Abstract
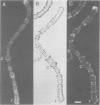
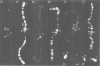
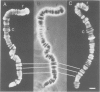
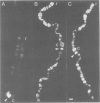
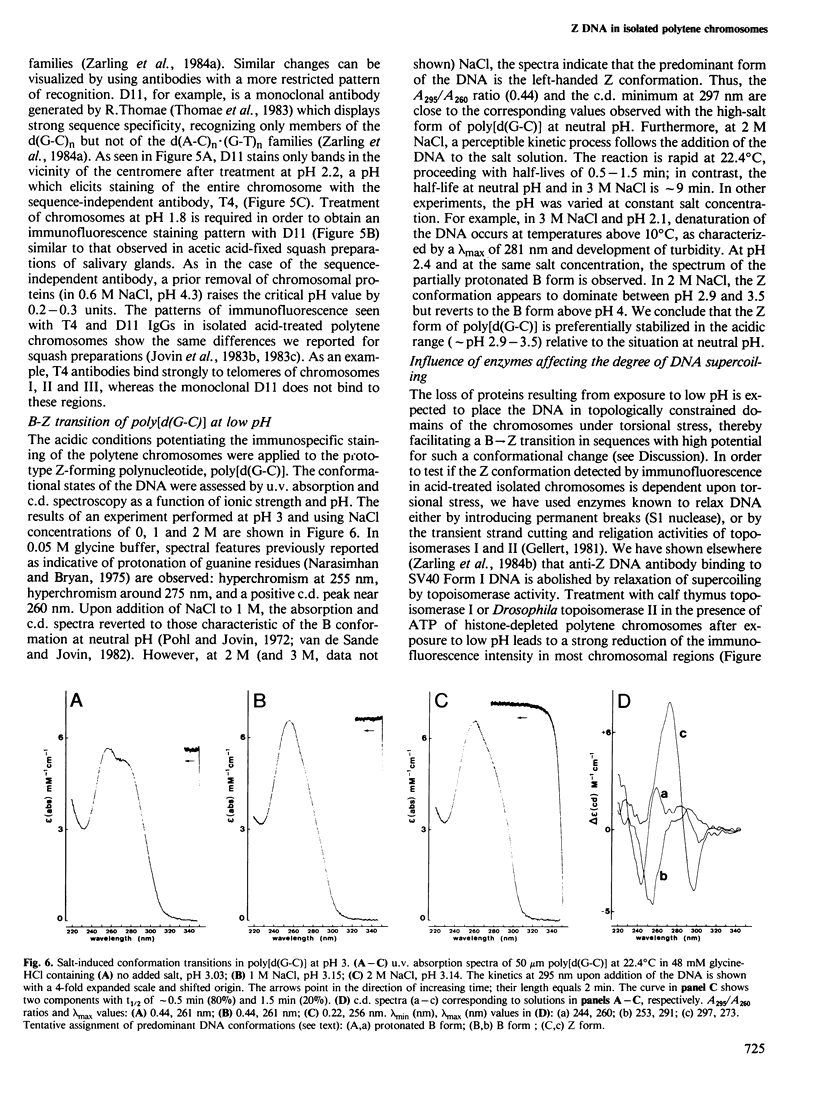
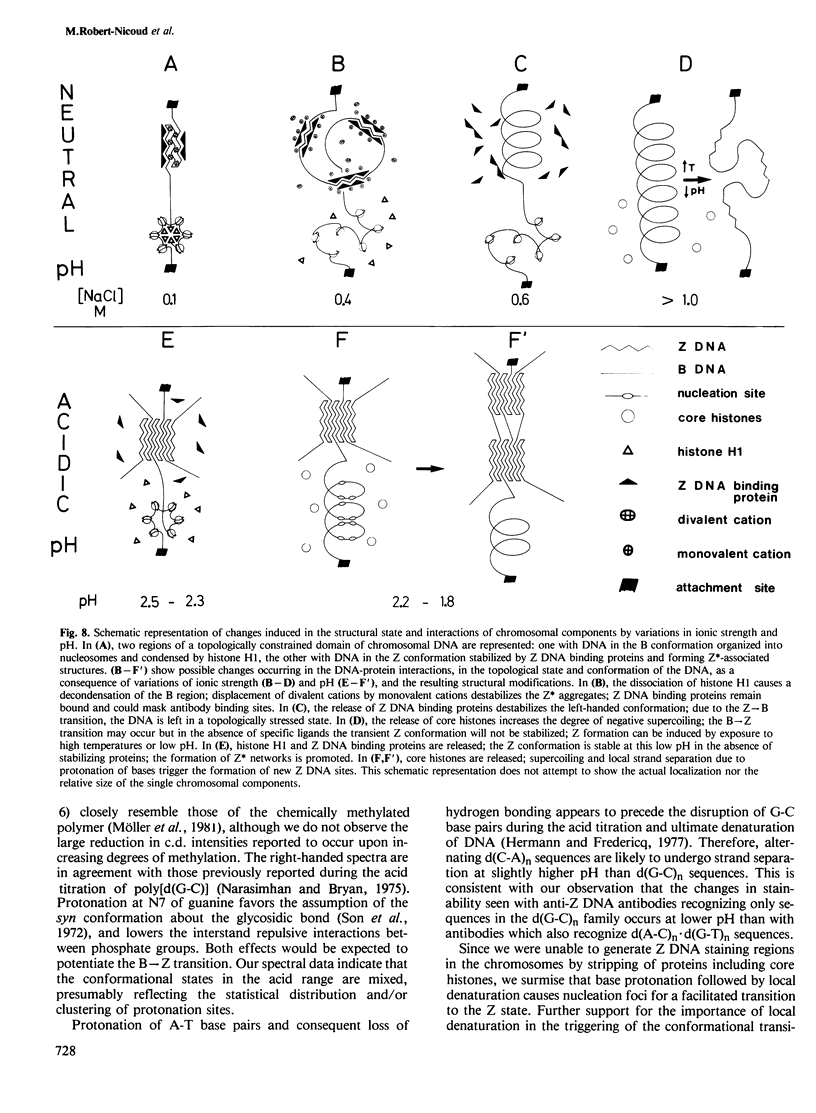
We have searched for the presence of left-handed Z DNA in unfixed polytene chromosomes isolated from the salivary glands of Chironomus thummi larvae. Physiological as well as fixation conditions were explored to assess the effects of a variety of factors known to influence the B-Z equilibrium. At neutral pH and physiological ionic strength, a weak immunofluorescence staining confined to the periphery of chromosomal bands is elicited but only by using high concentrations of anti-Z DNA immunoglobulin (IgG). The accessibility of internal highly condensed structures, as monitored with antibodies against core histones, is very limited under these conditions. Increasing the ionic strength exposes core histone determinants but results in a decondensation of the bands. The staining for Z DNA is still weak and primarily restricted to regions resisting decondensation or undergoing collapse. Dramatic changes in anti-Z DNA immunofluorescence intensities occur upon short exposure to low pH. Adjustment of the pH between 2.5 and 2.0 leads to an abrupt large increase in antibody binding, at first confined to a few specific bands and then generalized to bands throughout the chromosomes in a pattern very similar to that elicited in classical acid-fixed squash preparations. The acid-mediated effects are influenced by ionic strength, temperature and prior removal of histones; they can be mimicked by exposure to high temperature at neutral pH. The 'transition pH' assessed with a monoclonal IgG specific for left-handed d(G-C)n sequences is slightly lower than in the case of polyclonal antibodies which also recognize d(A-C)n X d(G-T)n.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Robert-Nicoud M., Zarling D. A., Greider C., Weimer E., Jovin T. M. Left-handed Z-DNA in bands of acid-fixed polytene chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4344–4348. doi: 10.1073/pnas.80.14.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azorin F., Nordheim A., Rich A. Formation of Z-DNA in negatively supercoiled plasmids is sensitive to small changes in salt concentration within the physiological range. EMBO J. 1983;2(5):649–655. doi: 10.1002/j.1460-2075.1983.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behe M., Zimmerman S., Felsenfeld G. Changes in the helical repeat of poly(dG-m5dC) . poly(dG-m5dC) and poly(dG-dC) . poly(dG-dC) associated with the B-Z transition. Nature. 1981 Sep 17;293(5829):233–235. doi: 10.1038/293233a0. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976 Nov;9(3):393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Bolund L. A., Johns E. W. The selective extraction of histone fractions from deoxyribonucleoprotein. Eur J Biochem. 1973 Jun 15;35(3):546–553. doi: 10.1111/j.1432-1033.1973.tb02871.x. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Wang J. C. Cruciform formation in a negatively supercoiled DNA may be kinetically forbidden under physiological conditions. Cell. 1983 Jul;33(3):817–829. doi: 10.1016/0092-8674(83)90024-7. [DOI] [PubMed] [Google Scholar]
