Abstract
Carbon-carbon (C-C) bond formation is paramount in the synthesis of biologically relevant molecules, modern synthetic materials and commodity chemicals such as fuels and lubricants. Traditionally, the presence of a functional group is required at the site of C-C bond formation. Strategies that allow C-C bond formation at inert carbon-hydrogen (C-H) bonds allow scientists to access molecules which would otherwise be inaccessible and to develop more efficient syntheses of complex molecules.1,2 Herein we report a method for the formation of C-C bonds by directed cleavage of traditionally non-reactive C-H bonds and their subsequent coupling with readily available alkenes. Our methodology allows for the selective C-C bond formation at single C-H bonds in molecules that contain a multitude of seemingly indifferentiable such bonds. Selectivity arises through a relayed photoredox catalyzed oxidation of an N-H bond. We anticipate our findings to serve as a starting point for functionalization at inert C-H bonds through a hydrogen atom transfer strategy.
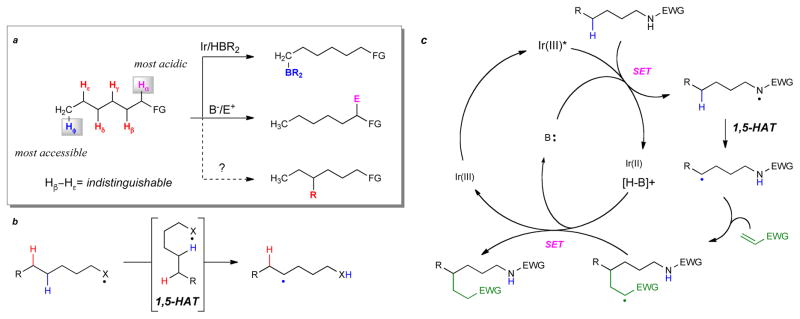
The challenges associated with targeting inert sp3 C-H bonds for C-C bond formation are their high bond strength and selectivity issues arising from their ubiquitous presence as the scaffolding of organic chemistry (Fig. 1a).3 Sterically accessible C-H bonds, typically termini of alkyl chains, may be activated using Rh or Ir based catalysts in emerging borylation chemistry.4 Otherwise, inert sp3 C-H bonds are nearly impossible to distinguish in a rational and selective way, with few exceptions.5–11
Figure 1.
Selectivity issues with C-H bond functionalization. a, Unactivated sp3 C-H bonds are similar in electronics and sterics, making them non-trivial to differentiate. b, Our proposed 1,5-hydrogen atom transfer strategy to cleave an unactivated C-H bond with a heteroatom radical. c, Proposed photocatalytic cycle for carbon-carbon bond formation at unactivated C-H bonds. Deprotonation of an acidified N-H bond and in situ oxidation delivers the nitrogen radical with subsequent HAT and functionalization occurring. Upon trapping, the resultant radical is reduced and protonated closing the catalytic cycle and delivering product. EWG: electron-withdrawing group; FG: functional group; HAT: hydrogen atom transfer; SET: single electron transfer.
In the context of our work on Rh(III) catalyzed C-H activation,12 we sought an alternative method to functionalize sp3 C-H bonds of aliphatic amines. A 1,5-hydrogen atom transfer13,14 (1,5 HAT) strategy occurred to us as a potential solution (Fig. 1b). Heteroatom radicals are known to abstract hydrogen atoms through the 1,5-HAT process in a selective way. Given the ubiquity and relevance of nitrogen in molecules of interest, we sought to generate nitrogen radicals15 from highly acidified amidyl N-H bonds16 using photoredox catalysis17 to functionalize these distal, unactivated positions, and couple them with a subsequent C-C bond forming step (Fig. 1c). Such a transformation would not require pre-functionalization18 of the X-H bond and could comprise a net addition of the C-H bond across the alkene, an atom economic process.
