Abstract
Pain encompasses both a sensory as well as an affective dimension and these are differentially processed in the brain and periphery. It is therefore important to develop animal models to reflect the non-reflexive assays in pain. In this study, we compared effects of the mu opioid receptor agonist morphine, the nonsteroidal anti-inflammatory drug ketoprofen and the kappa receptor opioid agonist U50,488H and antagonist JDTic on acetic acid-induced stretching and acetic acid-induced aversion in the condition place aversion (CPA) test in male ICR mice. Intraperitoneal administration of acetic acid (0.32–1%) was equipotent in stimulating stretching and CPA. Ketoprofen, morphine and U50,488H all inhibited the acid-induced stretching. Ketoprofen and morphine also blocked the acid-induced CPA but U50,488H failed to do so. The reversal ability of ketoprofen and morphine on acid-induced CPA is unique to pain-stimulated place aversion since these drugs failed to reduce non-noxious LiCl-induced CPA. Overall, this study characterized and validated a preclinical mouse model of pain-related aversive behavior that can be used to assess genetic and biological mechanisms of pain as well as improving the predictive validity of preclinical studies on candidate analgesics.
Keywords: acetic acid, conditioned place aversion, mice, pain, analgesics
1. Introduction
Pain has been described as a multi-dimensional state composed of sensory, affective, and cognitive components (Apkarian et al., 2004; Ji et al., 2010; Neugebauer et al., 2009). Furthermore, pain states that require clinical intervention are often accompanied by changes in affective behaviors (Hummel et al., 2008; Joshi and Honore, 2006; Mogil, 2009; Whiteside et al., 2013). Thus, animal models that measure pain-related changes in affective behaviors may serve as important tools in the development of more efficacious analgesic drugs. Recent behavioral studies suggest that these affective components of pain can be evaluated in rodents. For example, depression of positively reinforced operant responding maintained by delivery of food (Martin et al., 2004) or electrical brain stimulation (Do Carmo et al., 2009; Leitl et al., 2014) was reported after injury or treatment with experimental noxious stimuli. In addition, recent studies in rats showed that a aversion to a noxious stimuli can also be assessed with a conditioned place aversion (CPA) test after intraperitoneal (i.p.) injection of acetic acid (AA) (Deyama et al., 2010) or intraplantar injection of complete Freund’s adjuvant (Johansen et al., 2001; Zhang et al., 2013). These studies revealed that several limbic brain areas, such as the anterior cingulate cortex (Deyama et al., 2007; Johansen and Fields, 2004; Johansen et al., 2001), central amygdala (Deyama et al., 2010), and bed nucleus of the stria terminalis (Deyama et al., 2008, 2007), mediate this CPA. There has been limited studies exploring the induction of CPA after noxious stimulus delivery in mice (Browne and Woolf, 2014; Daou et al., 2013), but such studies might be useful given the molecular and genetic applicability of mouse models to human disease phenotypes (Rosenthal and Brown, 2007).
Toward that end, the purpose of this study was to evaluate the expression and pharmacological modulation of CPA produced in mice by a commonly used acute visceral noxious stimulus (i.p. AA). We hypothesized that AA would induce place aversion, and that AA-induced CPA would be sensitive to blockade by pretreatment with two clinically effective analgesics, the nonsteroidal anti-inflammatory drug ketoprofen or the mu opioid receptor agonist morphine. Effects of the kappa opioid receptor agonist U50,488H and the kappa antagonist JDTic were also evaluated. Kappa agonists that readily cross the blood-brain barrier to produce centrally mediated effects after systemic administration constitute one class of drugs that produces antinociception in many conventional preclinical assays of pain (Broadbear et al., 1994; Horan et al., 1991); however, centrally acting kappa agonists have failed to meet safety and efficacy criteria for clinical use (Pande et al., 1996a, 1996b). Consequently, kappa agonists exemplify the potential for “false positive” outcomes in conventional preclinical assays of candidate analgesics (Mogil, 2009; Negus et al., 2006; Vierck et al., 2008; Whiteside et al., 2013), and U50,488H was tested here as a negative control. We hypothesized that U50,488H would fail to block AA-induced CPA. Conversely, it has been suggested that negative affective components of pain may involve activation of endogenous kappa opioid systems in limbic brain regions (Cahill et al., 2014). This hypothesis predicts that AA-induced CPA might be blocked by a kappa antagonist like JDTic.
The present study also included two other components. First, the expression and pharmacological modulation of AA-induced CPA were compared to the expression and pharmacological modulation of the AA-induced stretching response. The stretching (or “writhing”) response is a commonly used behavioral endpoint in studies of visceral pain elicited by i.p. injection of AA or other chemical irritants (Koster et al., 1959). However, we have categorized the stretching response as an example of a “pain-stimulated behavior,” which can be defined as a behavior that increases in rate, frequency or intensity after delivery of a noxious stimulus (Negus et al., 2010, 2006). Exclusive reliance on pain-stimulated behaviors in preclinical research can be problematic because they are sensitive not only to treatments that reduce sensory sensitivity to the noxious stimulus, but also to treatments that produce motor impairment. We hypothesized that AA-induced stretching would be blocked by ketoprofen, morphine and U50,488H but not by JDTic. Second, ketoprofen and morphine effects on AA-induced CPA were compared to their effects on CPA induced by lithium chloride (LiCl), a non-noxious aversive stimulus (Lett, 1985). Insofar as LiCl-induced aversion is not thought to involve nociception, we hypothesized that neither ketoprofen nor morphine would block LiCl-induced aversion.
