; McFarland and Kalivas, 2001; Neisewander et al., 2000); examining if this overlap in mechanism would allow cocaine to trigger meth reinstatement was the second goal of this study.
2. Methods and Materials
2.1 Subjects
Sprague-Dawley rats were purchased (Harlan Laboratories; Indianapolis, IN, USA) at approximately 9 weeks of age (total n=56). Rats were housed individually with TEK-Fresh® cellulose bedding in clear polycarbonate cages (35.5 × 32 × 18 cm; length × width × depth). The colony room was temperature- and humidity-controlled and on a 6:00 AM light/6:00 PM dark cycle. All experimental procedures were conducted during light phase of the cycle. Each rat’s 90% free-feeding weight was calculated following 3 days of acclimation to the colony room (average weight: females=210 grams, males=295 grams). The 90% weight was maintained for the duration of the experiment. Rats were allowed ad libitum access to water in the home cages. Protocols were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
2.2 Apparatus
Conditioning chambers purchased from Med Associates (ENV-008CT; Georgia, VT, USA) were utilized for all behavioral testing. Chambers measured 30.5 × 24.1 × 21 cm and were enclosed in a sound-attenuating cubicle. A variable-speed syringe pump (PMH-100VS; Med-Associates) was located outside each cubicle. Tygon® tubing was threaded from the pump syringe, through a leash, into the chamber to be attached to the catheter port that exited below the scapula of the rat. A recessed receptacle (5.2×5.2×3.8 cm) was centered on 1 sidewall of each chamber. A dipper arm, when raised, provided access to 0.1 ml of 26% (w/v) sucrose in this recessed receptacle. The chambers were also fitted with 2 retractable levers; 1 located on each side of the receptacle. A white cue-light (2.54 cm diameter; 28V, 100-mA) was mounted 7 cm above each lever. A house-light (two white 28V, 100-mA lamps) located 10 cm above the Perspex chamber ceiling was also in the cubicle.
2.3 Drugs
(+)-Methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline. Meth was infused IV at a volume of 35.74 μl over 1 sec at 0.05 mg/kg/infusion for a 250 g rat (i.e. 0.3497 mg/ml). Meth dose is reported in salt form. (−)-Nicotine hydrogen tartrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Nicotine was dissolved in 0.9% sterile saline and adjusted to a pH of 7.0 ± 0.2 using a dilute NaOH solution. Nicotine doses are reported as the base form. Injections of nicotine were subcutaneous (SC) at 1 ml/kg. (−)-Cocaine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in 0.9% sterile saline, and injected IP at 1 ml/kg (salt form).
2.4 Preliminary lever training
Following colony room acclimation and 90% food restriction, rats were trained to lever press using our lab’s standard protocol (Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016). At the start of each session the house light was illuminated and 1 of the 2 levers was insertion into the chamber (randomly selected). A lever press or a lapse of 15 sec resulted in access to sucrose (4-sec duration), retraction of the lever, and commencement of a timeout (average=60 sec; range=30 to 89 sec). A randomly selected lever was again inserted following the timeout with the condition that the same lever could not be presented more than twice in a row. This protocol was repeated for 60 sucrose deliveries resulting in daily sessions ranging from 65 to 80 min duration, depending on individual performance. Training continued until a lever press was made on at least 80% of the lever insertions for two consecutive days. All rats met criterion between sessions 3 to 5. This autoshaping protocol was used to ensure robust lever pressing and equivalent training on both levers.
2.5 Catheter Surgery and Recovery
Indwelling jugular catheters were implanted using the standard procedures in our lab (previously described in Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016). Rats were anesthetized with a 2:1 ketamine HCl (100 mg/kg; MWI, Boise, ID) plus xylazine HCl (20 mg/kg; MWI, Boise, ID) cocktail (intramuscular; IM). Rats were administered buprenorphine (0.1 mg/kg, SC; MWI, Boise, ID) for pain management and atipamezole (0.5 mg/kg, IM; MWI, Boise, ID) to terminate anesthesia. Buprenorphine was again administered 24 h post-surgery. Rats were allowed to recover for 7 days. They remained in their home cages and catheters were flushed daily with a cocktail of 0.2-ml baytril (5.0 mg/ml; MWI, Boise, ID) to prevent infections and heparin (30 Units/ml; MWI, Boise, ID) to prevent catheter non-patency as a result of blood clotting. Catheter patency was checked on the last day of recovery and completion of the self-administration phase by IV infusion of 0.05-ml xylazine (20 mg/ml). All rats that displayed motor ataxia within 20 sec were considered patent (cf. Charntikov et al., 2015; Charntikov et al., 2013; Reichel et al., 2008; Pittenger et al., 2016). Rats were excluded from the study if their catheter was not patent.