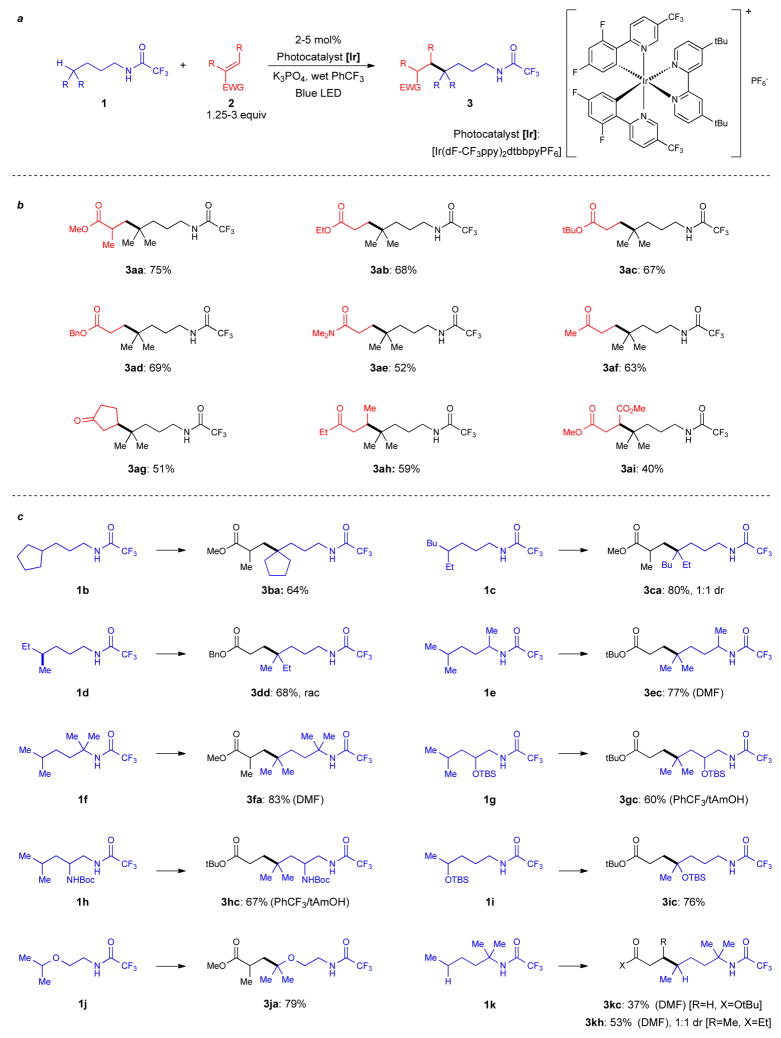
We have developed reaction conditions for carbon-carbon bond formation at a tertiary C-H bond that is five bonds away from a nitrogen atom bearing a readily removable trifluoroacetyl group (Fig. 2a and see supplementary information for further details). This group on nitrogen acidifies the N-H bond (pKa ~13.8)19 sufficiently to allow deprotonation using basic K3PO4 and providing a driving force for 1,5-HAT. Less acidifying groups on nitrogen such as difluoroacetyl or pentafluorobenzoyl do not lead to desired product. At this point, the excited state of the photocatalyst [Ir(dF-CF3ppy)2dtbbpy]PF6 (Fig. 2a) (E1/2red[*IrIII/IrII] = +1.21 V vs SCE in MeCN)20 oxidizes the amidyl anion (Ep = +0.77 V vs SCE in MeCN) to generate a nitrogen radical. After 1,5-HAT, a tertiary carbon radical is generated and subsequently trapped with methyl methacrylate. The use of PhCF3 as a solvent and high reaction concentrations (0.4 M) were necessary to achieve high yield (Fig. 2b) (see supplementary information for details).
Figure 2.
Photoredox catalyzed C-C bond formation at unactivated sp3 C-H bonds. a, Trifluoroacetamide as the directing group for C-H functionalization. b, Scope of C-C bond formation with respect to the alkene. c, Scope of C-C bond formation with respect to the amine. Solvent is benzotrifluoride unless otherwise indicated. Bn: benzyl; Bu: butyl; dr: diastereomeric ratio; Et: ethyl; Me: methyl; rac: racemic; tBu: tert-butyl; TBS: tert-butyldimethylsilyl; Boc: tert-butyloxycarbonyl; Ph: phenyl; DMF: dimethylformamide; tAmOH: tert-amyl alcohol; dF-CF3ppy: 2-(2,4-difluorophenyl)-5-trifluoromethylpyridine; dtbbpy: 4,4′-di-tert-butyl-2,2′-bipyridine.