2. Materials and Methods
2.1. Animals
Male adult ICR mice (20–25g) obtained from Harlan Laboratories (Indianapolis, IN) were used throughout the study. Animals were housed in an AAALAC approved facility in groups of four and had free access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.2. Drugs
U50,488H [trans- (±) -3,4-Dichloro-N-methyl-N-[2- (1-pyrrolidinyl) cyclohexyl] benzeneacetamide methanesulfonate salt], AA and LiCl were purchased from Sigma-Aldrich (St. Louis, MO). Ketoprofen (100 mg/ml in distilled water with 0.25% Benzyl alcohol) was purchased from Fort Dodge (Fort Dodge, IA). Morphine sulfate [morphine hemi[sulfate pentahydrate]] was supplied by the National Institute on Drug Abuse (Washington, DC). JDTic [(3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide], synthesized as previously described (Thomas et al., 2003), was a generous gift from the Research Triangle Institute (Research Triangle Park, NC). The AA was diluted with sterile water and ketoprofen working solutions were diluted with physiological saline (0.9% sodium chloride). All other drugs were dissolved in physiological saline and injected at a total volume of 1ml/100 g body weight unless noted otherwise. All doses are expressed as the free base of the drug. All test drugs were injected subcutaneously (s.c.), AA and LiCl were injected i.p.. All experiments were performed blindly to the drugs. Test drugs were prepared by another researcher than the examiner.
2.3. Acetic acid-induced stretching
Each mouse was placed in a Plexiglas box (29 × 19 × 13 cm each) and allowed to acclimate for 20 min. Then, mice were treated as described below, and the number of stretches was counted in 10-min bins for 60 min. A stretch was operationally defined as a contraction of the abdomen followed by an extension of the hind limbs.
To evaluate AA potency, mice were treated i.p. with either sterile water as vehicle of AA or AA (0.32–1.0%) immediately prior to the 60-min observation period. To evaluate AA time course, mice were also treated i.p. with 1% AA 30 or 60 min prior to the 60-min observation period. To evaluate test drug effects, mice were pretreated with s.c. saline as vehicle of test drugs, the NSAID ketoprofen (1, 2, 4 mg/kg; 15 min pretreatment), the µ-opioid receptor agonist morphine (0.1, 0.32, 1.0, 3.2 mg/kg; 15 min pretreatment), or the ĸ-opioid receptor (KOR) agonist U-50488H (0.1, 0.32, 1.0, 3.2, 10 mg/kg; 10 min pretreatment) prior to i.p. injection of 1% AA, and observation began immediately after AA injection. Each mouse was used for only one experiment.
2.4. Acetic acid (AA) conditioned place aversion (CPA) studies
CPA was evaluated using an unbiased design as previously described (Papke et al., 2015). In brief, separate groups of mice were handled for three days prior to initiation of conditioning. The CPA apparatus (Med-Associates, St. Albans, VT, ENV3013) consisted of white and black chambers (20 × 20 × 20 cm each), which differed in floor texture (white mesh and black rod). The compartments were separated by a smaller grey chamber with a smooth PVC floor and partitions that allowed access to the black and white compartments. The black and white compartments also had different floor textures, and removable doors separated the center grey compartment from the two white and black side compartments. Experiments were conducted using a 3-day protocol. On day 1, mice were placed in the grey center compartment for a 5 min habituation period followed by a 15 min test period to determine baseline time spent in each compartment by removing the doors. A pre-preference score was recorded, and mice within each group were then randomly assigned such that an even number of mice received the experimental treatment on the black and white side. On day 2, the doors were in place to separate the compartments, and mice were exposed to two 40-min conditioning sessions no less than 4 hr apart. Prior to one conditioning session, mice received one of the treatments described below and were placed into either the black or white compartment as dictated by their assignment on day 1. Prior to the other conditioning session, mice received vehicle injections and were placed into the other compartment. On day 3, the doors were again removed after habituation, and the day 1 procedure was repeated.
To evaluate AA potency, mice were treated i.p. with either sterile water or AA (0.32–1.0%) immediately prior to treatment conditioning sessions. To evaluate AA time course, mice were also treated i.p. with 1% AA 30, 60 or 180 min prior to treatment conditioning sessions. To evaluate test drug effects, mice were pretreated with s.c. saline as vehicle of test drugs, ketoprofen (1, 2, 4 mg/kg; 15 min pretreatment), morphine (0.1, 0.32, 1, 3.2 mg/kg; 15 min pretreatment), or U-50488 (0.1, 0.32, 1, 3.2, 10 mg/kg; 10 min pretreatment) prior to i.p. administration of 1% AA or sterile water as vehicle of AA, and treatment conditioning sessions began immediately after AA/vehicle injection. In a separate experiment, treatment conditioning sessions were preceded first by injection of the kappa antagonist JDTic (10 mg/kg) followed after 2 hours by U-50488 (10 mg/kg) and 10 min later by 1% AA.
Test drugs were administered before acid, because the sequence of treatments is consistent with the experimental design commonly used to assess effectiveness of test drugs to block development of place conditioning. This experimental design permits evaluation of test drug effectiveness to block development of place conditioning.
Data were expressed as time in seconds spent in the treatment-paired compartment post-conditioning minus time spent in that compartment pre-conditioning. A positive number indicated a treatment-induced place preference, whereas a negative number indicated a treatment-induced place aversion.