2.6 Post-surgery Lever Training
Following recovery, rats were place on a variable ratio 3 (VR3) schedule of sucrose reinforcement during daily training sessions (1 h; 3 consecutive days). During VR3 training, on average, every 3rd lever press (range 1 to 5) was followed by 4-sec access to sucrose. Levers were inserted individually with the condition that the same lever was not inserted more than 2 times in a row. These procedures were again designed to result in responding on both levers, each having a similar reinforcement history (Charntikov et al., 2015; Charntikov et al., 2013; Pittenger et al., 2016).
2.7 Self-administration
Following preliminary training and surgery, male and female rats were split into 2 treatment conditions prior to meth self-administration: repeated nicotine or saline (Female Repeated: n=14; Female Saline: n=14; Male Repeated: n=12; Male Saline: n=13). Rats in the repeated nicotine condition received nicotine injections (SC; 0.4 mg/kg) 4 h after their daily meth self-administration session (cf. Neugebauer et al., 2010). This temporal arrangement was selected so that the nicotine stimulus could not serve as a drug-context that may induce reinstatement later in the experiment. Previous research suggests that nicotine can serve as a reinstatement trigger through non-associative mechanisms in male rats. That is, repeated nicotine administration does not need to co-occur with the self-administration session (Neugebauer et al., 2010). Rats in the saline condition received saline injections 4 h after their self-administration sessions. Meth self-administration sessions and assigned injections continued for 21 days.
2.8 Extinction
Extinction sessions commenced 24 h after the last self-administration session. These sessions were identical to self-administration sessions except meth was no longer available. Requisite VR3 responding on the active lever still produced the same cues and the timeout. To match the procedures of Neugebauer and colleagues (2010), all injections administered following the sessions were saline (i.e., nicotine was no longer administered). Extinction was conducted daily for 14 sessions.
2.9 Nicotine-Triggered Reinstatement
Twenty-four hours after the last extinction session, rats began nicotine-triggered reinstatement testing. Reinstatement testing proceeded over 3 days. Reinstatement sessions were identical to extinction sessions (i.e., meth not available). Five min prior to reinstatement sessions, rats were administered 0.0 (saline), 0.2, or 0.4 mg/kg nicotine. Random assignment for each rat was used to construct the order in which each dose was tested.
2.10 Re-Extinction
Three additional days of extinction were then given. These re-extinction sessions were identical to earlier extinction sessions and occurred across consecutive days.
2.11 Cocaine-Triggered Reinstatement
Following re-extinction, rats began cocaine-triggered reinstatement testing. Similar to reinstatement with nicotine, testing proceeded over 3 days. Reinstatement sessions were identical to extinction sessions (i.e., meth not available). Fifteen min prior to reinstatement sessions, rats were administered 0.0 (saline), 5, or 10 mg/kg cocaine IP. Random assignment for each rat was again used to construct the order in which the doses were tested.
2.12 Dependent Measures
Drug intake was calculated for each rat in each self-administration session using the equation Drug Received = [((Average Weight Used to Calculate Meth Stock/Current Weight)*Stock Meth Dose)*Infusions Earned]. The amount of drug received served as the primary dependent measure during acquisition to account for the small difference in weight between males and females. That is, the females that received a slightly higher meth dose (0.059 mg/kg/inf) titrated meth intake with fewer lever presses than the males that received a lower meth dose (0.042 mg/kg/inf). Accordingly, a better measure for acquisition was meth intake. Active lever presses served as the primary dependent measure when meth was not available (i.e., extinction, nicotine- and cocaine-triggered reinstatement). To show inactive lever responding relative to active lever responding during self-administration, a discrimination index was calculated using the following formula: Discrimination Index = [Active Lever Presses/ (Inactive Lever Presses + Active Lever Presses)]. A Discrimination Index value of 0.5 indicates equal responding on the active and inactive lever (i.e., no discrimination between levers); a value >0.5 indicates more pressing on the active lever. Lever pressing on the inactive lever was near zero following early acquisition and remained for the rest of the experiment (data not displayed).
2.13 Statistical Analyses
Active lever responding, drug intake, and discrimination index in acquisition were analyzed by two separate 3-way mixed measures analysis of variance (ANOVA) with Sex (Female vs Male) and Nicotine Treatment (Saline vs Repeated Nicotine) as between-subjects factors and Session as a within-subjects factor. Active lever responding in extinction and re-extinction were analyzed by the same 3-way mixed measures ANOVA. Nicotine- and cocaine-primed reinstatement were analyzed by two separate 3-way mixed measures ANOVA with Sex (Female vs Male) and Nicotine Treatment Group (Saline vs Repeated Nicotine) as between-subjects factors and Reinstatement Dose [0 (saline), 0.2 , 0.4 mg/kg for nicotine reinstatement; o (saline), 5, 10 mg/kg for cocaine reinstatement] as a within-subjects factor. To adjust for multiple comparisons, Tukey HSDs were utilized for post-hoc analysis of behavioral data. Statistical significance was declared at p <0.05.
