Abstract
Abuse of addictive substances, including cigarettes, is much greater in HIV-1-infected individuals than in the general population and challenges the efficiency of highly active anti-retroviral therapy (HAART). The HIV-1 transgenic (HIV-1Tg) rat, an animal model used to study drug addiction in HIV-1-infected patients on HAART, displays abnormal neurobehavioral responses to addictive substances. Given that the cholinergic system plays an essential part in the central reward circuitry, we evaluated the expression profile of nine nicotinic acetylcholine receptor (nAChR) subunit genes in the central nervous system (CNS) of HIV-1Tg rats. We found that nAChR subunits were differentially expressed in various brain regions in HIV-1Tg rats compared to F344 control rats, with more subunits altered in the ventral tegmental area (VTA) and nucleus accumbens (NAc) of the HIV-1Tg rats than in other brain regions. We also found that chronic nicotine treatment (0.4 mg/kg/day) decreased the mRNA expression of nAChR subunits α6, β3, and β4 in the VTA of HIV-1Tg rats, whereas expression of α4 and α6 subunits in the NAc increased. No such changes were observed in F344 rats. Together, our data suggest that HIV-1 proteins alter the expression of nAChRs, which may contribute to the vulnerability to cigarette smoking addiction in HIV-1 patients.
Keywords: Nicotine, nAChRs, HIV-1, Addiction
Introduction
Abuse of addictive substances is prevalent in the HIV-1-infected population and increases the risk of morbidity and mortality. About 80 % of individuals infected with HIV-1 have used illicit drugs, and 25 % of this patient population is in need of treatment for alcohol and illicit drug abuse (SAMHSA 2010). Substance use and abuse are considered to be the two main risk factors in the spread of HIV-1 infection and accelerate the rate of progression to AIDS and the development of HIV-1-associated neurocognitive disorders (HAND) (Des Jarlais et al. 1992). Further, several studies have revealed an association between active drug use and poor adherence to antiretroviral therapy in HIV-1-infected patients which increased HIV/AIDS-associated morbidity and mortality (Mellins et al. 2009). For example, the prevalence of cigarette smoking is almost threefold higher in HIV-1 patients compared to the general population (Mamary et al. 2002), and smoking is defined as one of the major risk factor for non-AIDS-related mortality among HIV-1-infected individuals. Cigarette smoking increases the risk of lower respiratory tract infection, chronic obstructive pulmonary disease, lung cancer, and cardiovascular disease in HIV-1-positive patients (Rahmanian et al. 2011). These smoking-related diseases become major causes of mortality in HIV-1-infected individuals whose HIV-1 infection is under control with highly active anti-retroviral therapy (HAART) (Helleberg et al. 2013). Thus, it is important to understand the neuronal mechanisms that maintain nicotine dependence in HIV-1-infected patients; such understanding would help to develop effective pharmacological treatments for smoking cessation.
Nicotinic cholinergic signaling at nicotinic acetylcholine receptors (nAChRs) plays an important role in the regulation of the central reward pathway (Tuesta et al. 2011). The mesocorticolimbic dopaminergic projections from the ventral tegmental area (VTA) to corticolimbic brain regions are considered to be a major common pathway for addiction. Because of their effects on mesolimbic dopamine transmission, nAChRs modulate the reinforcing effects of drugs of abuse such as cocaine, amphetamines, alcohol, cannabinoids, and opiates (Tuesta et al. 2011). Neuronal nAChRs have diverse expression profiles in the mesolimbic system, and they modulate the afferent and efferent connections in the VTA-NAc dopaminergic system (Pierce and Kumaresan 2006). The VTA receives glutamatergic, GABAergic, and cholinergic afferents from the cortical regions, such as the prefrontal cortex (PFC), hippocampus (HIP), hypothalamus (HYPO), and later dorsal tegmentum (LDT), which provide important substrates for pathophysiology of addiction. The afferents to the NAc are the richest in dopamine followed by GABAergic and glutamatergic afferents from the VTA and PFC, respectively. The cellular localization and subunit composition of nAChRs vary on afferent and efferent connections of the VTA-NAc circuitry, which impact on the function of the brain’s reward system and addictive properties of drugs.
Nicotine, the major psychoactive component in tobacco smoke, exerts its central actions on the brain’s reward system after binding to nAChRs (Galzi and Changeux 1995; Leonard and Bertrand 2001; Leslie et al. 2013). These nAChR subunits arrange in various homomeric and heteromeric combinations of 12 different nAChR subunits, including α2-α10 and β2-β4 in the mammalian brain. Typically, α7, α8, and α9 subunits form homopentameric complexes lacking β subunits, and the α2–α6 containing subunits form heteropentameric complexes with β2-β4 subunits in the CNS (Galzi and Changeux 1995; Leonard and Bertrand 2001). The most abundant subtypes in the brain are α4, β2, and α7 subunits and followed by α3 and α5 subunits. Studies have shown that brain region differences in the messenger RNA (mRNA) expression levels of nAChRs can be contributed to the differences in the configuration of distinct receptor subunits and the cellular localization of the receptors (Galzi and Changeux 1995; Leonard and Bertrand 2001; Leslie et al. 2013). However, because of a lack of receptor agonists and antagonists with selectivity for all putative nAChR subtypes, the precise identification of functional nAChR subtypes that regulate the behavioral and physiological actions of nicotine in vivo remain to be further investigated.
