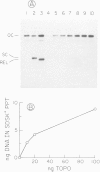
Abstract
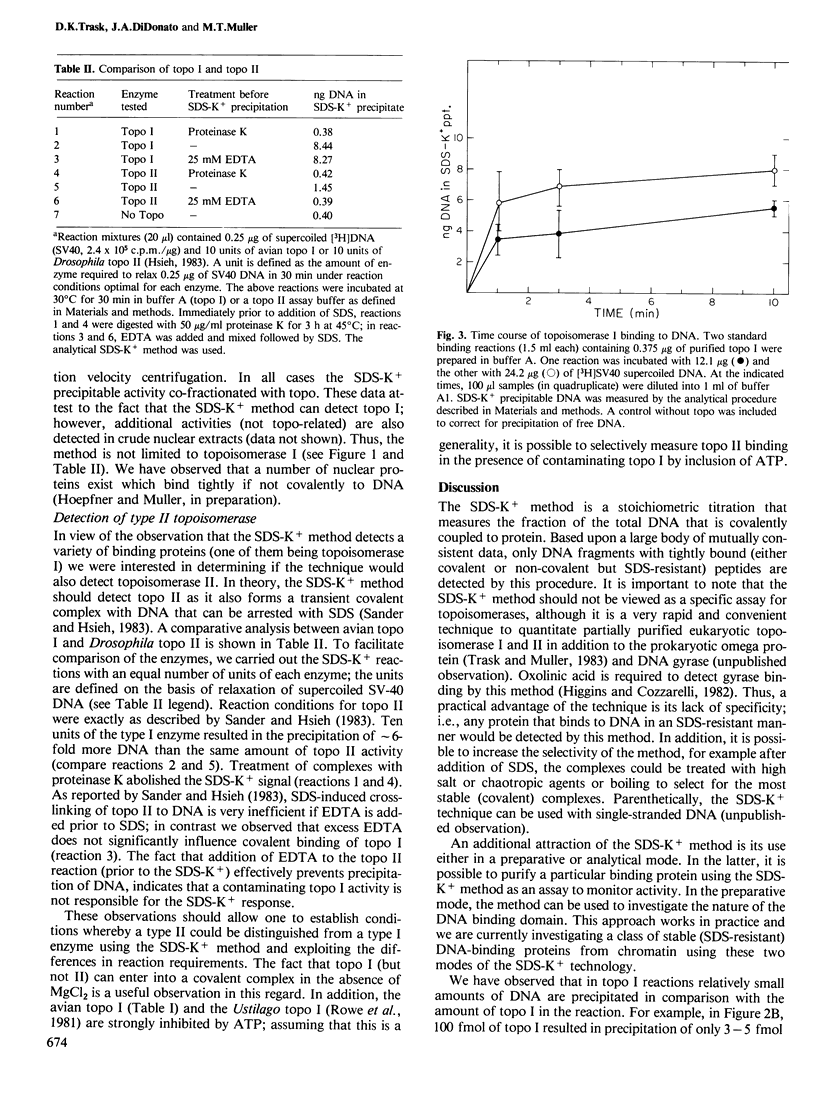
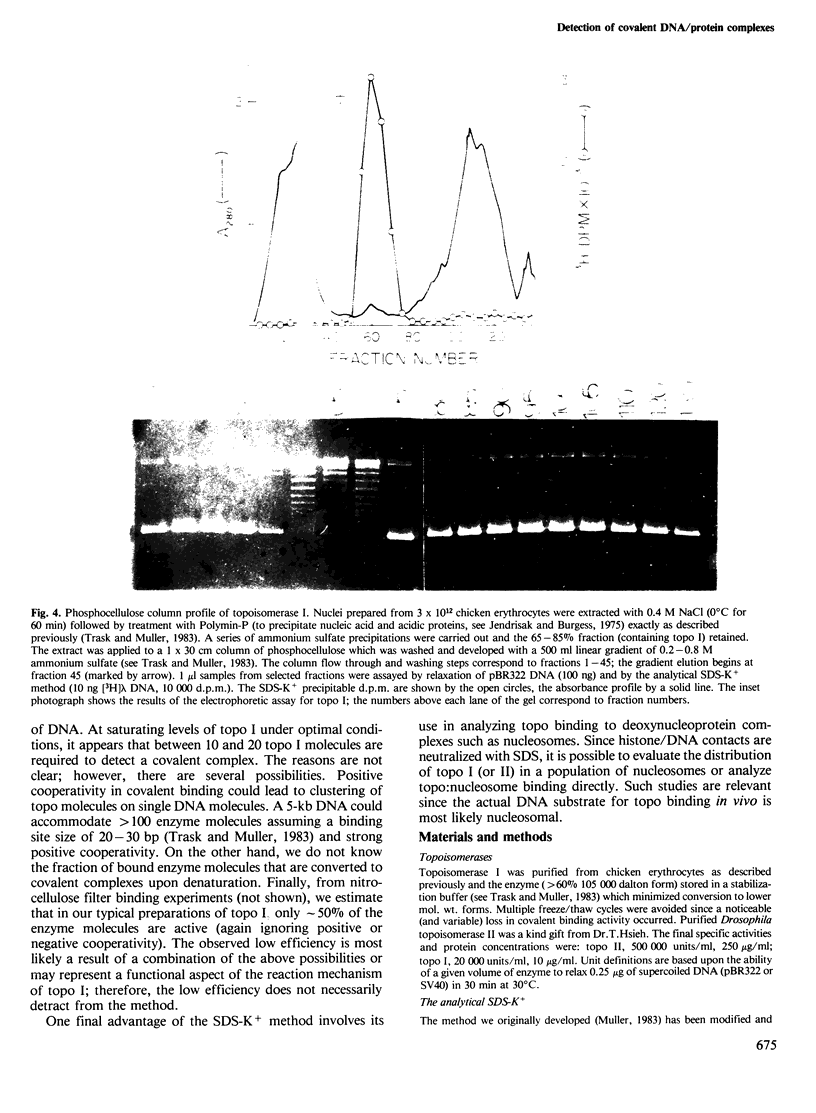
A rapid and simple method has been developed which allows detection and isolation of covalent DNA/protein adducts. The method is based upon the use of an ionic detergent, SDS, to neutralize cationic sites of weakly bound proteins thereby resulting in their dissociation off the helix. Proteins tightly or covalently bound to DNA that are not dissociable by SDS, result in the precipitation of the DNA fragment by the addition of KCl; however, free nucleic acid does not precipitate. The method is particularly useful as an analytical tool to titrate the binding of prototypic covalent binding proteins, topoisomerase I and II; thus, quantitation of topoisomerase activity is possible under defined conditions. As an analytical tool the method can be used as a general assay in the purification of as yet unidentified topoisomerases or other activities that bind DNA covalently. Moreover, the technology can be adapted for use in a preparative mode to separate covalent complexes from free DNA in a single step.
Full text
PDF
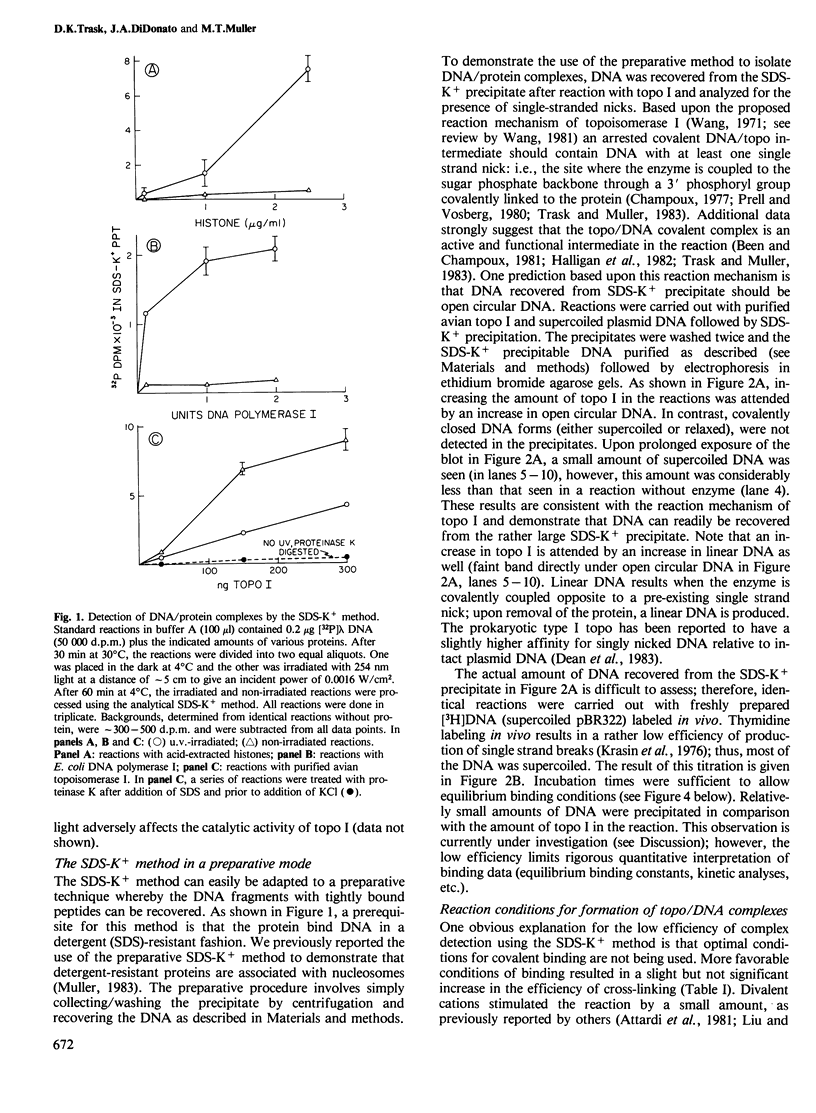
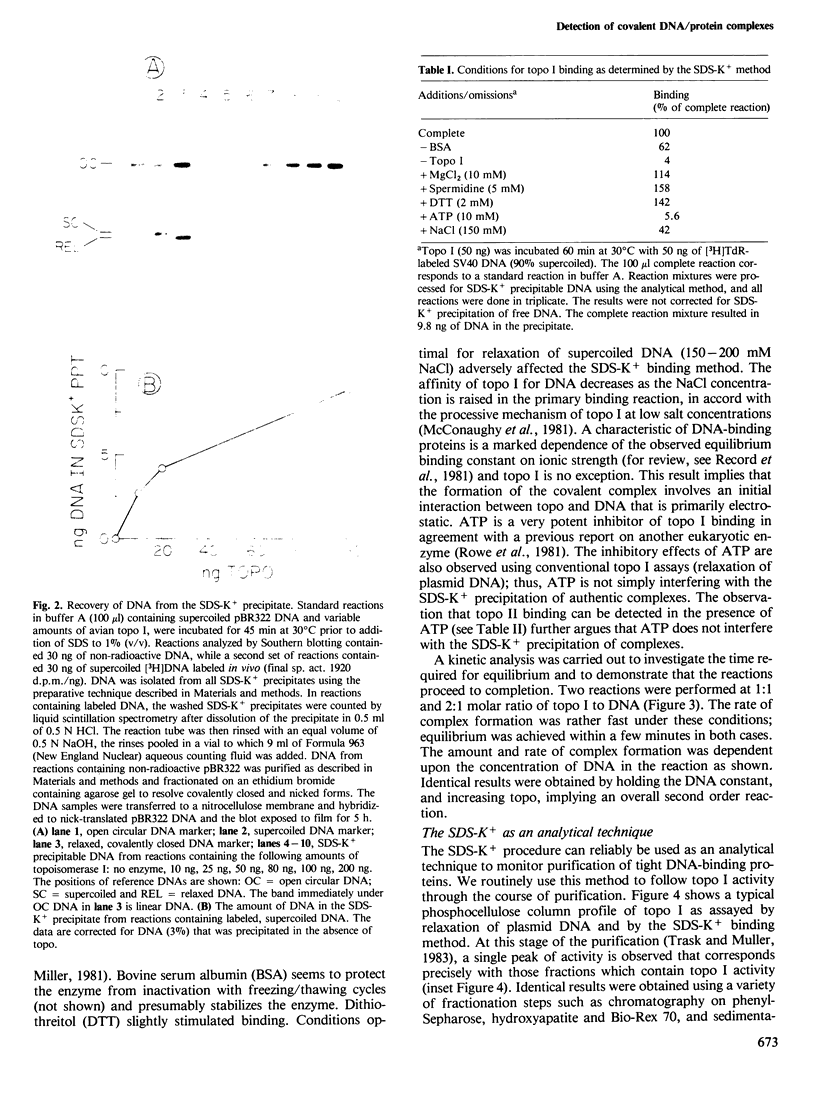

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi D. G., De Paolis A., Tocchini-Valentini G. P. Purification and characterization of Xenopus laevis type I topoisomerase. J Biol Chem. 1981 Apr 25;256(8):3654–3661. [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. DNA breakage and closure by rat liver type 1 topoisomerase: separation of the half-reactions by using a single-stranded DNA substrate. Proc Natl Acad Sci U S A. 1981 May;78(5):2883–2887. doi: 10.1073/pnas.78.5.