2.5. Lithium chloride (LiCl) conditioned place aversion (CPA) studies
LiCl-CPA was evaluated using the same design described above with the exception that conditioning occurred during twice-daily sessions over three days (six total conditioning sessions), and treatment conditioning sessions were preceded by the treatments described below. Regarding the structure of sessions across days, baseline time spent in each compartment was determined on day 1, two 20-min conditioning sessions (one saline conditioning session and one treatment conditioning session) were presented each day on days 2–4, and results of conditioning were evaluated during a test session on day 5. Regarding treatments before treatment conditioning sessions, mice received s.c. saline as vehicle of test drugs, ketoprofen (4 mg/kg) or morphine (0.32 mg/kg) 15 min prior to i.p. injection of 150 mg/kg LiCl or vehicle.
2.6. Statistical analysis
Statistical analysis of all behavioral studies was performed using one-way analysis of variance (ANOVA) with Tukey post hoc test when appropriate. The criterion for significance was p < 0.05. ED50 values with 95% CL for behavioral data were calculated by unweighted least-squares linear regression as described by Tallarida and Murray (Tallarida and Murray, 1987).
3. Results
3.1. Acetic acid-induced stimulation of stretching and conditioned place aversion
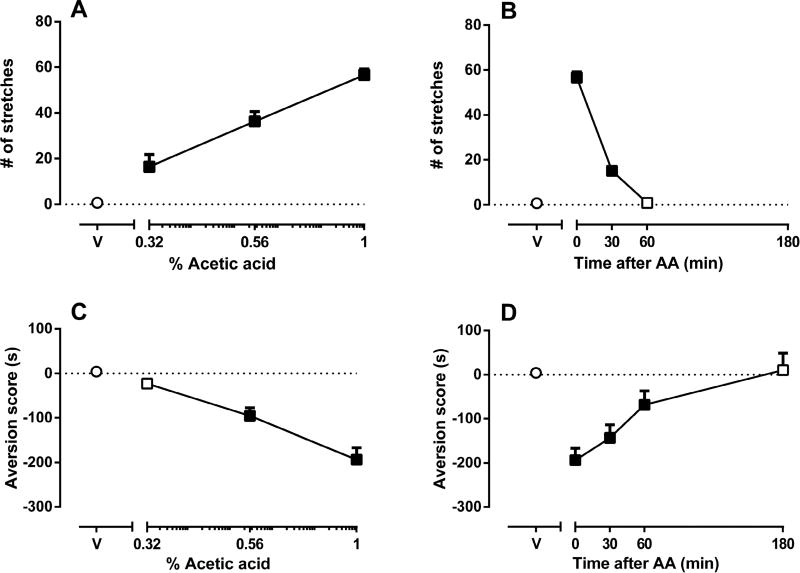
AA produced a concentration-dependent increase in stretching. Sterile water (acid vehicle) did not elicit stretching behavior. One-way ANOVA indicated that the number of stretches following 0.32, 0.56 and 1% AA was significantly greater than the number of stretches following i.p. water [F(3,20)=44.04; p<0.001] (Fig 1A) with an ED50 value of 0.54 (0.46–0.62) mg/kg. AA also produced concentration-dependent CPA [F(3,26)=21.5; p<0.001] (Fig 1C) with an ED50 value of 0.64 (0.54–0.76) mg/kg. Aversion scores following 0.56 and 1% AA were significantly increased relative to i.p. water. The time spent in the pretest day was: vehicle-paired side = 341 ± 16 sec and drug (AA 1%)-paired side = 343 ± 15 sec. The time spent in the post-conditioning day was: vehicle-paired side = 508 ± 77 sec and drug (AA 1%)-paired side = 199 ± 33 sec. These results show that the mice did not show aversion to the vehicle-paired chamber.
Figure 1. Effects of acetic acid (AA) concentration and treatment interval on acid-stimulated stretching and acid-induced conditioned place aversion (CPA) in mice.
Figure 1A shows the concentration-effect curve for intraperitoneal acetic acid (AA)-stimulated stretching, with AA concentration (log scale) on the x-axis and the number of stretches on the y-axis. Figure 1B shows the time course of 1% AA-stimulated stretching, with treatment time in min between AA injection and initiation of the observation period on the x-axis and the number of stretches on the y-axis. Figure 1C shows the concentration-effect curve for intraperitoneal AA-induced CPA with AA concentration (log scale) on the x-axis and the aversion score (in sec) on the y-axis. Figure 1D shows the time course of AA-induced CPA, with the treatment time in min between AA injection and initiation of the treatment conditioning session on the x-axis and the aversion score (in sec) on the y-axis. Data points above “V” represent effects of sterile water (vehicle of AA) control treatment on stretching (A,B) or aversion score (C,D), and filled symbols indicate significantly different from water as determined by one-way ANOVA followed by Tukey’s post hoc test (p<0.05). Data are expressed as means ± S.E.M. from 6–10 mice.
Because 1% AA produced robust stretching and place aversion, we used this concentration to determine the time course of AA effects on stretching behavior and place aversion. Figure 1B shows number of stretches as a function of treatment interval. Treatment with 1% AA at 0 and 30 min produced significant stretching relative to control (i.p. water) [F(3,20)=358.1; p<0.001] (Fig 1B). However, the effects of acid on stretching behavior dissipated after 60 min. Figure 1D shows CPA scores as a function of treatment interval. Treatment with 1% AA at 0, 30 and 60 min produced significant increases in aversion scores relative to control [F(4,35)=8.469; p<0.001] (Fig 1D). The effects of AA on CPA were no longer apparent after 180 min. On the basis of these results, subsequent experiments were conducted using a 0 min treatment with 1% AA.