Recent human genetic studies have revealed the functional importance of multiple nAChR subunits, such as the CHRNA5/A3/B4 gene cluster on chromosome 15 (Bierut 2010; Li and Burmeister 2009; Tuesta et al. 2011; Wen et al. 2016) and CHRNA2/CHRNA6 on chromosome 8 (Culverhouse et al. 2014; Haller et al. 2012; Hoft et al. 2009; Saccone et al. 2009; Thorgeirsson et al. 2010; Wang et al. 2014; Zeiger et al. 2008), in the etiology of smoking dependence. A key role for nAChRs containing α4, α6, and β2 subunits is implicated in regulating the positive reinforcing effects of nicotine, whereas the presence of α5 subunit appears to prevent upregulation of receptors and regulate the inhibitory effects of nicotine in vivo (Tuesta et al. 2011). Studies in animal models of nicotine addiction provide additional information related to the effects of nicotine on the nAChR functional regulation. Systemic injection of nicotine leads to an upregulation of nAChRs in the mesocorticolimbic pathways in rodents. The functional upregulation of nAChRs in the mesolimbic pathway appears to be restricted to particular midbrain neurotransmitter systems (Nashmi and Lester 2007; Nashmi et al. 2007). In addition, Rowell and Li (1997) reported that daily nicotine injections increased the number of binding sites, whereas a parallel increase was not observed in the mRNA levels of genes encoding nAChR subunits, suggesting a posttranslational effect of nicotine on its molecular target. Recent advances in our understanding of nicotine addiction have resulted in greater efficiency in treating smokers among the general population (Cao et al. 2013; Midde et al. 2011; Nesil et al. 2015; Song et al. 2016; Vigorito et al. 2013; Zhu et al. 2015). However, such progress has not been achieved in the HIV-1-infected smoking population. Therefore, it is critically important to investigate the effects of nicotine in an appropriate animal model in order to explain the complex interactions between HIV-1 infection and nicotine dependency in the brain.
The HIV-1 transgenic (HIV-1Tg) rat mimics several characteristics of HIV-1-infected patients receiving HAART, which inhibits viral replication without eliminating the presence of viral proteins (Peng et al. 2010). The HIV-1Tg rat carries a gag-pol-deleted HIV-1 genome under the control of HIV-1 viral long terminal repeats (LTRs) and expresses seven of nine viral proteins (Homji et al. 2012b; Liu et al. 2009). The HIV-1Tg rat develops symptoms similar to those of HIV-1-infected humans, including deficits in learning and memory (Reid et al. 2001; Vigorito et al. 2007), abnormal sensitivity psychostimulants such as ethanol, cocaine, morphine, and methamphetamine (Homji et al. 2012a; Liu et al. 2009; McIntosh et al. 2015; Moran et al. 2013; Sarkar and Chang 2013), and aberrant gene expression in the brain (Li et al. 2013). HIV-1Tg rats are more sensitive to the addictive properties of morphine and methamphetamine compared to F344 control strain rats (Homji et al. 2012b; Liu et al. 2009). Previous studies have further shown that HIV-1Tg rats respond differently to nicotine than do F344 control rats, both at the behavioral and gene expression levels (Cao et al. 2013; Midde et al. 2011; Nesil et al. 2015; Song et al. 2016; Vigorito et al. 2013; Zhu et al. 2015). Together, these findings indicate that the cholinergic system is altered in the HIV-1Tg rat; however, the expression of nAChRs, in particular, in this animal model has not been investigated. Thus, the primary purpose of this study was to examine nAChR expression in the brain regions involved in the neuronal reward circuitry of the HIV-1Tg rats.
Materials and methods
Animals
Young adult (6–8 weeks old) male HIV-1Tg rats and F344 strain control rats were purchased from Harlan, Inc. (Indianapolis, IN). All rats were group-housed in standard plastic cages, maintained in a temperature-controlled environment with a 12-h light/dark cycle, and fed a standard rat diet and water ad libitum. All experimental procedures were conducted during the light cycle and approved by the Animal Care and Use Committee of the University of Virginia.
