Abstract
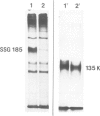
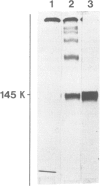
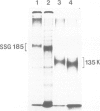
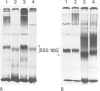
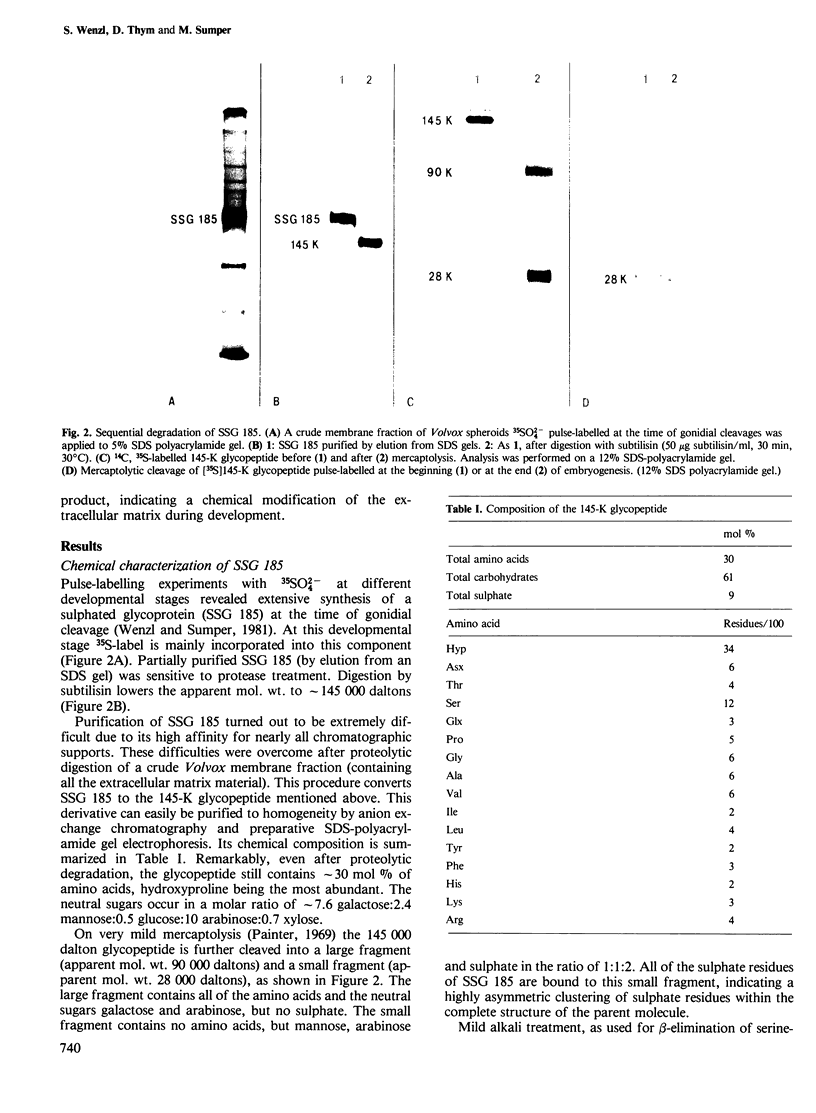
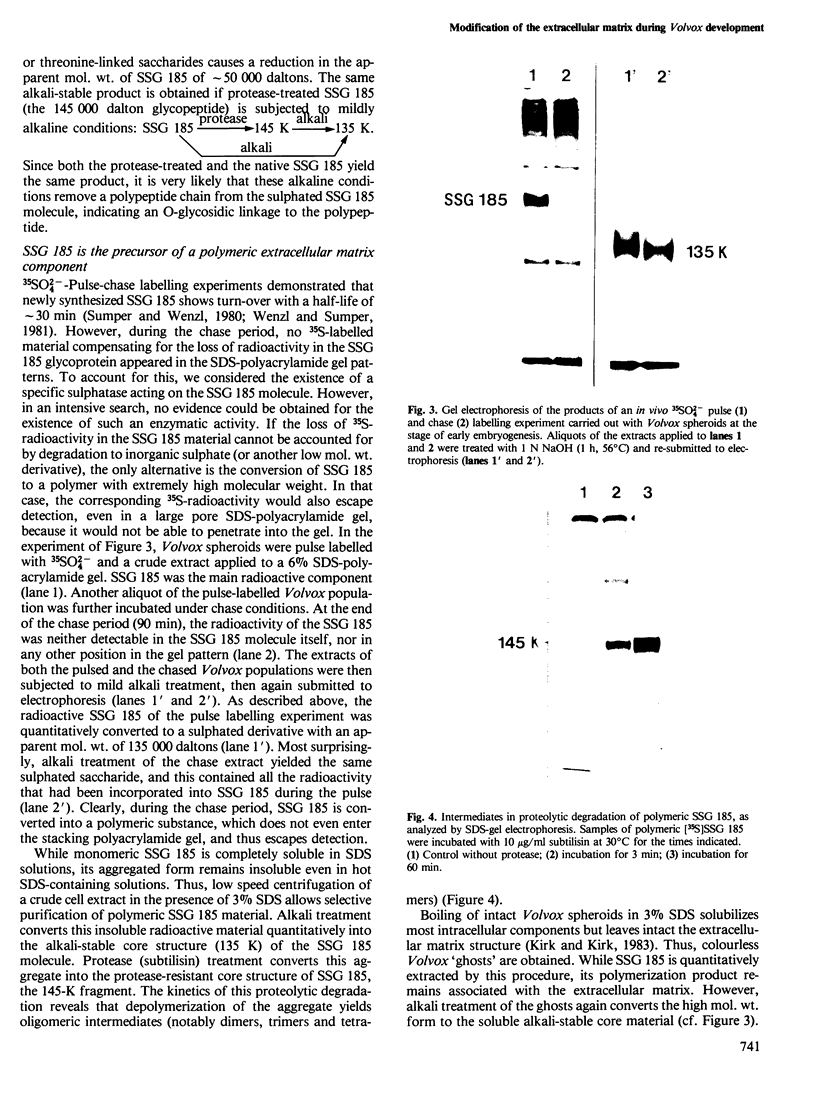
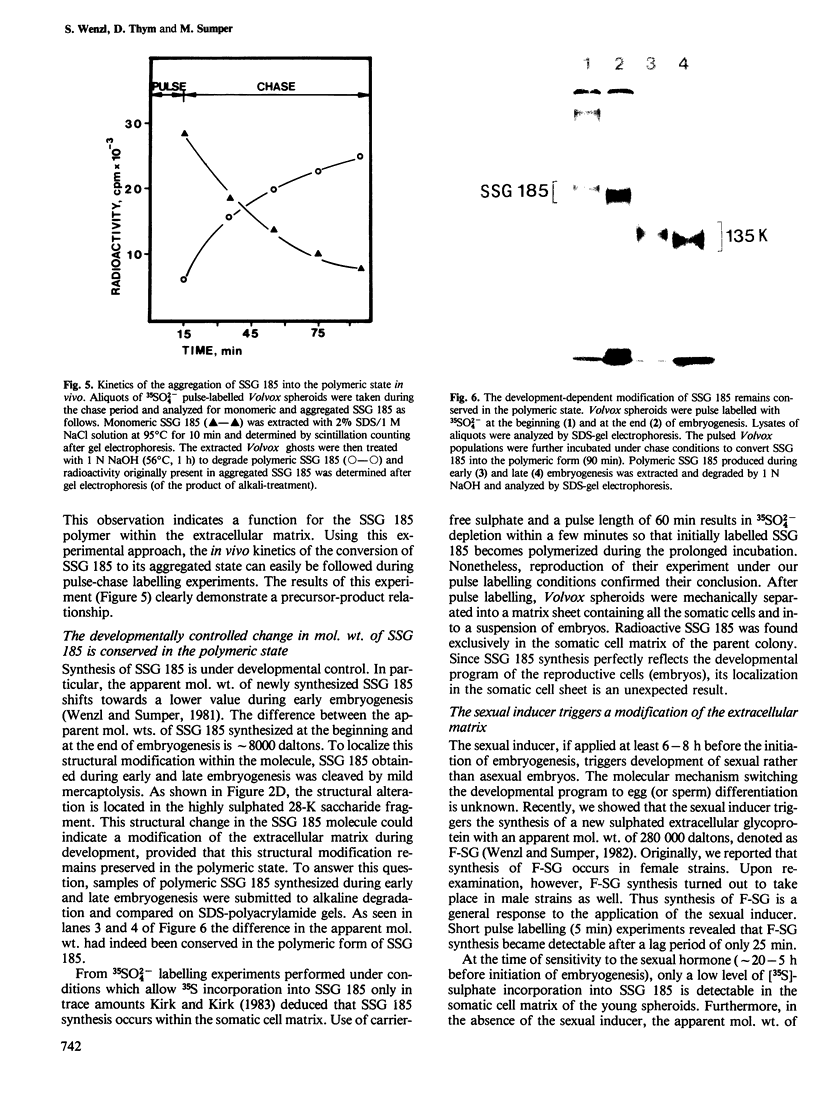
We report the chemical characterization of the highly sulphated glycoprotein SSG 185 from Volvox carteri. SSG 185 is a hydroxyproline-containing, extracellular glycoprotein. The sulphate residues are clustered within the parent saccharide structure of SSG 185, since on mercaptolysis all the sulphate residues are recovered in a small saccharide fragment containing mannose, arabinose and sulphate (in a molar ratio of 1̇1̇2). SSG 185 is a short-lived molecule, serving as a precursor for a high mol. wt. component of the extracellular matrix. Synthesis of SSG 185 is developmentally controlled. Different SSG 185 variants, with unknown modifications in the sulphated saccharide fragment, are synthesized at different developmental stages or under the influence of the sexual inducer. These modifications remain conserved in the aggregated state of SSG 185, indicating the development-dependent modification of the extracellular matrix.