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W., Jones C. J., Coombs D. H., Pearson G. D., Ward D. C. Proteins tightly bound to HeLa cell DNA at nuclear matrix attachment sites. Mol Cell Biol. 1983 Sep;3(9):1567–1579. doi: 10.1128/mcb.3.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F., Krasnow M. A., Otter R., Matzuk M. M., Spengler S. J., Cozzarelli N. R. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):769–777. doi: 10.1101/sqb.1983.047.01.088. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Davis J. L., Edwards K. A., Liu L. F. Intra- and intermolecular strand transfer by HeLa DNA topoisomerase I. J Biol Chem. 1982 Apr 10;257(7):3995–4000. [PubMed] [Google Scholar]
- Harrison C. A., Turner D. H., Hinkle D. C. Laser crosslinking of E. coli RNA polymerase and T7 DNA. Nucleic Acids Res. 1982 Apr 10;10(7):2399–2414. doi: 10.1093/nar/10.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N. P., Cozzarelli N. R. The binding of gyrase to DNA: analysis by retention by nitrocellulose filters. Nucleic Acids Res. 1982 Nov 11;10(21):6833–6847. doi: 10.1093/nar/10.21.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T. Purification and properties of type II DNA topoisomerase from embryos of Drosophila melanogaster. Methods Enzymol. 1983;100:161–170. doi: 10.1016/0076-6879(83)00052-x. [DOI] [PubMed] [Google Scholar]