Nicotine treatment and tissue collection
(−)-Nicotine hydrogen tartrate (Sigma-Aldich Corp., St Louis, MO) was dissolved in 0.9 % physiological saline. F344 and HIV-1Tg rats were injected subcutaneously (s.c.) with saline or nicotine (0.4 mg/kg/day) once per day for 27 days before tissue collection (Nesil et al. 2015; Song et al. 2016). The concentration of nicotine was calculated as a nicotine-free base. This dose of nicotine was determined based on Matta et al. (2007) and based on our previous studies (Nesil et al. 2015; Song et al. 2016), which showed that 0.04-mg/kg chronic injections of nicotine significantly induced alterations at the transcriptional level in the brain regions of HIV-1Tg rats. Using a rat brain matrix (Kent Scientific, Torrinton, CT), 1-mm slices were taken from each brain. Slices that contained the VTA, NAc, PFC, HIP, and hypothalamus (HYP) were identified based on a rat brain atlas (Paxinos and Watson 1998). Tissue from the brain regions of interest was collected from each rat using a 1.5-mm brain punch (Stoelting, Wood Dale, IL) and stored at −80 ° C until use.
qRT-PCR array
The primers for each nAChR subunit gene were designed using Primer Express (v. 3.0) software (Applied Biosystems, Carlsbad, CA) and spanned introns to avoid amplifying genomic DNA. The amplicon sequences were subjected to a BLAST search to ensure the specificity of the primers for the target gene and were synthesized by Fisher Scientific (Pittsburgh, PA). All of the primers were tested for their specificity by checking the cycle number and the dissociation curve before inclusion in the qRT-PCR array. The primer sequences of each nAChR subunit gene are given in Table 1.
Table 1.
Primer sequences for nAChR subunits used in quantitative RT-PCR assay
| Gene symbol | Subunit Gene name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|
| Chrna2 | nAChR α2 | CTGGTGCCAGCAGTGTTGAC | GATTGTAGCCTCCAAACAGGTGTT |
| Chrna3 | nAChR α3 | TGGCTAATGTGTCCCATCCA | TCAGCCACAGGTTGGTTTCC |
| Chrna4 | nAChR α4 | GCCAGTAGCCAATATCTCAGATGTG | GTTCTTCTCGTCCACGTCAATG |
| Chrna5 | nAChR α5 | TGGCCTTGCGATATCTCAGTT | CACATCTATCCATTCCTGCTTCAA |
| Chrna6 | nAChR α6 | GAAGCCTGACATCGTTCTGTATAATAA | CGTACTTAAGAAGAGCTTTGGTCTTG |
| Chrna7 | nAChR α7 | CAGGCTATTGTCTGTATCTACCTCCAT | TGCACATCAAAAGGGAACCA |
| Chrnb2 | nAChR β2 | ACTGTACAGCTCATGGTATCATTGG | CCATGTGAGGCGGTAATCTTC |
| Chrnb3 | nAChR β3 | CCTAAGGGTCTTCTTGGTTCTCAGT | GTCTGAGGAGTGCGTCTTCGT |
| Chrnb4 | nAChR β4 | CCTGAACAAAACCCGGTACAA | GTTCTCGCTCATTCACACTGATG |
Total RNA was extracted from the punched tissues of specific brain regions of each animal using TRIZol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. qRT-PCR was conducted as previously described (Cao et al. 2013; Li et al. 2004). Briefly, the RT product was amplified in a volume of 10 μl containing 5 μl of 2× Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) and a combination of 2.5 μl sense and anti-sense primers at a final concentration of 250 nM in a 384-well plate using the 7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA). Expression of all nAChR subunit genes was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). This gene is the most stable internal control identified by Normfinder based on our previous deep sequencing expression data (Cao et al. 2013). Expression was then analyzed using a comparative Ct method (Winer et al. 1999).
Statistical analysis
Data was analyzed using SPSS 22 (IBM, Armonk, NY). The effects of strains and treatment on gene expression of each subunit gene within each brain region were analyzed with Multi-Way ANOVA. Significant group differences were further examined by using post-hoc Bonferroni test.