3. Results
3.1 Self-Administration
Rats showed robust active lever responding (Figure 1A). For active lever pressing there was a significant main effect of Sex [F(1, 49)=4.057; p=0.049] with females responding less than the males (Figure 1A). There was also a main effect of Session [F(20, 980)=3.670; p<0.001] with responding escalating during this phase. The main effect of Nicotine Treatment (F<1; p=0.464) and all of the interactions (all Fs<1) were not significant. To account for the difference in meth dose between the sexes analysis of the drug intake in acquisition was conducted (Figure 1B). There was no longer a main effect of Sex (F<1, p=0.727). That is, females and males titrated consumption of meth to similar levels. There was again a main effect of Session [F(20, 980)=2.073; p=0.025] with drug intake at the end of self-administration higher than levels in early sessions. Nicotine treatment 4 h after each session did not alter the amount of meth received (F<1, p=0.499) and again none of the interactions were significant (Fs<1).
Figure 1.
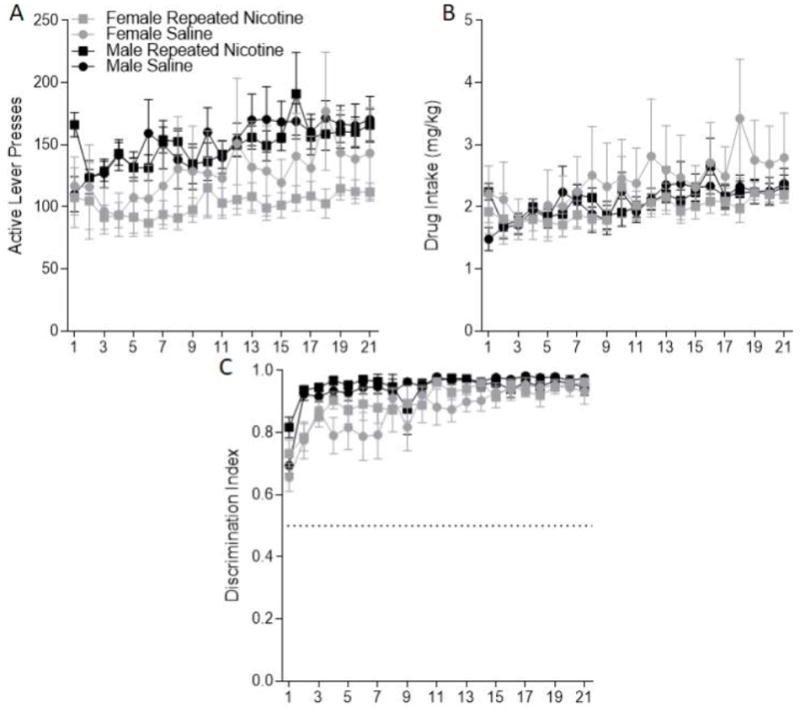
A: Active Lever presses (±SEM) during self-administration sessions for females (grey) and males (black) in the repeated nicotine (square) and saline (circle) conditions is displayed. B: Total meth intake (±SEM) accounting for body weight during each self-administration session is displayed. C: Discrimination Index (±SEM) during self-administration sessions is displayed.
Rats quickly discriminated between the active and inactive lever (Figure 1C) showing better lever discrimination as self-administration progressed [main effect of Session: F(20,980)=13.500, p<0.001]. There was a main effect of Sex [F(1,49)=7.745, p=0.007] and a significant Sex x Session interaction [F(20,980)=1.810, p=0.016]. The females showed statistically lower lever discrimination during initial self-administration sessions; females reached male levels by session 12. Nicotine treatment 4 h after each session did not have a significant effect on the discrimination index [F<1, p=0.441] and there were no other significant interactions [Sex x Group x Session: F<1, p=0.609; Sex x Nicotine Treatment: F<1, p=0.497; Nicotine Treatment x Session F(20,980)=1.401, p=0.112].