Keywords: extracellular matrix, sulphated glycoproteins, sexual inducer, Volvox
Full text
PDF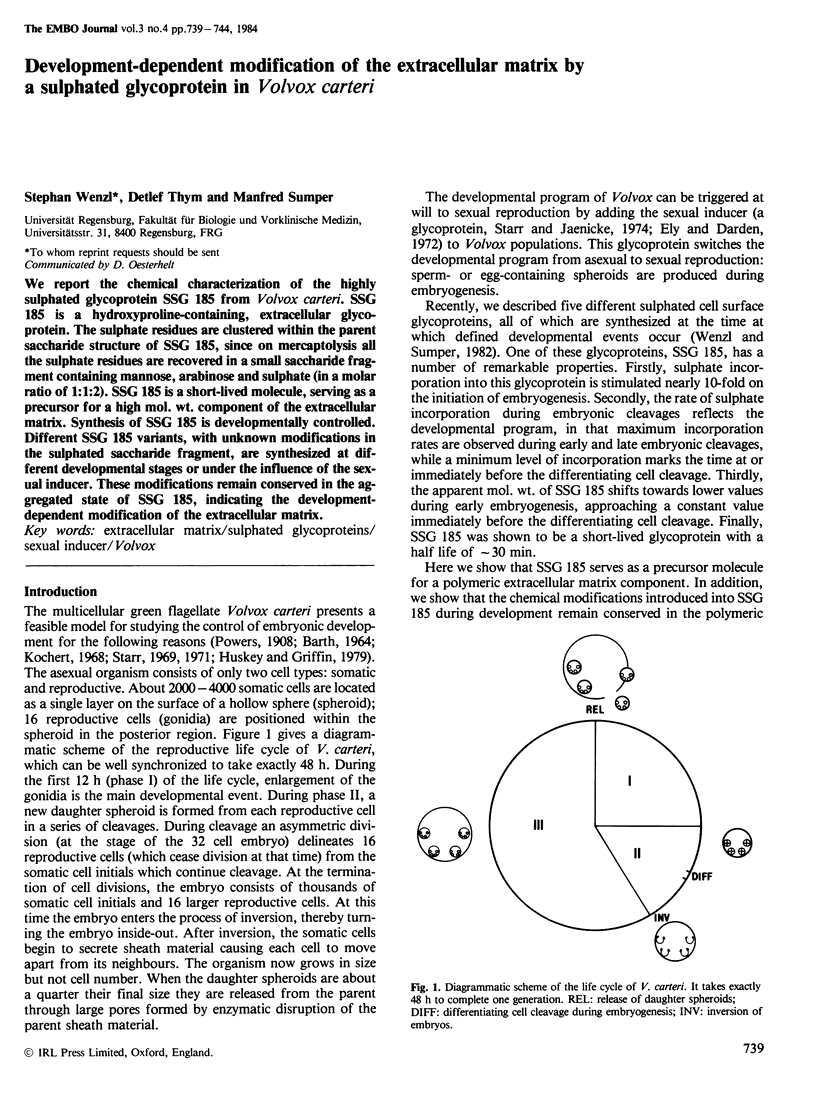

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Ely T. H., Darden W. H. Concentration and purification of the male-inducing substance from Volvox aureus M5. Microbios. 1972 Jan;5(17):51–58. [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Huskey R. J., Griffin B. E. Genetic control of somatic cell differentiation in Volvox analysis of somatic regenerator mutants. Dev Biol. 1979 Oct;72(2):226–235. doi: 10.1016/0012-1606(79)90113-1. [DOI] [PubMed] [Google Scholar]
- Kirk D. L., Kirk M. M. Protein synthetic patterns during the asexual life cycle of Volvox carteri. Dev Biol. 1983 Apr;96(2):493–506. doi: 10.1016/0012-1606(83)90186-0. [DOI] [PubMed] [Google Scholar]
- Kochert G. Differentiation of reproductive cells in Volvox carteri. J Protozool. 1968 Aug;15(3):438–452. doi: 10.1111/j.1550-7408.1968.tb02154.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Starr R. C., Jaenicke L. Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1050–1054. doi: 10.1073/pnas.71.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M., Wenzl S. Sulphation-desulphation of a membrane component proposed to be involved in control of differentiation in Volvox carteri. FEBS Lett. 1980 Jun 2;114(2):307–312. doi: 10.1016/0014-5793(80)81140-9. [DOI] [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Wenzl S., Sumper M. Sulfation of a cell surface glycoprotein correlates with the developmental program during embryogenesis of Volvox carteri. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3716–3720. doi: 10.1073/pnas.78.6.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]