Results
Expression of nAChR subunits in the VTA of HIV-1Tg and F344 control rats
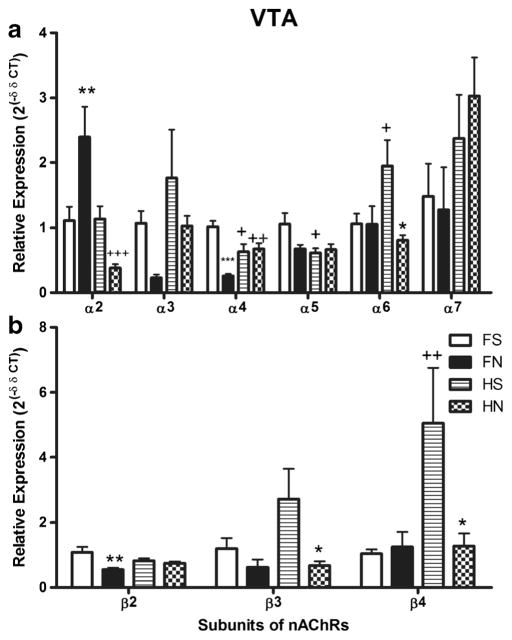
In the VTA (Fig. 1), there existed significant differences in mRNA expression of nAChR subunits between strains, treatments, and subunits (p < 0.05 for all comparisons). The saline-treated HIV-1Tg rats showed significantly lower expression of α4 and α5 subunits (p < 0.05), but higher expression of α6 (p < 0.05) and β4 (p < 0.01) subunits compared to saline-treated F344 rats. Nicotine treatment significantly increased the expression of the α2 subunit (p < 0.01) and decreased the expression of β2 (p < 0.01) and α4 (p < 0.001) subunits in the F344 rats. In contrast, nicotine significantly decreased the expression of α6, β3, and β4 subunits (p < 0.05) in HIV-1Tg rats. Nicotine-treated HIV-1Tg rats showed lower expression of α2 (p < 0.001) and α4 (p < 0.01) subunits compared to nicotine-treated F344 rats.
Fig. 1.
A comparison of mRNA expression differences of nAChR subunits α2–α7 (panel A) and β2–β4 (panel B) in the ventral tegmental area (VTA) of HIV-1Tg rats and F344 control rats (n = 4–6 per group). The first type of comparisons shown in the figure is the difference of either nicotine-treated or saline-treated HIV-1Tg rats compared with F344 rats, where we used +, ++, and +++ to indicate the p values of < 0.05, < 0.01, and < 0.001, respectively. The second type of comparison shown in the figure is the effect of nicotine on HIV-1Tg rats or on F344 background rats, where we use *, **, and *** to indicate a p value of < 0.05, < 0.01, and < 0.001, respectively. FS saline-treated F344 rats, FN nicotine-treated F344 rats. HS saline-treated HIV-1Tg rats, HN nicotine-treated HIV-1Tg rats
Expression of nAChR subunits in the NAc of HIV-1Tg and F344 control rats
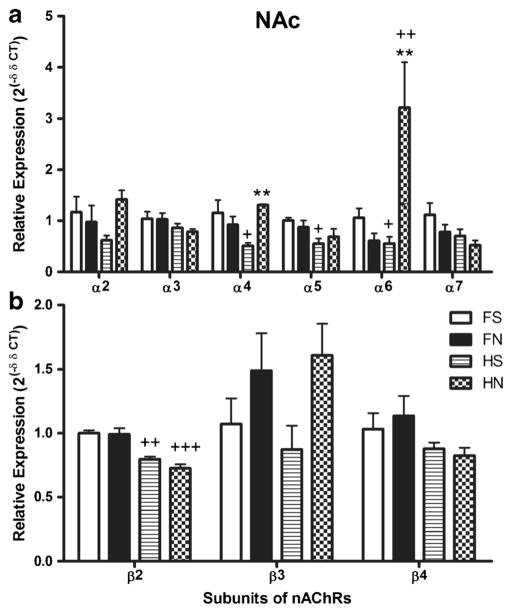
In the NAc (Fig. 2), there also existed significant differences in mRNA expression of nAChR subunits between strains, treatments, and subunits (p < 0.05 for all comparisons). The saline-treated HIV-1Tg rats showed significantly lower expression of α4, α5, α6 (p < 0.05 for all the three subunits), and β2 (p < 0.01) subunits compared to saline-treated F344 rats. Nicotine treatment significantly increased the expression of α4 and α6 subunits in the HIV-1Tg rats (p < 0.01). In contrast, nicotine treatment did not significantly change the expression of nAChR subunits in F344 rats. Nicotine-treated HIV-1Tg rats showed higher expression of the α6 subunit (p < 0.01) and lower expression of the β2 (p < 0.001) subunit compared to nicotine-treated F344 rats.
Fig. 2.