- Di Capua E., Stasiak A., Koller T., Brahms S., Thomae R., Pohl F. M. Torsional stress induces left-handed helical stretches in DNA of natural base sequence: circular dichroism and antibody binding. EMBO J. 1983;2(9):1531–1535. doi: 10.1002/j.1460-2075.1983.tb01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand R., Job C., Zarling D. A., Teissère M., Jovin T. M., Job D. Comparative transcription of right- and left-handed poly[d(G-C)] by wheat germ RNA polymerase II. EMBO J. 1983;2(10):1707–1714. doi: 10.1002/j.1460-2075.1983.tb01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameister H. RNA synthesis in isolated polytene nuclei from Chironomus tentans. Chromosoma. 1977 Jul 8;62(3):217–242. doi: 10.1007/BF00286045. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Facile transition of poly[d(TG) x d(CA)] into a left-handed helix in physiological conditions. Nature. 1983 Apr 14;302(5909):632–634. doi: 10.1038/302632a0. [DOI] [PubMed] [Google Scholar]
- Harvey S. C. DNA structural dynamics: longitudinal breathing as a possible mechanism for the B in equilibrium Z transition. Nucleic Acids Res. 1983 Jul 25;11(14):4867–4878. doi: 10.1093/nar/11.14.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann P., Fredericq E. The role of the AT pairs in the acid denaturation of DNA. Nucleic Acids Res. 1977 Aug;4(8):2939–2947. doi: 10.1093/nar/4.8.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. J., Stollar B. D. Dependence of Z-DNA antibody binding to polytene chromosomes on acid fixation and DNA torsional strain. Nature. 1983 Sep 22;305(5932):338–340. doi: 10.1038/305338a0. [DOI] [PubMed] [Google Scholar]
- Hsieh T. Purification and properties of type II DNA topoisomerase from embryos of Drosophila melanogaster. Methods Enzymol. 1983;100:161–170. doi: 10.1016/0076-6879(83)00052-x. [DOI] [PubMed] [Google Scholar]
- Hägele K. Differential staining of polytene chromosome bands in Chironomus by Giemsa banding methods. Chromosoma. 1977 Feb 3;59(3):207–216. doi: 10.1007/BF00292778. [DOI] [PubMed] [Google Scholar]
- Igó-Kemenes T., Zachau H. G. Domains in chromatin structure. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):109–118. doi: 10.1101/sqb.1978.042.01.012. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Liu J. F., Wang J. C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science. 1978 Mar 24;199(4335):1345–1346. doi: 10.1126/science.628842. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., McIntosh L. P., Arndt-Jovin D. J., Zarling D. A., Robert-Nicoud M., van de Sande J. H., Jorgenson K. F., Eckstein F. Left-handed DNA: from synthetic polymers to chromosomes. J Biomol Struct Dyn. 1983 Oct;1(1):21–57. doi: 10.1080/07391102.1983.10507425. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., van de Sande J. H., Zarling D. A., Arndt-Jovin D. J., Eckstein F., Füldner H. H., Greider C., Grieger I., Hamori E., Kalisch B. Generation of left-handed Z-DNA in solution and visualization in polytene chromosomes by immunofluorescence. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):143–154. doi: 10.1101/sqb.1983.047.01.019. [DOI] [PubMed] [Google Scholar]