3.2. The effects of ketoprofen, morphine and U50,488H on AA-induced stretching
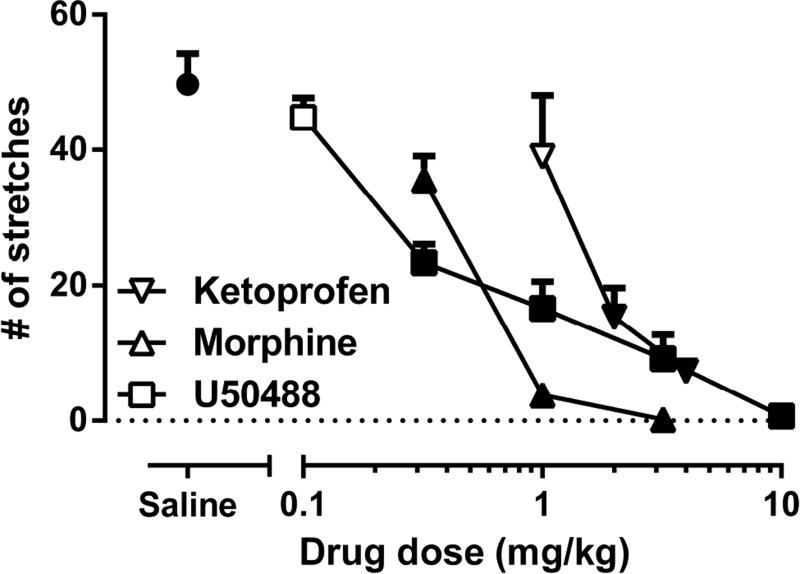
Fig. 2 shows that AA-stimulated stretching was dose-dependently blocked by ketoprofen (1–4 mg/kg; F(3,20)=64.94; p<0.001), morphine (0.32–3.2 mg/kg; F(3,20)=64.94; p<0.001), and U50,488H (0.1–10 mg/kg; F(5,30)=58.01; p<0.001). The potency of these drugs in blocking AA-induced stretching as expressed in ED50 values are shown in Table 1. Neither saline nor the test drugs stimulated stretching in the absence of acid treatment (data not shown).
Figure 2. The effects of ketoprofen, morphine and U50,488H on AA-induced stretching.
Subcutaneous pretreatment of saline, ketoprofen (1, 2, 4 mg/kg), morphine (0.32, 1 3.2 mg/kg), and U50,488H (0.1, 0.32, 1, 3.2, 10 mg/kg) on intraperitoneal 1% acetic acid (AA)-induced stretching. Data point “saline” represents number of stretches following acid administration after saline pretreatment. Data are expressed as means ± S.E.M. from 6–8 mice. Filled symbols indicate significantly different from saline pretreatment as determined by one-way ANOVA followed by Tukey’s post hoc test (p<0.05).
Table 1.
Potency of ketoprofen, morphine, and U50,488H in blocking 1% acetic acid (AA)-induced stretching and conditioned place aversion (CPA). ED50 values (±CL) were calculated from the dose-response curve of the respective compounds and expressed as mg/kg.
| Test Drug | AA-induced stretching | AA-induced CPA |
|---|---|---|
| Ketoprofen (mg/kg) | 1.63 (1.23–2.17) | 1.17 (0.69–1.99) |
| Morphine (mg/kg) | 0.45 (0.22–0.49) | 0.13 (0.06–0.29) |
| U50,488H (mg/kg) | 0.25 (0.19–0.32) | inactive |
3.3. The effects of ketoprofen, morphine and U50,488H on AA-induced CPA
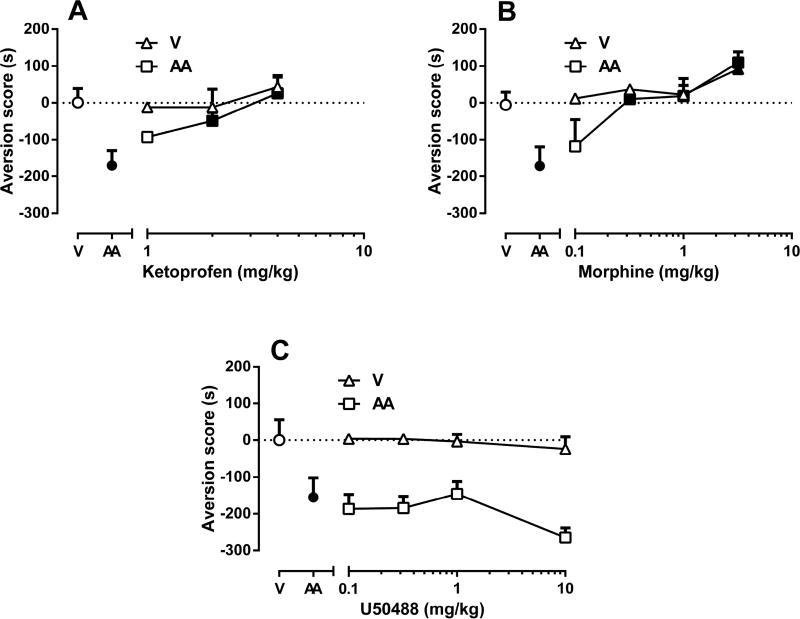
Fig. 3 shows the effects of ketoprofen, morphine and U50,488H pretreatment on AA-induced CPA (conditioned immediately after i.p. injection of 1% AA, square symbols) and on control place preference (conditioned immediately after i.p. sterile water treatment, triangle symbols). Ketoprofen pretreatment reversed the CPA effect of AA in a dose-dependent manner [F(3,27)=5.692; p<0.01] (Fig 3A) but did not alter place preference or aversion in control mice [F(3,25)=0.8359; p=0.4869]. Thus, ketoprofen selectively attenuated the AA-induced CPA.