With the optimized conditions, we investigated the variation in amine molecules with inert C-H bonds and alkenes that are applicable to the developed reaction (Fig. 2b). A range of electron deficient alkenes were successfully trapped by the tertiary radical carbon generated through our photocatalyzed 1,5-HAT methodology using trifluoroacetamide 1a. Ethyl acrylate 2a, an alkene devoid of an α-substituent, coupled with trifluoroacetamide 1a to give the product 3ab in good yield. These results were mirrored with other acrylate esters such as t-butyl and benzyl to give products 3ac and 3ad respectively. It should be noted that the reactive benzylic C-H bonds in benzyl acrylate, or in the corresponding product 3ad, remain intact under the reaction conditions. N,N-dimethyl acrylamide 2e, which has a lower reactivity compared to acrylates, couples with trifluoroacetamide 1a in reasonable yield. Coupling with vinyl ketones was possible giving products 3af, 3ag and 3ah in good yields. The use of methyl vinyl ketone requires lower reaction concentrations and higher catalyst loading in order to disfavor a competitive aza-Michael reaction pathway, which is formed in >50% conversion in the absence of the photoredox catalyst. Less reactive β-substituted vinyl ketones require a higher alkene concentration. Product 3ai was obtained in moderate yield using dimethyl maleate.
Variation of the steric environment around the inert C-H bonds had little effect on the outcome of the reaction; products 3ba, 3ca and 3dd were all obtained in good yield (Fig. 2c). Subjection of enantiomerically pure alkane possessing a stereogenic C-H bond results in formation of racemic product 3dd, consistent with our proposed mechanism. Substrates which bear substituents α to the nitrogen were also competent in the reaction delivering products 3ec and 3fa in good yields. In these cases, optimal yields were obtained by switching from PhCF3 to the more polar solvent DMF. Heteroatom functionality including a TBS protected oxygen and a Boc protected amine can be incorporated into the trifluoroacetamide substrate to give products 3gc and 3hc in good yields. Importantly, the presence of a Boc protected primary amine in substrate 1h does not affect the outcome of the reaction presumably because the Boc-N-H bond is not acidic enough to be deprotonated by the K3PO4. Substrates 1i and 1j, with oxygen atoms adjacent to the C-H bond, participate well in the reaction to give products 3ic and 3ja. Methylene C-H bonds may also be functionalized using this approach as demonstrated with substrate 1k to deliver product 3kc albeit in slightly lower yield. Increased yields are observed using a β-substituted Michael acceptor delivering products such as 3kh.
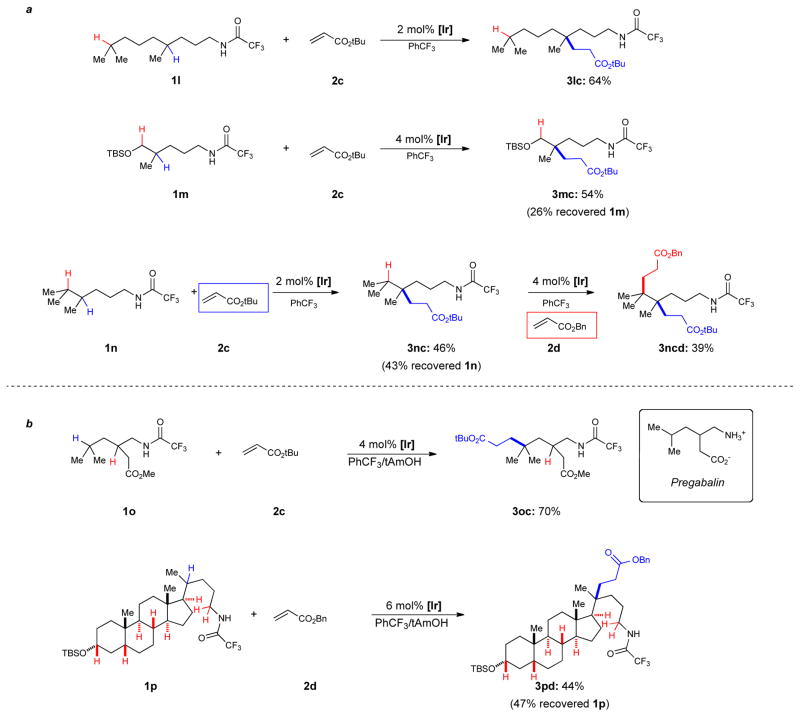