- KEYL H. G. Untersuchungen am Karyotypus von Chironomus thummi. I. Karte der Speicheldrüsen-Chromosomen von Chironomus thummi thummi und die cytologische Differenzierung der Subspezies Ch. th. thummi und Ch. th. piger. Chromosoma. 1957;8(6):739–756. [PubMed] [Google Scholar]
- Klevan L., Schumaker V. N. Stabilization of Z-DNA by polyarginine near physiological ionic strength. Nucleic Acids Res. 1982 Nov 11;10(21):6809–6817. doi: 10.1093/nar/10.21.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klysik J., Stirdivant S. M., Singleton C. K., Zacharias W., Wells R. D. Effects of 5 cytosine methylation on the B-Z transition in DNA restriction fragments and recombinant plasmids. J Mol Biol. 1983 Jul 25;168(1):51–71. doi: 10.1016/s0022-2836(83)80322-2. [DOI] [PubMed] [Google Scholar]
- Kroeger H., Trösch W., Müller G. Changes in nuclear electrolytes of Chironomus thummi salivary gland cells during development. Exp Cell Res. 1973 Aug;80(2):329–339. doi: 10.1016/0014-4827(73)90304-2. [DOI] [PubMed] [Google Scholar]
- Lang M. C., Malfoy B., Freund A. M., Daune M., Leng M. Visualization of Z sequences in form V of pBR322 by immuno-electron microscopy. EMBO J. 1982;1(10):1149–1153. doi: 10.1002/j.1460-2075.1982.tb00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. M., Cole R. D. Selective displacement of histone H1 from whole HeLa nuclei: effect on chromatin structure in situ as probed by micrococcal nuclease. Biochemistry. 1979 May 29;18(11):2160–2166. doi: 10.1021/bi00578a005. [DOI] [PubMed] [Google Scholar]
- Lemeunier F., Derbin C., Malfoy B., Leng M., Taillandier E. Identification of left-handed Z-DNA by indirect immunofluorescence in polytene chromosomes of Chironomus thummi thummi. Exp Cell Res. 1982 Oct;141(2):508–513. doi: 10.1016/0014-4827(82)90245-2. [DOI] [PubMed] [Google Scholar]
- Lezzi M., Robert M. Chromosomes isolated from unfixed salivary gland of Chironomus. Results Probl Cell Differ. 1972;4:35–57. doi: 10.1007/978-3-540-37164-9_2. [DOI] [PubMed] [Google Scholar]
- Lipps H. J., Nordheim A., Lafer E. M., Ammermann D., Stollar B. D., Rich A. Antibodies against Z DNA react with the macronucleus but not the micronucleus of the hypotrichous ciliate stylonychia mytilus. Cell. 1983 Feb;32(2):435–441. doi: 10.1016/0092-8674(83)90463-4. [DOI] [PubMed] [Google Scholar]
- McIntosh L. P., Grieger I., Eckstein F., Zarling D. A., van de Sande J. H., Jovin T. M. Left-handed helical conformation of poly[d(A-m5C).d(G-T)]. Nature. 1983 Jul 7;304(5921):83–86. doi: 10.1038/304083a0. [DOI] [PubMed] [Google Scholar]
- Miller F. D., Rattner J. B., van de Sande J. H. Nucleosome-core assembly on B and Z forms of poly[d(G-m5C)]. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):571–575. doi: 10.1101/sqb.1983.047.01.067. [DOI] [PubMed] [Google Scholar]
- Morgenegg G., Celio M. R., Malfoy B., Leng M., Kuenzle C. C. Z-DNA immunoreactivity in rat tissues. Nature. 1983 Jun 9;303(5917):540–543. doi: 10.1038/303540a0. [DOI] [PubMed] [Google Scholar]
- Murray K. The acid extraction of histones from calf thymus deoxyribonucleoprotein. J Mol Biol. 1966 Feb;15(2):409–419. doi: 10.1016/s0022-2836(66)80116-x. [DOI] [PubMed] [Google Scholar]