Figure 3. The effects of ketoprofen, morphine and U50,488H on AA-induced CPA.
Subcutaneous pretreatment of (A) ketoprofen (1, 2, 4 mg/kg), (B) morphine (0.32, 1 3.2 mg/kg), and (C) U50,488H (0.1, 0.32, 1, 3.2, 10 mg/kg) on intraperitoneal 1% acetic acid (AA)-induced conditioned place aversion (CPA). For each panel, the abscissa shows drug dose in mg/kg (log scale, V=vehicle), and the ordinate shows preference score expressed as the time spent in drug-paired side of the box. Vehicle was saline for test drugs and sterile water for AA. “AA” represents time spent acid-induced aversion score (in sec) on test day. Data are expressed as means ± S.E.M. from 6–10 mice. Filled circles points indicate that the AA treatment is significantly different than the V treatment. Filled triangles points indicate significant difference from the V treatment and filled squares points indicate significant difference from the AA treatment by one-way ANOVA followed by Tukey’s post hoc test (p<0.05).
Fig. 3B shows that morphine produced a dose-dependent reduction of AA-induced aversion [F(4,34)=6.734; p<0.001]. Indeed, morphine pretreatment (0.32–1 mg/kg) significantly enhanced time spent in drug-paired compartments for AA-treated mice. It is important to note that at these doses, morphine did not induce place preference or aversion in control mice. However, the highest dose of morphine (3.2 mg/kg) produced significant place preference in water-treated mice [F(3,25)=4.195; p<0.05].
Fig. 3C shows total time spent within U50,488H-paired side of boxes as a function of U50,488H pretreatment dose (0.1, 0.32, 1 and 10 mg/kg). In contrast to the effects of U50,488H in AA-induced stretching, U50,488H failed to significantly reduce AA-induced CPA [F(3,25)=1.667; p=0.1996] (Fig 3C). U50,488H did not induce place preference or aversion [F(2,20)=0.2651; p=0.7698] in control mice. Although there was also a trend for U50,488H at the highest dose of 10 mg/kg to increase AA-induced CPA, this effect did not achieve statistical significance (p= 0.71).
The potency of ketoprofen, morphine and U50,488H in blocking AA-induced CPA as expressed in ED50 values are shown in Table 1. While ketoprofen and morphine potencies did not statistically differ (See ± CL values in Table 1) between the two AA measures, U50,488H completely blocked AA-induced stretching but failed to block AA-induced CPA.
3.4. The effects of JDTic on AA-induced stretching and CPA
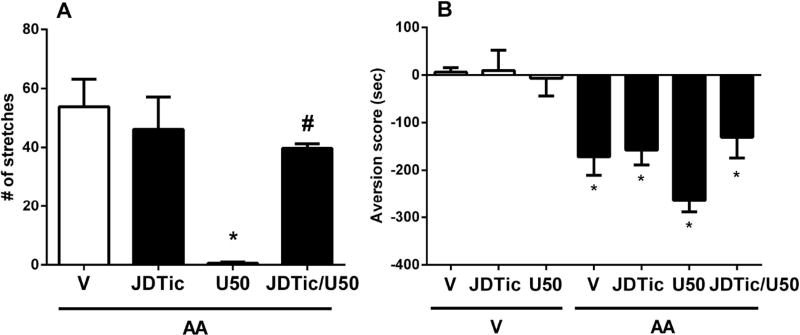
Fig. 4 shows the effects of the selective kappa receptor antagonist JDTic (10 mg/kg) on AA-induced stretching and CPA. One-way ANOVA indicated main effects of JDTic dose and the associated co-treatment (saline, U50,488H) [F(3,19)=9.017; p<0.001, Fig 4A and F(6,44)=11.26; p<0.001, Fig 4B]. JDTic failed to reduce AA-induced number of stretches (p>0.05, Fig 4A) or place aversion (p>0.05, Fig 4B). However, pretreatment of JDTic blocked the antinociceptive effect of U50,488H (10 mg/kg) in AA-induced stretching (p<0.001; Fig 4A). There was also a trend of U50,488H enhancing AA-induced CPA, which JDTic attenuated.
Figure 4. The effects of JDTic on AA-induced stretching and CPA.
The effects of subcutaneous JDTic (10 mg/kg) pretreatment to vehicle, acetic acid (AA, 1%, intraperitoneal) and U,50488H (10 mg/kg) administrations on (A) AA-induced stretching and (B) conditioned place aversion (CPA). Vehicle was saline for test drugs and sterile water for AA. Data are expressed as means ± S.E.M. from 5–9 mice. * indicates a significant effect compared to correspondent vehicle by one-way ANOVA followed by Tukey’s post hoc test (*p<0.05). #indicates a significant effect compared to U50,488H by one-way ANOVA followed by Tukey’s post hoc test (#p<0.05).