3.2 Extinction and Re-extinction
Active lever pressing was attenuated in extinction (Figure 2). There was a significant main effect of Session [F(13,637)=38.783, p<0.001], with responding in the early sessions significantly higher than responding in the subsequent session. Notably, there was a significant main effect of Nicotine Treatment [F(1,49)=6.959, p=0.011]. Rats that received nicotine repeatedly throughout the self-administration phase of the experiment responded less than rats treated with saline. Extinction in females and males was similar with no main effect of Sex [F(1,49)=1.559, p=0.217]. In re-extinction (Figure 2), the main effect of Sex (F<1, p=0.479) and Nicotine Treatment [F(1,49)=2.170, p=0.147] were not significant, but there was an effect of Session [F(1,98)=3.826, p=0.0251]. Session 2 was slightly lower than session 3, with no other differences in re-extinction sessions. Visual inspection shows this variation was quite small and the groups did not differ.
Figure 2.
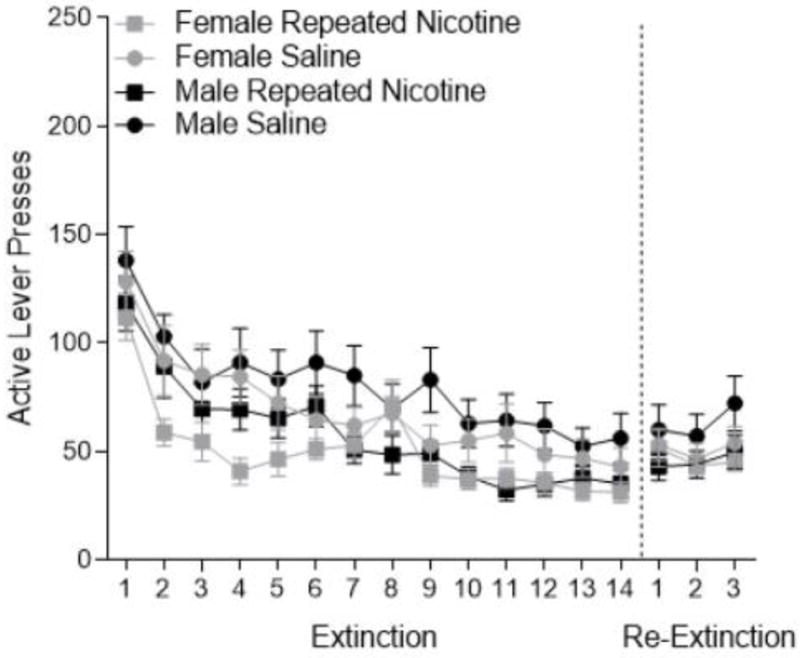
Active lever presses (± SEM) in extinction (left side) and re-extinction (right side) for female (grey) and male (black) rats in the repeated nicotine (square) and saline (circle) treatment conditions.
3.3 Nicotine-Triggered Reinstatement
Overall, nicotine reinstated robust meth-seeking behavior (Figure 3). Analysis of nicotine-induced reinstatement of meth-seeking revealed a main effect of Reinstatement Dose [F(2, 98)=38.311, p<0.001], with no significant effect of Sex or Nicotine Treatment (Fs<1). Notably, there was a significant Nicotine Treatment x Reinstatement Dose interaction [F(2,98)=5.691, p=0.005]. In both the Nicotine Treatment and Saline groups, the 0.2 mg/kg and 0.4 mg/kg nicotine trigger induced more active lever pressing compared to saline (no trigger). However, reinstatement following 0.4 mg/kg nicotine was potentiated in rats that had received nicotine repeatedly during self-administration when compared to rats that had no previous experience with nicotine. That is, when nicotine was previously experienced more reinstatement of meth-seeking was induced by 0.4 mg/kg nicotine. There were no other significant interactions (Fs<1).
Figure 3.