A comparison of mRNA expression differences of nAChR subunits α2–α7 (panel A) and β2–β4 (panel B) in the nucleus accumbens (NAc) of HIV-1Tg rats and F344 control rats (n = 4–6 per group). The first type of comparisons shown in the figure is the difference of either nicotine-treated or saline-treated HIV-1Tg rats compared with F344 rats, where we used +, ++, and +++ to indicate the p values of < 0.05, < 0.01, and < 0.001, respectively. The second type of comparison shown in the figure is the effect of nicotine on HIV-1Tg rats or on F344 background rats, where we use *, **, and *** to indicate a p values of < 0.05, < 0.01 and < 0.001, respectively. FS saline-treated F344 rats, FN nicotine-treated F344 rats, HS saline-treated HIV-1Tg rats, HN nicotine-treated HIV-1Tg rats
Expression of nAChR subunits in the PFC, HIP, and HYP of HIV-1Tg and F344 control rats
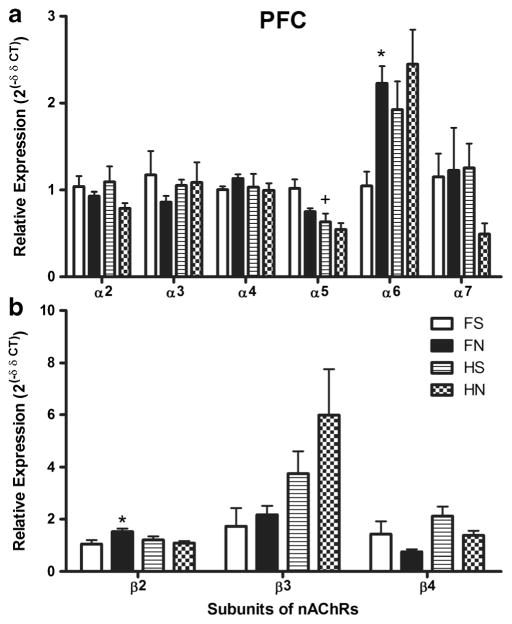
There were significant differences in mRNA expression of nAChR subunits between strains, treatments, and subunit (p < 0.05) in the PFC (Fig. 3). Saline-treated HIV-1Tg rats showed significantly lower expression of the α5 subunit than saline-treated F344 rats (p < 0.05). Nicotine significantly increased the expression of α6 and β2 subunits (p < 0.05) in F344 control rats. In contrast, nicotine did not significantly change the expression of nAChR subunits in HIV-1Tg rats.
Fig. 3.
A comparison of mRNA expression differences of nAChR subunits α2–α7 (panel A) and β2–β4 (panel B) in the prefrontal cortex (PFC) of HIV-1Tg rats and F344 control rats (n = 4–6 per group). The first type of comparisons shown in the figure is the difference of either nicotine-treated or saline-treated HIV-1Tg rats compared with F344 rats, where we used +, ++, and +++ to indicate the p values of < 0.05, < 0.01, and < 0.001, respectively. The second type of comparison shown in the figure is the effect of nicotine on HIV-1Tg rats or on F344 background rats, where we use *, **, and *** to indicate a p values of < 0.05, < 0.01, and < 0.001, respectively. FS saline-treated F344 rats, FN nicotine-treated F344 rats, HS saline-treated HIV-1Tg rats, HN nicotine-treated HIV-1Tg rats
In the HIP (Table 2), HIV-1Tg rats treated with saline showed significantly lower expression of the α6 subunit than saline-treated F344 rats (p < 0.05), whereas nicotine did not change the expression of any subunit in either F344 or HIV-1Tg rats. In the HYP, there existed significant strain difference in the expression of α3 subunit (P = 0.01, Table 3). Nicotine-treated HIV-1Tg rats (Table 3) showed a significantly higher expression of the α3 subunit than in the nicotine-treated F344 rats or in the saline-treated HIV-1Tg rats (p < 0.01 for both comparisons). In addition, nicotine-treated F344 rats showed a significantly lower expression of the β2 subunit than the saline-treated F344 or nicotine-treated HIV-1Tg rats (p < 0.05 for both comparisons).
Table 2.
nAChR subunit mRNA expression in the hippocampus of HIV-1Tg and F344 rats treated with nicotine or saline
| nAChR subunit | F344_saline | F344_nicotine | HIV-1Tg_saline | HIV-1Tg_nicotine |
|---|---|---|---|---|
| α2 | 1.31 ± 0.38 | 2.48 ± 0.71 | 2.01 ± 0.51 | 1.30 ± 0.24 |
| α3 | 1.12 ± 0.26 | 1.21 ± 0.29 | 1.23 ± 0.29 | 0.41 ± 0.08 |
| α4 | 1.24 ± 0.34 | 1.40 ± 0.36 | 1.33 ± 0.41 | 0.83 ± 0.19 |
| α5 | 1.04 ± 0.14 | 1.23 ± 0.23 | 0.88 ± 0.05 | 0.76 ± 0.04 |
| α6 | 1.44 ± 0.65 | 0.63 ± 0.10 | 0.39 ± 0.09a | 0.26 ± 0.07 |
| α7 | 1.22 ± 0.34 | 2.15 ± 0.62 | 1.66 ± 0.42 | 2.49 ± 0.53 |
| β2 | 1.04 ± 0.13 | 1.26 ± 0.20 | 1.02 ± 0.18 | 0.81 ± 0.09 |
| β3 | 1.65 ± 0.67 | 1.69 ± 0.73 | 1.25 ± 0.29 | 0.82 ± 0.18 |
| β4 | 1.03 ± 0.12 | 1.37 ± 0.17 | 1.20 ± 0.09 | 0.88 ± 0.18 |
p < 0.05 strain differences in same drug treatment
Table 3.