3.5. The effect of antinociceptive test drugs on non-noxious LiCl-induced CPA
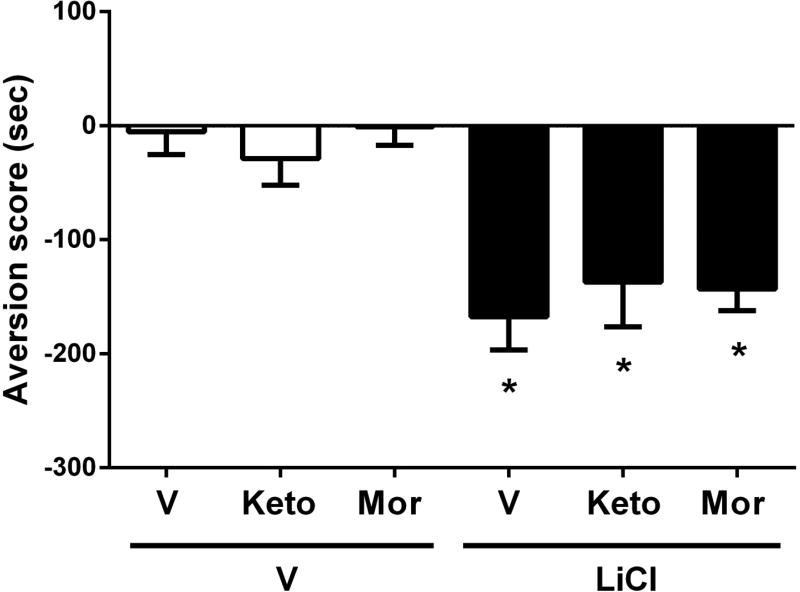
To determine if the effects of analgesic compounds on AA-CPA is specific for pain-induced CPA or not, we tested the effects of these drugs on CPA response which produced by i.p. injection of LiCl (150 mg/kg). LiCl (i.p. 150 mg/kg) induces place aversion due to nausea, without visceral nociception (Lett, 1985). While LiCl did not induce stretching (number of stretches after LiCl at 150 mg/kg = 0 ± 0), it did produce a significant aversive effect after 3 day conditioning. The overall analysis of time spent within drug-paired side of boxes revealed a significant aversive effect for LiCl treatment [F(5,46)=8.987; p<0.001] (Fig 5). As shown in Figure 5, active doses of ketoprofen (4 mg/kg) and morphine (0.32 mg/kg) in the AA-CPA test were not able to reduce the LiCl-induced CPA.
Figure 5. The effect of ketoprofen, morphine on non-noxious LiCl-induced CPA.
Subcutaneous pretreatment of ketoprofen (4 mg/kg) and morphine (0.32 mg/kg) on intraperitoneal lithium chloride (LiCl, 150 mg/kg)-induced conditioned place aversion (CPA). Vehicle was saline for test drugs and LiCl. Data are expressed as means ± S.E.M. from 6–10 mice. * indicates a significant effect compared to correspondent vehicle by one-way ANOVA followed by Tukey’s post hoc test (*p<0.05).
4. Discussion
Since pain is an unpleasant sensory and emotional experience, it is important to evaluate the negative affective component of pain as well as measuring the nociceptive behaviors. The purpose of the present paper was to develop and characterize a mouse model evaluating drug effects on an aversive behavior induced by a noxious stimulus. Our main findings were that i.p. injection of AA induced in the mouse an aversive behavior as measured in the CPA test in a dose- and time-dependent manner. In addition, the opioid analgesic morphine and the NSAID ketoprofen blocked both AA-induced CPA and AA-induced stretching. Conversely, the kappa agonist U50,488H blocked stretching but failed to block AA-induced CPA, and the kappa antagonist JDTic failed to block either AA-induced CPA or stretching. Furthermore, ketoprofen and morphine failed to reduce non-noxious LiCl-induced CPA, suggesting that effects of these analgesic drugs on CPA were specific to pain-related place aversion. Our results suggest that complementary use of procedures that measure sensory and CPA behaviors may enhance predictive validity in translational research with candidate analgesics.
Animal studies of pain are largely performed by measuring reflexive responses to noxious stimuli. It has been discussed that although reflexive responses are important to investigate the sensory aspect of pain, the affective pain aspect is equally important though difficult to assess due to the lack of validated animal models (Li, 2013). Recent studies showed that the aversive aspects of pain can be assessed using a rat model of the CPA procedure. Specifically, CPA could be elicited in rats by i.p. administration of AA or intraplantar administration of complete Freund’s adjuvant, formalin, or carrageenan (Deyama et al., 2007; Johansen et al., 2001; LaBuda and Fuchs, 2000; Tanimoto et al., 2003; van der Kam et al., 2008; Wang et al., 2009). In this study, we used a CPA procedure to investigate the affective component of pain in mice. In agreement with previous studies, i.p. AA served as a visceral noxious stimulus that induced a significant CPA that was dose- and time-dependent. AA also produced dose- and time-dependent stimulation of stretching in mice. The potencies of AA to elicit CPA and stretching were similar. However, the time-course was shorter for AA-induced stretching than for AA-induced CPA (the stretching behavior lasted for 60 min while the aversive response lasted for 180 min). This finding agrees with a previous report that AA was equipotent but longer lasting to depress both locomotor activity and liquid food consumption than to stimulate stretching in mice (Stevenson et al., 2009, 2006). While many factors could account for this discrepancy, one possible explanation is that the two measures are mediated by different pathways. While peripheral and visceral mechanisms mediate the stretching behavior, the central nuclei of amygdala and the anterior cingulate cortex were reported to be key neuronal substrates underlying AA- and formalin-induced CPA in rats (Johansen et al., 2001; Tanimoto et al., 2003; Zhang et al., 2013). Another more remote possibility is that the post-60 min aversion could represent a time window during which the previous pain (gone at 60 min) is still associated to a visual and/or tactile cue in the CPA test.