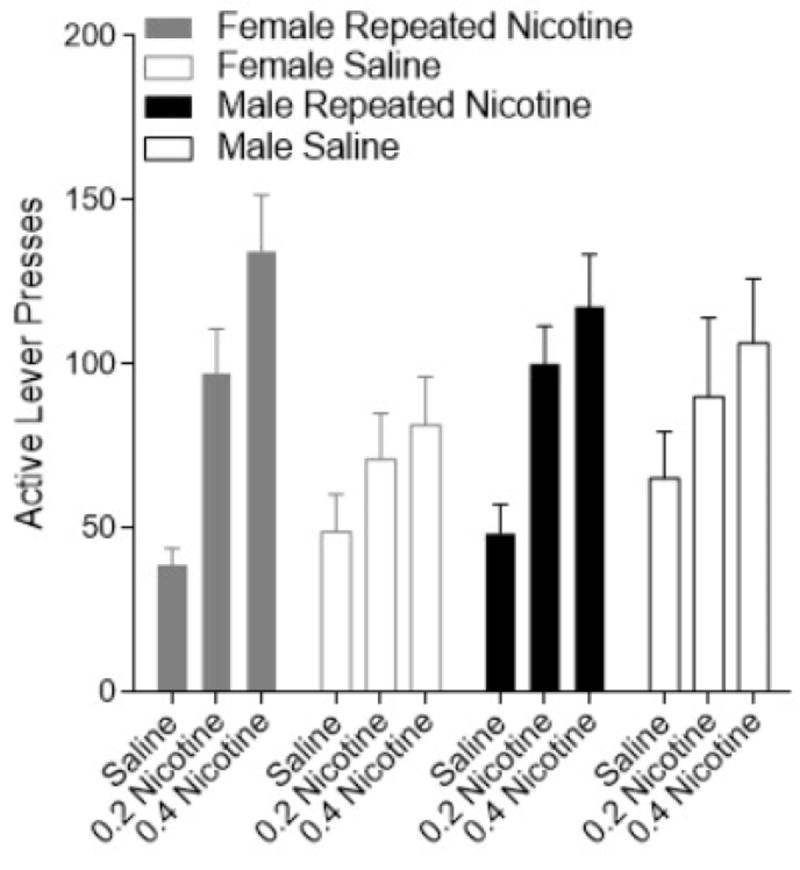
Active lever presses (±SEM) for female (grey) and male (black) rats during nicotine-triggered reinstatement. Filled bars delineate the repeated nicotine condition and open bars delineate the saline condition.
3.4 Cocaine-Triggered Reinstatement
During cocaine-triggered reinstatement of meth-seeking (Figure 4) there was a significant main effect of Reinstatement Dose [F(2,980=45.753, p<0.001]. Cocaine robustly induced meth-seeking behavior with 10 mg/kg cocaine inducing the most reinstatement of active lever responding; 5 mg/kg triggering an intermediate level. The main effect of Sex and Nicotine Treatment were not significant (Fs<1). Additionally, none of the interactions were significant [Sex x Nicotine Treatment x Reinstatement Dose: F<1, p=0.901; Sex x Nicotine Treatment: F<1, p=0.574; Sex x Reinstatement Dose: F(2,98)=2.531, p=0.085; Group x Reinstatement Dose: F(2,98)=1.114, p=0.333].
Figure 4.
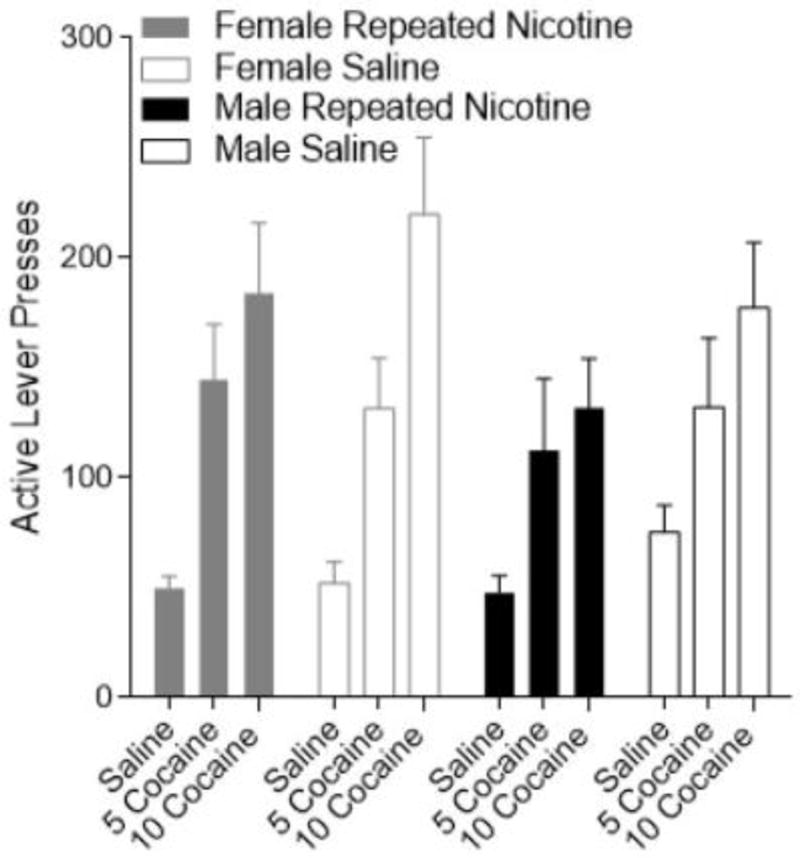
Active lever presses (±SEM) for female (grey) and male (black) rats during cocaine-triggered reinstatement. Filled bars delineate the repeated condition and open bars delineate the saline condition.