Substrates which contain more than one tertiary C-H bond were explored in the reaction (Fig. 3a). Trifluoroacetamide 1l, which contains two tertiary C-H bonds, can be selectively functionalized at the position proximal to the N-H bond. Functionalization of the remote tertiary C-H bond is not observed. This outcome supports our hypothesis that an intramolecular 1,5-HAT process is operating. When there is potential for a competitive 1,6-HAT,21 as in substrates 1m and 1n, the 1,5-HAT transfer is favored over 1,6-HAT resulting in products 3mc and 3nc. Although 1,5-HAT occurs in preference to 1,6-HAT, products arriving from sequential 1,5-HAT and then 1,6-HAT are observed when reactions are run to completion. Re-subjecting product 3nc to the reaction conditions but with a different alkene coupling partner results in product 3ncd in moderate yield. This reaction sequence allows for the coupling of two distinct alkene partners at the two tertiary C-H bonds in substrate 1n with control over regioselectivity to give a molecule containing two contiguous all-carbon quaternary centers. Medicinally relevant molecules such as Pregabalin22 can also be functionalized (Fig. 3b). Lastly, steroid derived trifluoroacetamide 1p, a molecule containing six distinct tertiary C-H bonds and three C-H bonds adjacent to heteroatoms, can be selectively functionalized at a single position, determined by its proximity to the N-H bond.
Figure 3.
Regioselective functionalization of sp3 C-H Bonds. a, The presence of a remote tertiary C-H bond does not affect the outcome of the reaction; good selectivity is obtained even with potential competition between 1,5 and 1,6 hydrogen atom transfer. b, Applications to medicinally relevant molecules. Bn: benzyl; Me: methyl; tBu: tert-butyl; TBS: tert-butyldimethylsilyl; tAmOH: tert-amyl alcohol; Ph: phenyl.
Our mechanistic studies (see supplementary information for a full discussion) suggest that a stepwise deprotonation/oxidation event is at least partly responsible for the generation of the nitrogen radical. Both Cyclic Voltammetry and Stern Volmer studies show that the trifluoroacetamide conjugate base quenches the excited Ir photocatalyst. The close match of trifluoroacetamide and K3PO4 pKa under these biphasic conditions means that appreciable amounts of the trifluoracetamide conjugate base are present in solution. The observation of aza-Michael product with methyl vinyl ketone supports the assertion that the amidyl anion is formed in appreciable amounts. Furthermore, weaker electron withdrawing groups on nitrogen such as difluoroacetyl do not lead to either desired product or aza-Michael adduct implicating the need for the presence of negative charge on nitrogen in order to get desired reactivity. Thus, the bulk of the evidence supports the stepwise pathway is operative. On the other hand, the use of Cs2CO3, a weaker base (pKa = 10.3), also provides the desired product albeit in lower yield. This raises the possibility that the proton-coupled electron transfer pathway23,24 can be operative in tandem with the stepwise mechanism.
In summary, we have developed a photoredox catalyzed amide directed selective sp3 C-H bond functionalization with concomitant carbon-carbon bond formation. The proposed mechanism features the formation of a nitrogen centered radical by photoredox mediated oxidation of an in situ formed amidyl anion, and subsequent 1,5 hydrogen atom transfer to relay the reactive radical species to a specific carbon atom. The subsequent radical can be trapped with a range of electron deficient alkenes. This finding addresses one of the shortcomings in aliphatic C-H bond functionalization.