Following demonstration of AA-induced CPA, we assessed the degree to which various drugs could selectively reverse acid-induced aversion. The NSAID ketoprofen and the opioid morphine were chosen because these two drug classes are widely used to treat moderate to severe pain in humans and have efficacy in a broad range of rodent models of pain. The NSAID ketoprofen and µ-opiod agonist morphine dose dependently reversed the aversion and sensory components of AA-induced visceral pain. Since drugs are applied before AA during the conditioning phase, the decrease in aversion may result from the preventive effects of these drugs on CPA. Examination of the dose-response curves in the two tests suggests that the two drugs seem to have similar potency in blocking AA-induced CPA and stretching. While the low dose of morphine (0.32 mg/kg) was able to totally block AA-CPA, it did not cause a significant conditioned place preference (CPP) in the mouse on its own. However, and not surprisingly for a rewarding drug, the highest dose of morphine (3.2 mg/kg) did. These findings are consistent with a previous report that found morphine to dose-dependently reduce carrageenan-induced CPA with a minimal effective dose of 0.03 mg/kg in the rat (van der Kam et al., 2008). In a recent study on the pain-related depression of nesting, ketoprofen and morphine blocked the pain-related depression of nesting which demonstrates pain-related depression is analgesic-reversible (Negus et al., 2015). Similarly, they reversed the formalin-induced depression of intracranial self-stimulation (ICSS) that shows morphine is effective on negative affective signs of pain (Leitl et al., 2014). In addition, they decreased inflammation-induced decrease in voluntary wheel running in mice (Cobos et al., 2012).
Although ketoprofen and morphine were effective to attenuate pain-related CPA in our study, the centrally acting kappa agonist U50,488H failed to attenuate AA-induced CPA at doses that did block AA-induced stretching. Similarly, the other centrally acting kappa agonists U69,593 and salvinorin A failed to block lactic acid-induced depression of ICSS in rats at doses that blocked acid-induced stretching (Negus et al., 2012, 2010) and U69593 also failed to block lactic acid-induced depression of nesting in mice (Negus et al., 2015). It has been reported that brain-penetrating κ-agonists caused side effects such as sedation and dysphoria (Rivière & Junien, 2000). Insofar as centrally acting kappa agonists have also failed to win approval for clinical treatment of pain in humans (Rivière & Junien, 2000), these results suggest that these assays of acid-induced CPA or depression of nesting in mice, or depression of ICSS in rats, may be useful to improve translation of preclinical to clinical evaluation of candidate analgesic drugs.
Since pain may activate endogenous kappa signaling and that dynorphin and the kappa system have been suggested to make a contribution to the negative affective component of pain (Cahill et al., 2014), we therefore used JDTic as an antagonist for this receptor to investigate possible beneficial effects on acid-induced CPA in the mouse. However, JDTic did not block either acid-induced stretching or CPA at doses that did block behavioral effects of U50,488H. In agreement with our results, kappa antagonists (norbinaltorphimine and JDTic) were also ineffective in reducing acid-induced depression of ICSS in rats or nesting in mice (Leitl et al., 2014, 2013; Negus et al., 2015). These results do not support a role for activation of endogenous kappa opioid systems in these examples of these pain-related aversive behaviors.
Because CPA produced by LiCl is characterized as a sickness-associated aversion and a suitable model to assess antiemetic properties of drugs which differentiates from pain-like effects (Frisch et al., 1995; Lett, 1985; Rinaman et al., 2009), we used LiCl-induced CPA as a control. Finally, while ketoprofen and morphine were able to attenuate AA-induced CPA, they failed to reduce CPA induced by the non-noxious aversive stimulus LiCl. This finding provides further support for the selectivity of CPA in rodents for assessing an aversive component of pain. Overall, this study characterized and validated a preclinical mouse model of pain-related aversive behavior that can be used to assess genetic and biological mechanisms of pain as well as improving the predictive validity of preclinical studies on candidate analgesics.
Highlights.