4. Discussion
Concordant with previous work, males and females robustly self-administered meth (Cox et al., 2013; Holtz et al., 2012; Pittenger et al., 2016; Pittenger et al., 2017; Reichel et al., 2012). Differences in active lever pressing were detected, however these were likely due to the slight difference in meth dose. When meth intake was adjusted for body weight, males and females did not differ. The lack of difference in meth intake between the sexes was similar to previous work in limited access self-administration sessions (Cox et al., 2013; Reichel et al., 2012). Repeated nicotine administration did not alter meth self-administration. This was not surprising as nicotine was explicitly unpaired with the self-administration session (injected 4-h after). These findings replicate previous work with repeated nicotine unpaired from meth self-administration in male rats and extends them to female rats (Neugebauer et al., 2012).
In extinction, no differences were detected between males and female. This finding supports the notion that although responding was different due to the slight difference in meth dose in acquisition, these differences did not persist during non-reinforcement. Given that the reinstatement tests are non-reinforced, this is an important finding that allows comparison of the sexes without accounting for differences in responding during acquisition.
In extinction, an interesting effect of nicotine was seen with the group that received nicotine during the self-administration phase. That is, responding in the repeated nicotine group was lower compared to responding in the group that had not received nicotine. There are a couple possible explanations for this finding. The first is that the repeated nicotine group displayed lower responding as a result of withdrawal from cessation of nicotine exposure. Recall, nicotine was no longer administered during the extinction phase. This cessation may have resulted in a behavioral response that interfered with lever responding. However, this reduction in responding persisted throughout the extinction phase; while work has demonstrated deficits in reward responsiveness and decreases in brain reward function during nicotine withdrawal, these effects are typically transient (Epping-Jordan et al., 1998; Pergadia et al., 2014; Shram et al., 2008).
A second possible explanation for the decrease in extinction responding in the repeated nicotine group is that nicotine enhanced learning during the extinction phase. Previous work does suggest that nicotine can function as a cognitive enhancer, augmenting learning, memory, and attention (Couey et al., 2007; Levin et al., 2006; Mansvelder et al., 2006; Newhouse et al., 2004) with effects demonstrated in extinction (Elias et al., 2010; Kaplan et al., 2011). As extinction is new learning (Bouton and King, 1983, Rescorla, 2004), the nicotine treatment may be enhancing this learning. This explanation does have a major issue; the enhancement of learning associated with nicotine is found when nicotine is administered concurrently with the learning (Elias et al., 2010; Gould and Higgins, 2003; Gould and Wehner, 1999; Tian et al., 2008). In the present report, nicotine was administered during the self-administration phase and halted prior to the extinction phase. Thus, nicotine would not be expected to enhance learning in this phase. This lack of enhanced extinction learning was found in previous work with nicotine administration during the acquisition of both meth self-administration and meth conditioned place preference (Berry et al., 2012; Neugebauer et al, 2010). The divergent findings reported here may reflect between study differences, including the drug model (Berry et al., 2012) or reinforcement schedule (Neugebauer et al., 2010). Future work investigating if nicotine administered concurrently with extinction in a meth self-administration paradigm further facilitates learning will be of interest.
Nicotine triggered meth reinstatement in females and males. Reinstatement was induced by 0.2 and 0.4 mg/kg in the saline and repeated nicotine groups. However, reinstatement was potentiated following the 0.4 mg/kg dose in the group that had a prior history with nicotine. Meth reinstatement induced by acute nicotine did not align with the result of Neugebauer et al. (2012) that did not find reinstatement following acute nicotine, but do match those of Hiranita et al. (2006). The potentiated ability of nicotine to reinstate meth-seeking following repeated administration is quite interesting. This effect may be a result of neurochemical sensitization following repeated nicotine administration. Repeated nicotine administration can increase reactivity in response to nicotine in overlapping neural circuitry known to play a role in the expression of meth-seeking behavior [for a review see Vezina et al. (2007)]. We hypothesize that this amplified reactivity following nicotine sensitization results in the modest meth-seeking reinstatement induced by acute nicotine and the robust meth-seeking reinstatement induced by repeated nicotine administration demonstrated herein and in other studies (Neugebauer et al., 2012; Hiranita et al., 2006).