- Krasin F., Person S., Ley R. D., Hutchinson F. DNA crosslinks, single-strand breaks and effects on bacteriophage T4 survival from tritium decay of (2-3H)adenine, (8-3H)adenine and (8-3H)guanine. J Mol Biol. 1976 Feb 25;101(2):197–209. doi: 10.1016/0022-2836(76)90372-7. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B. H., Huang C. Y., Pogo A. O. Isolation and characterization of the nuclear matrix in Friend erythroleukemia cells: chromatin and hnRNA interactions with the nuclear matrix. Cell. 1979 Dec;18(4):1079–1090. doi: 10.1016/0092-8674(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Markovitz A. Ultraviolet light-induced stable complexes of DNA and DNA polymerase. Biochim Biophys Acta. 1972 Nov 9;281(4):522–534. doi: 10.1016/0005-2787(72)90153-0. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., Young L. S., Champoux J. J. The effect of salt on the binding of the eucaryotic DNA nicking-closing enzyme to DNA and chromatin. Biochim Biophys Acta. 1981 Aug 27;655(1):1–8. doi: 10.1016/0005-2787(81)90059-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Carbon J., Herzberg M., Davis R. W., Berg P. Isolation and characterization of individual clones of simian virus 40 mutants containing deletions duplications and insertions in their DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):69–84. doi: 10.1101/sqb.1974.039.01.012. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Nucleosomes contain DNA binding proteins that resist dissociation by sodium dodecyl sulfate. Biochem Biophys Res Commun. 1983 Jul 18;114(1):99–106. doi: 10.1016/0006-291x(83)91599-1. [DOI] [PubMed] [Google Scholar]
- Neuer B., Plagens U., Werner D. Phosphodiester bonds between polypeptides and chromosomal DNA. J Mol Biol. 1983 Feb 25;164(2):213–235. doi: 10.1016/0022-2836(83)90076-1. [DOI] [PubMed] [Google Scholar]