- Mähr R., Perriard J. C., Lezzi M., Eppenberger H. M. Synthesis of RNA in isolated polytene nuclei from salivary gland cells of Chironomus thummi. Exp Cell Res. 1979 Nov;124(1):7–14. doi: 10.1016/0014-4827(79)90251-9. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Nichols S. R., Rich A. 7-Methylguanine in poly(dG-dC).poly(dG-dC) facilitates z-DNA formation. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4777–4781. doi: 10.1073/pnas.78.8.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan V., Bryan A. M. The unique protonated conformation of poly d (G-C) as detected by circular dichroism studies. FEBS Lett. 1975 Jun 1;54(1):49–52. doi: 10.1016/0014-5793(75)81065-9. [DOI] [PubMed] [Google Scholar]
- Nickol J., Behe M., Felsenfeld G. Effect of the B--Z transition in poly(dG-m5dC) . poly(dG-m5dC) on nucleosome formation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1771–1775. doi: 10.1073/pnas.79.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Lafer E. M., Peck L. J., Wang J. C., Stollar B. D., Rich A. Negatively supercoiled plasmids contain left-handed Z-DNA segments as detected by specific antibody binding. Cell. 1982 Dec;31(2 Pt 1):309–318. doi: 10.1016/0092-8674(82)90124-6. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Pardue M. L., Lafer E. M., Möller A., Stollar B. D., Rich A. Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature. 1981 Dec 3;294(5840):417–422. doi: 10.1038/294417a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Tesser P., Azorin F., Kwon Y. H., Möller A., Rich A. Isolation of Drosophila proteins that bind selectively to left-handed Z-DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7729–7733. doi: 10.1073/pnas.79.24.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. K., Srinivasan A. R., Marky N. L., Balaji V. N. Theoretical probes of DNA conformation examining the B leads to Z conformational transition. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):229–241. doi: 10.1101/sqb.1983.047.01.028. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Civan M. M. Intracellular distribution of free potassium in Chironomus salivary glands. Science. 1975 Jun 27;188(4195):1321–1322. doi: 10.1126/science.1145200. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Nordheim A., Lafer E. M., Stollar B. D., Rich A. Z-DNA and the polytene chromosome. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):171–176. doi: 10.1101/sqb.1983.047.01.022. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Nordheim A., Rich A., Wang J. C. Flipping of cloned d(pCpG)n.d(pCpG)n DNA sequences from right- to left-handed helical structure by salt, Co(III), or negative supercoiling. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4560–4564. doi: 10.1073/pnas.79.15.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Thomae R., DiCapua E. Antibodies to Z-DNA interact with form V DNA. Nature. 1982 Dec 9;300(5892):545–546. doi: 10.1038/300545a0. [DOI] [PubMed] [Google Scholar]
- Rich A. Right-handed and left-handed DNA: conformational information in genetic material. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):1–12. doi: 10.1101/sqb.1983.047.01.003. [DOI] [PubMed] [Google Scholar]
- Robert M. Einfluss von Ionenstärke und pH auf die differentielle Dekondensation der Nukleoproteide isolierter speicheldrüsen-Zellkerne und -Chromosomen von Chironomus thummi. Chromosoma. 1971;36(1):1–33. doi: 10.1007/BF00326419. [DOI] [PubMed] [Google Scholar]