nAChR subunit mRNA expression in the hypothalamus of HIV-1Tg and F344 rats treated with nicotine or saline
| nAChR subunit | F344_saline | F344 nicotine | HIV-1Tg_saline | HIV-1Tg_nicotine |
|---|---|---|---|---|
| α2 | 1.10 ± 0.19 | 1.24 ± 0.18 | 1.18 ± 0.21 | 0.94 ± 0.34 |
| α3 | 1.15 ± 0.25 | 0.83 ± 0.18 | 1.02 ± 0.24 | 2.40 ± 0.40a,** |
| α4 | 1.01 ± 0.07 | 0.98 ± 0.11 | 1.70 ± 0.27 | 1.87 ± 0.40 |
| α5 | 1.06 ± 0.15 | 0.86 ± 0.10 | 0.91 ± 0.18 | 0.79 ± 0.18 |
| α6 | 1.73 ± 0.65 | 0.81 ± 0.53 | 1.77 ± 1.03 | 3.81 ± 2.12 |
| α7 | 1.48 ± 0.69 | 0.37 ± 0.08 | 1.70 ± 0.79 | 1.29 ± 0.45 |
| β2 | 1.02 ± 0.08 | 0.69 ± 0.08* | 0.91 ± 0.11 | 1.01 ± 0.10a |
| β3 | 1.28 ± 0.33 | 2.04 ± 0.91 | 1.09 ± 0.22 | 1.88 ± 0.66 |
| β4 | 1.01 ± 0.07 | 0.77 ± 0.10 | 1.01 ± 0.10 | 1.06 ± 0.15 |
p < 0.05;
p < 0.01 drug effect in same strain
p < 0.01 strain differences in same drug treatment
Discussion
Our previous studies have showed that HIV-1Tg rats responded to nicotine differently than F344 control rats in aspects related to learning and memory (Nesil et al. 2015), cellular response to oxidative stress (Song et al. 2016), and gene expression in the brain (Cao et al. 2013; Li et al. 2013). In the current study, we found that the cholinergic system was also altered in HIV-1Tg rats. This was confirmed by the unique mRNA expression of nAChR subunits in the HIV-1Tg rat, especially in the limbic brain regions that are involved in reward processing.
HIV-1 viral proteins alter nAChR subunit expression in the mesocorticolimbic system
HIV-1Tg rats showed altered mRNA levels of nAChR subunits in the mesocorticolimbic dopaminergic system compared to F344 control rats. We observed brain region- and subunit- specific alterations in the expression of several subunit genes in HIV-1Tg rats compared to F344 control rats. Among the brain regions examined, the VTA, the origin of dopaminergic neurons, and the NAc, one of the dopaminergic terminals, in the HIV-1Tg rat exhibited more altered subunits than other brain regions, such as the PFC and HIP. This suggests that cholinergic modulation of mesoaccumbens dopamine transmission is more vulnerable to HIV-1 proteins. While a few previous studies have reported that gp120 can directly bind to nAChRS and interfere with cholinergic neurotransmitter system (Bracci et al. 1992; Gonzalez-Lira et al. 2006), there have been no reports on the interaction of other HIV-1 proteins with nAChRs in the mammalian brain. Our observed alterations in the expression of nAChR subunits in the HIV-1Tg rats are most likely presenting the transcriptional effects of multiple HIV-1 proteins in the mesocorticolimbic system.
Expression of all altered subunits, including α4, α5, α6, and β2, was decreased in the NAc of HIV-1Tg rats. The nAChRs containing α4, α6, and β2 subunits are important in modulating dopamine transmission in the NAc and for nicotine self-administration (Exley et al. 2008, 2011; Pons et al. 2008). In contrast to the NAc, both up- and down-regulation of subunit expression was observed in the VTA of HIV-1Tg rats, with decreased expression of α4 and α5, and increased expression of α6 and β4 subunits. Both α6 (non-α4)- and α6α4-containing nAChRs were identified in the VTA and were reported to be involved in the activation of dopamine neurons (Zhao-Shea et al. 2011). Given the important role of cholinergic modulation on the central reward circuitry, alterations in nAChR subunit mRNA expression is a possible mechanism underlying the increased vulnerability to drug addiction in HIV-1-infected patients.