Acetic acid (AA) induced stretching and conditioned place aversion (CPA)
AA-stretching was blocked by ketoprofen, morphine and U50,488H but not by JDTic
AA-CPA was blocked by ketoprofen, morphine but not by U50,488H and JDTic
Neither ketoprofen nor morphine blocked LiCl-induced aversion
Acknowledgments
This work was supported by NIH grants R01DA-019377 (MID), R01NS070715 (SSN), and R01DA030404 (SSN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Apkarian A Vania, Sosa Yamaya, Krauss Beth R, Thomas P Sebastian, Fredrickson BE, Levy Robert E, Harden R. Norman, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–9. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Browne LE, Woolf CJ. Casting light on pain. Nat. Biotechnol. 2014;32:240–241. doi: 10.1038/nbt.2844. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Taylor AMW, Cook C, Ong E, Morãn Ja, Evans CJ. Does the kappa opioid receptor system contribute to pain aversion? Front. Pharmacol. 2014;5:1–15. doi: 10.3389/fphar.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou I, Tuttle aH, Longo G, Wieskopf JS, Bonin RP, Ase aR, Wood JN, De Koninck Y, Ribeiro-da-Silva a, Mogil JS, Seguela P. Remote Optogenetic Activation and Sensitization of Pain Pathways in Freely Moving Mice. J. Neurosci. 2013;33:18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Ohno A, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Activation of the beta-adrenoceptor-protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J. Neurosci. 2008;28:7728–7736. doi: 10.1523/JNEUROSCI.1480-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Nakagawa T, Kaneko S, Uehara T, Minami M. Involvement of the bed nucleus of the stria terminalis in the negative affective component of visceral and somatic pain in rats. Behav. Brain Res. 2007;176:367–371. doi: 10.1016/j.bbr.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Deyama S, Takishita A, Tanimoto S, Ide S, Nakagawa T, Satoh M, Minami M. Roles of β- and α2-adrenoceptors within the central nucleus of the amygdala in the visceral pain-induced aversion in rats. J. Pharmacol. Sci. 2010;114:123–126. doi: 10.1254/jphs.10139SC. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon Wa, Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur. J. Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch C, Hasenöhrl RU, Mattern CM, Häcker R, Huston JP. Blockade of lithium chloride-induced conditioned place aversion as a test for antiemetic agents: comparison of metoclopramide with combined extracts of Zingiber officinale and Ginkgo biloba. Pharmacol. Biochem. Behav. 1995;52:321–327. doi: 10.1016/0091-3057(95)00073-6. [DOI] [PubMed] [Google Scholar]
- Horan P, de Costa BR, Rice KC, Porreca F. Differential antagonism of U69,593-and bremazocine-induced antinociception by (-)-UPHIT: evidence of kappa opioid receptor multiplicity in mice. J. Pharmacol. Exp. Ther. 1991;257:1154–1161. [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons Ta, Whiteside GT. The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain. 2008;140:436–445. doi: 10.1016/j.pain.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010;30:5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SK, Honore P. Animal models of pain for drug discovery. Expert Opin. Drug Discov. 2006;1:323–34. doi: 10.1517/17460441.1.4.323. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp. Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon Wa, Banks ML, Negus SS. Pain-Related Depression of the Mesolimbic Dopamine System in Rats: Expression, Blockade by Analgesics, and Role of Endogenous κ-opioids. Neuropsychopharmacology. 2013;39:614–624. doi: 10.1038/npp.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon Wa, Negus S. Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol. Pain. 2014;10:62. doi: 10.1186/1744-8069-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT. The painlike effect of gallamine and naloxone differs from sickness induced by lithium chloride. Behav. Neurosci. 1985;99:145–150. doi: 10.1037/0735-7044.99.1.145. [DOI] [PubMed] [Google Scholar]
- Li JX. The application of conditioning paradigms in the measurement of pain. Eur. J. Pharmacol. 2013;716:158–168. doi: 10.1016/j.ejphar.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101:191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;209:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC. Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J. Pharmacol. Exp. Ther. 2012;340:501–9. doi: 10.1124/jpet.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J. Pharmacol. Exp. Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res. Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin. Neuropharmacol. 1996a;19:92–7. doi: 10.1097/00002826-199619010-00009. [DOI] [PubMed] [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clinical neuropharmacology. 1996b doi: 10.1097/00002826-199619050-00009. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bagdas D, Kulkarni AR, Gould T, AlSharari SD, Thakur Ga, Damaj MI. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology. 2015;91:34–42. doi: 10.1016/j.neuropharm.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Saboury M, Litvina E. Ondansetron blocks LiCl-induced conditioned place avoidance but not conditioned taste/flavor avoidance in rats. Physiol. Behav. 2009;98:381–385. doi: 10.1016/j.physbeh.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière PJM, Junien JL. Opioid receptors, targets for new gastrointestinal drug development Drug Development. In: Gaginella TS, Guglietta A, editors. Molecular Targets for GI Diseases. Totowa, NJ: Humana Press; 2000. pp. 203–238. [Google Scholar]
- Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat. Cell Biol. 2007;9:993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. Targeting Pain-Suppressed Behaviors in Preclinical Assays of Pain and Analgesia: Effects of Morphine on Acetic Acid-Suppressed Feeding in C57BL/6J Mice. J. Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: Drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci. 2009;85:309–315. doi: 10.1016/j.lfs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations. Springer-Verlag; New York: 1987. Springer-Verlag, New York. [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur. J. Neurosci. 2003;18:2343–2350. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Atkinson RN, Vinson NA, Catanzaro JL, Perretta CL, Fix SE, Mascarella SW, Rothman RB, Xu H, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. Identification of (3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1,2,3,4-tetrahydro- 3-isoquinolinecarboxamide as a novel potent and selective opioid kappa receptor antagonist. J. Med. Chem. 2003;46:3127–3137. doi: 10.1021/jm030094y. [DOI] [PubMed] [Google Scholar]
- Van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136:373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Wang HC, Wang YC, Huang ACW, Chai SC, Wu YS, Wang CC. Roles of corticosterone in formalin-induced conditioned place aversion in rats. Neurosci. Lett. 2009;464:122–126. doi: 10.1016/j.neulet.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Pomonis JD, Kennedy JD. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci. Lett. 2013;557:65–72. doi: 10.1016/j.neulet.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Zhang M, Li a, Pan L, Berman BM, Ren K, Lao L. DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience. 2013;252:359–366. doi: 10.1016/j.neuroscience.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]