- Robert M. Isolation and manipulation of salivary gland nuclei and chromosomes. Methods Cell Biol. 1975;9(0):377–390. doi: 10.1016/s0091-679x(08)60083-7. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Precious B., Martin S. R., Bayley P. M. Differential promotion and suppression of Z leads to B transitions in poly[d(G-C)] by histone subclasses, polyamino acids and polyamines. EMBO J. 1983;2(10):1647–1653. doi: 10.1002/j.1460-2075.1983.tb01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8421–8428. [PubMed] [Google Scholar]
- Sass H. Features of in vitro puffing and RNA synthesis in polytene chromosomes of Chironomus. Chromosoma. 1980;78(1):33–78. doi: 10.1007/BF00291908. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Stirdivant S. M., Wells R. D. Left-handed Z-DNA is induced by supercoiling in physiological ionic conditions. Nature. 1982 Sep 23;299(5881):312–316. doi: 10.1038/299312a0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Klysik J., Wells R. D. Conformational flexibility of junctions between contiguous B- and Z-DNAs in supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 May;80(9):2447–2451. doi: 10.1073/pnas.80.9.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son T. D., Guschlbauer W., Guéron M. Flexibility and conformations of guanosine monophosphates by the Overhauser effect. J Am Chem Soc. 1972 Nov 1;94(22):7903–7911. doi: 10.1021/ja00777a038. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Kłysik J., Wells R. D. Energetic and structural inter-relationship between DNA supercoiling and the right- to left-handed Z helix transitions in recombinant plasmids. J Biol Chem. 1982 Sep 10;257(17):10159–10165. [PubMed] [Google Scholar]
- Stockton J. F., Miller F. D., Jorgenson K. F., Zarling D. A., Morgan A. R., Rattner J. B., van de Sande J. H. Left-handed Z-DNA regions are present in negatively supercoiled bacteriophage PM2 DNA. EMBO J. 1983;2(12):2123–2128. doi: 10.1002/j.1460-2075.1983.tb01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomae R., Beck S., Pohl F. M. Isolation of Z-DNA-containing plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5550–5553. doi: 10.1073/pnas.80.18.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Lemeunier F., Taillandier E. Identification of left-handed Z-DNA by indirect immunomethods in metaphasic chromosomes of a mammal, Gerbillus nigeriae (Gerbillidae, Rodentia). Ann Genet. 1982;25(4):218–222. [PubMed] [Google Scholar]
- Viegas-Péquignot E., Derbin C., Malfoy B., Taillandier E., Leng M., Dutrillaux B. Z-DNA immunoreactivity in fixed metaphase chromosomes of primates. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5890–5894. doi: 10.1073/pnas.80.19.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley R. M., Szostak J. W., Petes T. D. Is there left-handed DNA at the ends of yeast chromosomes? Nature. 1983 Mar 3;302(5903):84–86. doi: 10.1038/302084a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]
- Weith A. Mg2+-dependent compactness of heterochromatic chromosome segments. Exp Cell Res. 1983 Jun;146(1):199–203. doi: 10.1016/0014-4827(83)90338-5. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]