Nicotine-induced alterations in nAChR mRNA expression
Chronic nicotine treatment altered more subunits in the VTA and NAc than in any other brain regions in both F344 and HIV-1Tg rats, indicating that mesolimbic dopamine transmission is more sensitive to cholinergic modulation. Chronic nicotine treatment down-regulated the expression of subunits α6, β3, and β4 in the VTA, but up-regulated the expression of subunits α4 and α6 in the NAc of HIV-1Tg rats. Since α6 containing nAChRs dominate the effects of nicotine on DA release in the NAc (Exley et al. 2008), the increased α6 expression in the NAc of HIV-1Tg rats indicates that nAChRs play a role in modulating dopamine neuronal activity and vulnerability to nicotine-induced neurobehavioral abnormalities (Leslie et al. 2013). Even though chronic nicotine treatment significantly decreased the expression of nAChR subunits α6, β3, and β4 in the VTA of HIV-1Tg rats, no expression differences of the three subunits were detected between HIV-1Tg and F344 strains. This indicates that the expression differences of these three subunits might be less related to the observed strain differences regarding the molecular alterations that affect the reinforcing and rewarding effects of nicotine in the HIV-1Tg rats. On the other hand, it could also indicate that nicotine restores the function of nAChRs in the VTA, as we reported previously on this animal model treated with nicotine (Cao et al. 2013). Most of the subunit genes were differentially expressed in the saline-treated HIV-1Tg compared to the saline-treated F344 control rats. These findings indicate that chronic nicotine treatment restores the expression of some of these subunits in the HIV-1Tg rat. However, not all of the nAChR subunits were able to “restored” by nicotine. The altered subunits by nicotine in the HIV-1Tg rats were not significantly changed in the F344 rats and vice versa, suggesting that chronic nicotine treatment affects expression of different nAChR subunits in the brain with the presence or absence of HIV-1 proteins.
To increase the statistical power for detecting significantly expressed genes and minimizing individual variations between experimental groups, only male rats were used in this study. Future studies should be conducted on female rats in order to determine the possible gender differences on the expression of nAChR subunit genes in the HIV-1Tg rats treated with and without nicotine. Given that only the expression at RNA level was investigated in this study, it remains to be determined whether these expression differences of different nAChR subunits can be detected at the protein level. However, compared with the approaches used for RNA profiling, it would be much more challenging to profile protein expression because of limited tissue collected from specific brain region of each individual rat and agents required for assaying protein expression level of such a complicated receptor family. Thus, it was our primary objective in this study to determine an expression profile of different nAChR subunits at the RNA level first such that we can design specific study targets for specific receptor subtypes in future studies.
In summary, our data show that the expression of nAChRs is altered in the HIV-1Tg rat. HIV-1 proteins could, thus, alter the sensitivity to addictive substances by modifying nAChRs in brain regions controlling the central neuronal reward pathway. Identification of nAChR subtypes in the midbrain dopaminergic pathways has a potential translational value for the development of therapies for HIV-associated neurocognitive disorders.
Acknowledgments
We thank Drs. Guohua Song and Zhongli Yang for helping to collect brain tissue during the animal experiments. We also thank Drs. David L. Bronson and Louaine L. Spriggs for their excellent editing of this manuscript. This project was supported, in part, by the US National Institutes of Health grants, DA-012844 to MDL, DA-016149 to SLC, and DA-026356 to SLC and MDL.
Footnotes
Compliance with ethical standards All experimental procedures were conducted during the light cycle and approved by the Animal Care and Use Committee of the University of Virginia.
References
- Bierut LJ. Convergence of genetic findings for nicotine dependence and smoking related diseases with chromosome 15q24–25. Trends Pharmacol Sci. 2010;31:46–51. doi: 10.1016/j.tips.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci L, Lozzi L, Rustici M, Neri P. Binding of HIV-1 gp120 to the nicotinic receptor. FEBS Lett. 1992;311:115–8. doi: 10.1016/0014-5793(92)81380-5. [DOI] [PubMed] [Google Scholar]
- Cao J, Wang S, Wang J, Cui W, Nesil T, Vigorito M, Chang SL, Li MD. RNA deep sequencing analysis reveals that nicotine restores impaired gene expression by viral proteins in the brains of HIV-1 transgenic rats. PLoS ONE. 2013;8:e68517. doi: 10.1371/journal.pone.0068517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culverhouse RC, Johnson EO, Breslau N, Hatsukami DK, Sadler B, Brooks AI, Hesselbrock VM, Schuckit MA, Tischfield JA, Goate AM, Saccone NL, Bierut LJ. Multiple distinct CHRNB3-CHRNA6 variants are genetic risk factors for nicotine dependence in African Americans and European Americans. Addiction. 2014;109:814–22. doi: 10.1111/add.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Friedman SR, Choopanya K, Vanichseni S, Ward TP. International epidemiology of HIV and AIDS among injecting drug users. AIDS. 1992;6:1053–68. doi: 10.1097/00002030-199210000-00001. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33:2158–66. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux JP, Maskos U, Cragg SJ, Faure P. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–82. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi JL, Changeux JP. Neuronal nicotinic receptors: molecular organization and regulations. Neuropharmacology. 