Cocaine also triggered reinstatement of meth-seeking with no differences between sexes. This finding suggests that nicotine is not unique in its ability to reinstate meth-seeking. The ability of nicotine and cocaine to serve as triggers for meth-seeking may be a result of their overlapping interoceptive stimulus effects with meth (Czoty et al., 2004; Desai et al., 2010a; Dasai et al., 2010b; Gatch et al., 2008) As discussed, it is well established that a meth injection can reinstate meth-seeking behavior (Pittenger et al., 2016; Pittenger et al., 2017). While nicotine and meth, as well as cocaine and meth differ in biological mechanism, they also share significant overlap. In fact, full substitution (>80% responding on the drug-appropriate lever) is found when nicotine (Desai et al., 2010a; Gatch et al., 2008) and cocaine (Czoty et al., 2004; Dasai et al., 2010b) were substituted for meth in a 2-lever discrimination task. Although the 2-lever discrimination paradigm can be limited by abbreviated test durations or cumulative dose procedures (see Reichel et al., 2012; Bevins et al., 2012), these findings do suggest that nicotine and cocaine may initially be perceived as the meth, thus inducing reinstatement behavior.
The experiment reported herein did not examine gonadal hormone levels, although pre-clinical work has shown, in general, estrogen enhances and progesterone inhibits acquisition and escalation of self-administration, resistance to extinction, and reinstatement of drug-seeking under certain conditions (for a review see Anker and Carroll, 2011). With meth specifically, studies have not detected differences in reinstatement based on phase of estrous cycle (Ruda-Kucerova et al., 2015; Cox et al., 2013). However, allopregnanolone, a progesterone metabolite, does reduce meth-primed reinstatement in female, but not male rats, suggesting gonadal hormones may be a factor (Holtz et al., 2012).
While future work will be needed to elucidate the precise mechanisms by which nicotine and cocaine trigger meth reinstatement, this study clearly shows that nicotine and cocaine induce meth-seeking in both females and males. However, the sex difference typically observed when meth serves as the meth-seeking trigger (Cox et al., 2013; Holtz et al., 2012; Reichel et al., 2012) was not seen with a nicotine- or cocaine-prime. These findings imply that females are more sensitive to reinstatement when the original drug is used as a drug-trigger, but this amplified sensitivity does not remain when a different drug is used as a trigger. Future work examining if this effect is specific to meth as a primary drug, or if this effect generalizes to other drugs of abuse that are readily self-administered will be of interest.
Highlights.
Repeated nicotine treatment did not alter meth self-administration.
Repeated nicotine treatment facilitated meth extinction.
Nicotine and cocaine induced meth-seeking in male and female rats.
Previous nicotine experience potentiated meth reinstatement induced by nicotine.
Acknowledgments
This research was supported in part by NIH research grant DA034389.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anker JJ, Carroll ME. Biological Basis of Sex Differences in Psychopharmacology. Springer; New York, USA: 2011. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. [DOI] [PubMed] [Google Scholar]
- 2.Beardsley PM, Shelton KL, Hendrick E, Johnson KW. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol. 2010;637(1-3):102–108. doi: 10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry JN, Neugebauer NM, Bardo MT. Reinstatement of methamphetamine conditioned lace preference in nicotine-sensitized rats. Behav Brain Res. 2012;235(2):158–165. doi: 10.1016/j.bbr.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevins RA, Barrett ST, Polewan RJ, Pittenger ST, Swalve N, Charntikov S. Disentangling the nature of the nicotine stimulus. Behav Processes. 2012;90(1):28–33. doi: 10.1016/j.beproc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- 6.Brecht ML, O’Brien A, Von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29(1):89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 7.Blum K, Chen TJH, Downs BW, Bowirrat A, Waite RL, Braverman ER, Madigan M, Oscar-Berman M, DiNublie N, Gold M. Neurogentics of dopaminergic receptor super-sensitivity in activation of brain reward circuitry and relapse: proposing “Deprivation-Amplification Relapse Therapy” (DART) Postgrad Med. 2009;121(6):176–196. doi: 10.3810/pgm.2009.11.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouton ME, King DA. Contextual control of the extinction of conditioned fear: Tests for the associative value of the context. J Exp Psychol Anim Behav Process. 1983;9(3):248–65. [PubMed] [Google Scholar]
- 9.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- 10.Charntikov S, Pittenger ST, Thapa I, Bastola DR, Bevins RA, Pendyala G. Ibudilast reverses the decrease in the synaptic signaling protein phosphatidylethanolamine-binding protein 1 (PEBP1) produced by chronic methamphetamine intake in rats. Drug Alcohol Depend. 2015;152:15–23. doi: 10.1016/j.drugalcdep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Charntikov S, Swalve N, Pittenger S, Fink K, Schepers S, Hadlock GC, et al. Iptakalim attenuates self-administration and acquired goal-tracking behavior controlled by nicotine. Neuropharmacology. 2013;75C:138–144. doi: 10.1016/j.neuropharm.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chornock WM, Stitizer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmocology (Berl) 1992;108(4):495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- 13.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands Sb, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heyman JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005a;48(10):3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 14.Coe JW, Brooks PR, Wirtz MC, Bashore CG, Bianco KE, Vetelino MG, Arnold EP, Lebel LA, Fox CB, Tingley FD, Schulz DW, Davis TI, Sands SB, Mansbach RS, Rollema H, O’Neil BT. 3,5-Bicyclic aryl piperidines: a novel class of alpha4beta2 neuronal nicotinic receptor partial agonists for smoking cessation. Bioorg Med Chem Lett. 2005b;15(22):4889–4897. doi: 10.1016/j.bmcl.