- Prell B., Vosberg H. P. Analysis of covalent complexes formed between calf thymus DNA topoisomerase and single-stranded DNA. Eur J Biochem. 1980 Jul;108(2):389–398. doi: 10.1111/j.1432-1033.1980.tb04734.x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Mazur S. J., Melançon P., Roe J. H., Shaner S. L., Unger L. Double helical DNA: conformations, physical properties, and interactions with ligands. Annu Rev Biochem. 1981;50:997–1024. doi: 10.1146/annurev.bi.50.070181.005025. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Rowe T. C., Rusche J. R., Brougham M. J., Holloman W. K. Purification and properties of a topoisomerase from Ustilago maydis. J Biol Chem. 1981 Oct 25;256(20):10354–10361. [PubMed] [Google Scholar]
- Sander M., Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8421–8428. [PubMed] [Google Scholar]
- Smart J. E., Stillman B. W. Adenovirus terminal protein precursor. Partial amino acid sequence and the site of covalent linkage to virus DNA. J Biol Chem. 1982 Nov 25;257(22):13499–13506. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Muller M. T. Biochemical characterization of topoisomerase I purified from avian erythrocytes. Nucleic Acids Res. 1983 May 11;11(9):2779–2800. doi: 10.1093/nar/11.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui K., Watanabe S., Katagiri M., Oda T. Identification of proteins interacting with newly replicated DNA in SV40-infected cells by UV-induced DNA-protein cross-linking. Nucleic Acids Res. 1983 Jul 25;11(14):4793–4807. doi: 10.1093/nar/11.14.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]