1995;34:563–82. doi: 10.1016/0028-3908(95)00034-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lira B, Rueda-Orozco PE, Galicia O, Montes-Rodriguez CJ, Guzman K, Guevara-Martinez M, Elder JH, Prospero-Garcia O. Nicotine prevents HIVgp120-caused electrophysiological and motor disturbances in rats. Neurosci Lett. 2006;394:136–9. doi: 10.1016/j.neulet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Haller G, Druley T, Vallania FL, Mitra RD, Li P, Akk G, Steinbach JH, Breslau N, Johnson E, Hatsukami D, Stitzel J, Bierut LJ, Goate AM. Rare missense variants in CHRNB4 are associated with reduced risk of nicotine dependence. Hum Mol Genet. 2012;21:647–55. doi: 10.1093/hmg/ddr498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727–34. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Schlaepfer IR, Huizinga D, Ehringer MA. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology. 2009;34:698–706. doi: 10.1038/npp.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homji NF, Mao X, Langsdorf EF, Chang SL. Endotoxin-induced cytokine and chemokine expression in the HIV-1 transgenic rat. J Neuroinflammation. 2012a;9:3. doi: 10.1186/1742-2094-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homji NF, Vigorito M, Chang SL. Morphine-induced conditioned place preference and associated behavioural plasticity in HIV-1 transgenic rats. Int J Clin Exp Med. 2012b;5:105–23. [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–23. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Mol Pharmacol. 2013;83:753–8. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nat Rev Genet. 2009;10:225–31. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Res Mol Brain Res. 2004;132:168–80. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Li MD, Cao J, Wang S, Wang J, Sarkar S, Vigorito M, Ma JZ, Chang SL. Transcriptome sequencing of gene expression in the brain of the HIV-1 transgenic rat. PLoS ONE. 2013;8:e59582. doi: 10.1371/journal.pone.0059582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chang L, Vigorito M, Kass M, Li H, Chang SL. Methamphetamine-induced behavioral sensitization is enhanced in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2009;4:309–16. doi: 10.1007/s11481-009-9160-8. [DOI] [PubMed] [Google Scholar]
- Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDs. 2002;16:39–42. doi: 10.1089/108729102753429389. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacol (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE. Increased sensitivity to cocaine self-administration in HIV-1 transgenic rats is associated with changes in striatal dopamine transporter binding. J Neuroimmune Pharmacol. 2015;10:493–505. doi: 10.1007/s11481-015-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, Santamaria EK, Bell J. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009;21:168–77. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midde NM, Gomez AM, Harrod SB, Zhu J. Genetically expressed HIV-1 viral proteins attenuate nicotine-induced behavioral sensitization and alter mesocorticolimbic ERK and CREB signaling in rats. Pharmacol Biochem Behav. 2011;98:587–97. doi: 10.1016/j.pbb.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013;239:139–47. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester H. Cell autonomy, receptor autonomy, and thermodynamics in nicotine receptor up-regulation. Biochem Pharmacol. 2007;74:1145–54. doi: 10.1016/j.bcp.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional alpha 4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–18. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain. 2015;8:43. doi: 10.1186/s13041-015-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc. 2011;8:313–9. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98:9271–6. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced up-regulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–9. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–66. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. The NSDUH Report: HIV/AIDS and Substance Use. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2010. [Google Scholar]
- Sarkar S, Chang SL. Ethanol concentration-dependent alterations in gene expression during acute binge drinking in the HIV-1 transgenic rat. Alcohol Clin Exp Res. 2013;37:1082–90. doi: 10.1111/acer.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine mediates expression of genes related to antioxidant capacity and oxidative stress response in HIV-1 transgenic rat brain. J Neurovirol. 2016;22:114–24. doi: 10.1007/s13365-015-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem Pharmacol. 2011;82:984–95. doi: 10.1016/j.bcp.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, LaShomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007;2:319–28. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- Vigorito M, Cao J, Li MD, Chang SL. Acquisition and long-term retention of spatial learning in the human immunodeficiency virus-1 transgenic rat: effects of repeated nicotine treatment. J Neurovirol. 2013;19:157–65. doi: 10.1007/s13365-013-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, DvdV A, Xu Q, Seneviratne C, Pomerleau OF, Pomerleau CS, Payne TJ, Ma JZ, Li MD. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum Genet. 2014;133:575–86. doi: 10.1007/s00439-013-1398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Jiang K, Yuan W, Cui W, Li MD. Contribution of variants in CHRNA5/A3/B4 gene cluster on chromosome 15 to tobacco smoking: from genetic association to mechanism. Mol Neurobiol. 2016;53:472–84. doi: 10.1007/s12035-014-8997-x. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–9. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ, Hewitt JK, Hopfer CJ, Lessem J, McQueen MB, Rhee SH, Ehringer MA. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum Mol Genet. 2008;17:724–34. doi: 10.1093/hmg/ddm344. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–32. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yuan Y, Midde NM, Gomez AM, Sun WL, Quizon PM, Zhan CG. HIV-1 transgenic rats display an increase in [H]dopamine uptake in the prefrontal cortex and striatum. J Neurovirol. 2015 doi: 10.1007/s13365-015-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]