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38(10):2343–2353. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 2004;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- 18.Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010a;335:807–16. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: Discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther. 2010b;333(3):834–843. doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias GA, Gulick D, Wilkinson DS, Gould Tj. Nicotine and extinction of fear conditioning. Neuroscience. 2010;165(4):1063–1073. doi: 10.1016/j.neuroscience.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein DH, Preston KL, Stewar J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189(1):1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend. 93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57Bl/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80(2):147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 25.Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- 26.Hiranita T, Nawata Y, Katsuya K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. PNAS. 2006;103(22):8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT. Environmental enrichment reduces methamphetamine cu-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav Brain Res. 2014;270:151–158. doi: 10.1016/j.bbr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2012;120(1-3):233–237. doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan GB, Moore KA. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav. 2011;99:217–228. doi: 10.1016/j.pbb.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: Physiological and subjective responses in alcoholics and nonproblem drinkers. J Stud Alcohol. 1985;46(4):267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- 31.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 32.Kufahl PR, Olive MF. Investigating methamphetamine craving using the extinction-reinstatement model in the rat. J Addict Res Ther. 2011;(S1):003. doi: 10.4172/2155-6105.s1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin E, McClernon F, Rezvani A. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2005;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 34.Mansvelder HD, van Aerde K, Couey J, Brussaard A. Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 2006;184:292–05. doi: 10.1007/s00213-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 35.McFarland K, Kalivas P. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neuroscience. 2001;21(21):8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 37.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LTL, Palmer A, Marshall FJ. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20(2):798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106(1):72–78. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004b;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Pergadia ML, Der-Avakian A, D’Souza MS, Madden PAF, Heath A, Shiffman S, Markou A, Pizzagalli DA. Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry. 2014;17(11):1238–1245. doi: 10.1001/jamapsychiatry.2014.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittenger ST, Barrett ST, Chou S, Bevins RA. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in female rats. Behav Brain Res. 2016;300:150–159. doi: 10.1016/j.bbr.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittenger ST, Barrett S, Chou S, Bevins RA. The effects of varenicline on methamphetamine self-administration and drug-primed reinstatement in male rats. Behav Brain Res. 2017;320:195–199. doi: 10.1016/j.bbr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston KL, Sullivan JT, Strain EC, Bigelow GE. Effects of cocaine alone and in combination with bromocriptine in human cocaine abusers. J Pharmocol Exp Ther. 1992;262(1):279–291. [PubMed] [Google Scholar]
- 44.Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223(4):371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89(3):463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- 47.Ruda-Kucerova J, Amchova P, Babinska Z, Dusek L, Micale V, Sulcuva A. Sex differences in the reinstatement of methamphetamine seeking after forced abstinence in Sprague-Dawley rats. Front Psychiatry. 2015;6:91. doi: 10.3389/fpsyt.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Self D. Neural substrates of drug craving and relapse in drug addiction. Ann Med. 1998;30:379–389. doi: 10.3109/07853899809029938. [DOI] [PubMed] [Google Scholar]
- 49.Self D, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 50.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 51.Shram MJ, Siu ECK, Li Z, Tyndale RF, Le AD. Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male wistar rats. Psychopharmacology (Berl) 2008;198(2):181–190. doi: 10.1007/s00213-008-1115-x. [DOI] [PubMed] [Google Scholar]
- 52.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55(11):1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 53.Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190(2):157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- 54.Stockwell TR, Hodgson HJ, Taylor RC. Alcohol dependence, beliefs and the priming effect. Behav Res Ther. 1982;20(5):513–522. doi: 10.1016/0005-7967(82)90072-9. [DOI] [PubMed] [Google Scholar]
- 55.Tian S, Gao J, Han J, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhance contextual fear conditioning in rats. Neuroscience. 2008;153(4):935–943. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry 15. 2007;31(8):1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology (Berl) 2000;150:361–373. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]