Supplementary Material
Acknowledgments
We thank NIGMS (GM80442) for support. J.C.K.C. thanks the Croucher Foundation (Hong Kong) for support. We thank Matthew Burns (CSU) for technical assistance. We thank Professor Robert R. Knowles (Princeton University) for sharing results prior to publication.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions. T.R. and J.C.K.C. conceived the concept and prepared the manuscript. T.R. directed the investigation. J.C.K.C. developed and studied the reaction.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Gutekunst WR, Baran PS. C-H functionalization logic in total synthesis. Chem Soc Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi J, Yamaguchi AD, Itami KC-H. Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew Chem Int Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 3.Newhouse T, Baran PS. If C-H bonds could talk: selective C-H bond oxidation. Angew Chem Int Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence JD, Takahashi M, Bae C, Hartwig JF. Regiospecific functionalization of methyl C-H bonds of alkyl groups in reagents with heteroatom functionality. J Am Chem Soc. 2004;126:15334–15335. doi: 10.1021/ja044933x. [DOI] [PubMed] [Google Scholar]
- 5.Shabashov D, Daugulis O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon-hydrogen bonds. J Am Chem Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, et al. Ligand-controlled C(sp3)-H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, He J, Liu T, Yu JQ. Ligand enabled γ-C(sp3)-H Olefination of Amines: En route to pyrrolidines. J Am Chem Soc. 2016;138:2055–2059. doi: 10.1021/jacs.5b13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang FL, Hong K, Li TJ, Park H, Yu JQ. Functionalization of C(sp3)-H bonds using a transient directing group. Science. 2016;351:252–256. doi: 10.1126/science.aad7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topczewski J, Cabrera P, Saper NI, Sanford MS. Palladium-catalyzed transannular C-H functionalization of alicyclic amines. Nature. 2016;531:220–224. doi: 10.1038/nature16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen MS, White MS. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 2010;327:566–571. doi: 10.1126/science.1183602. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt VA, Quinn RK, Brusoe AT, Alexanian EJ. Site-selective aliphatic C–H bromination using N-bromoamides and visible light. J Am Chem Soc. 2014;136:14389–14392. doi: 10.1021/ja508469u. [DOI] [PubMed] [Google Scholar]
- 11.McNally A, Haffemeyer B, Collins BSL, Gaunt MJ. Palladium-catalysed C-H activation of aliphatic amines to give strained nitrogen heterocycles. Nature. 2014;510:129–133. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]
- 12.Archambeau A, Rovis T. Rhodium(III)-catalyzed allylic C(sp3) activation of alkenyl sulfonamides: unexpected formation of azabicycles. Angew Chem Int Ed. 2015;54:13337–13340. doi: 10.1002/anie.201504150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson J, Pillai J, Lush RK. Radical translocation reactions in synthesis. Chem Soc Rev. 2001;30:94–103. [Google Scholar]
- 14.Cekovic Z. Reactions of carbon radicals generated by 1,5-transposition of reactive centers. J Serb Chem Soc. 2005;70:287–318. [Google Scholar]
- 15.Zard SZ. Recent progress in the generation and use of nitrogen-centered radicals. Chem Soc Rev. 2008;37:1603–1618. doi: 10.1039/b613443m. [DOI] [PubMed] [Google Scholar]
- 16.Nikishin GI, Troyansky EI, Lazareva MI. Regiospecific oxidative cyclization of N-methylsulfonylamines into pyrrolidines. Tetrahedron. 1985;41:4279–4288. [Google Scholar]
- 17.Noble A, MacMillan DWC. Photoredox α-vinylation of α-amino acids and N-aryl amines. J Am Chem Soc. 2014;136:11602–11605. doi: 10.1021/ja506094d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Li Y, Zhang F, Hu C, Chen Y. Generation of alkoxy radicals by photoredox catalysis enables selective C(sp3)-H functionalization under mild reaction condition. Angew Chem Int Ed. 2016;55:1872–1875. doi: 10.1002/anie.201510014. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ. Named Reactions for Functional Group Transformation. Wiley; 2007. pp. 423–437. [Google Scholar]
- 20.Lowry MS, et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem Mater. 2005;17:5712–5719. [Google Scholar]
- 21.Nechab M, Mondal S, Bertrand MP. 1,n-Hydrogen-atom transfer (HAT) reactions in which n≠5: an updated inventory. Chem Eur J. 2014;20:16034–16059. doi: 10.1002/chem.201403951. [DOI] [PubMed] [Google Scholar]
- 22.Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: A novel γ-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48. doi: 10.1016/j.clinthera.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Choi GJ, Knowles RR. Catalytic olefin hydroamidation enabled by proton-coupled electron transfer. J Am Chem Soc. 2015;137:9226–9229. doi: 10.1021/jacs.5b09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller DC, Choi GJ, Orbe HS, Knowles RR. Catalytic alkene carboaminations enabled by oxidative proton-coupled electron transfer. J Am Chem Soc. 2015;137:13492–13495. doi: 10.1021/jacs.5b05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.