Abstract
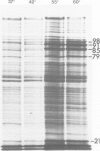
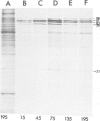
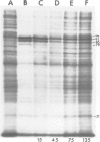
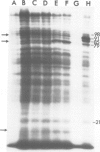
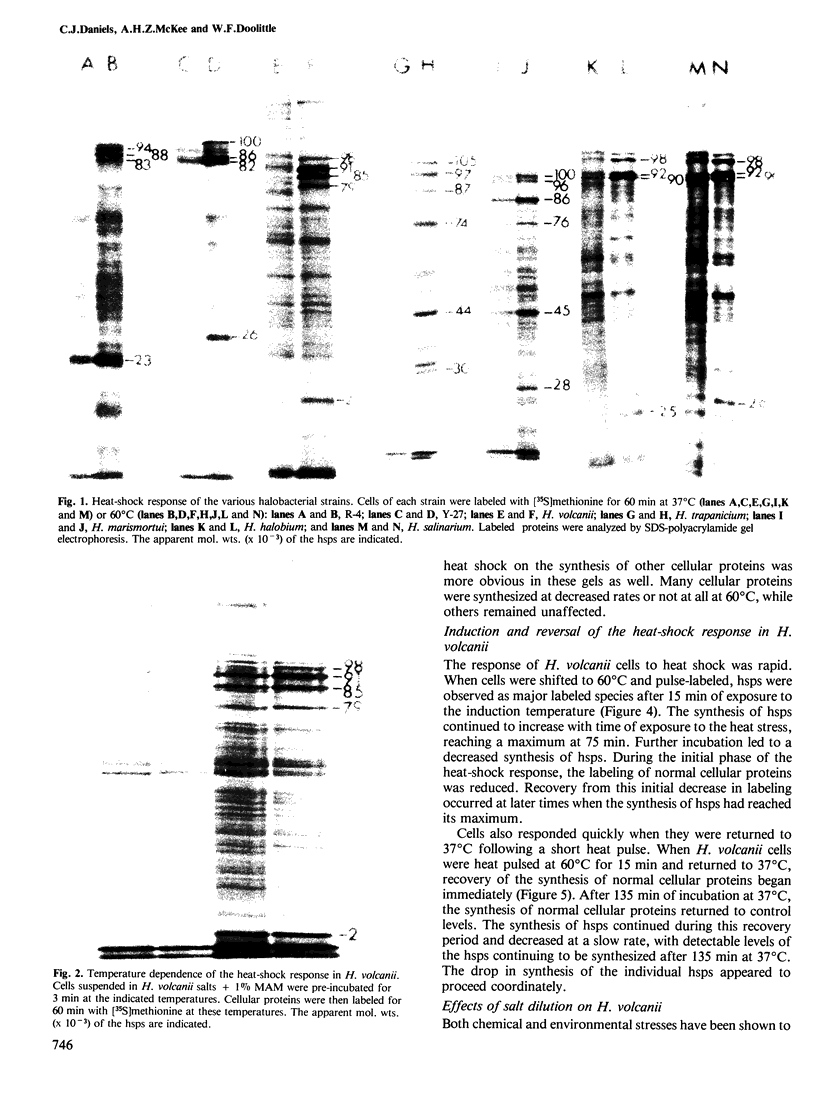
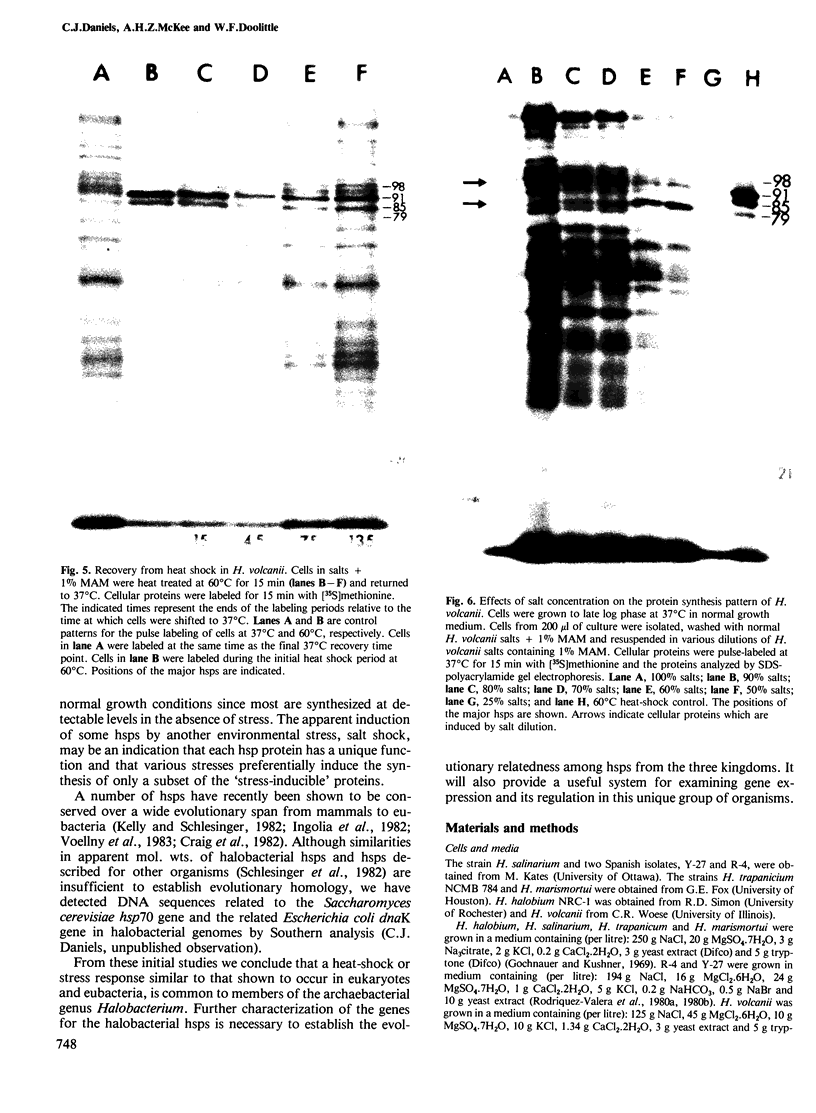
The response to heat shock was examined in seven archaebacterial strains from the genus Halobacterium. Upon heat shock each strain preferentially synthesized a limited number of proteins which fell into three narrow mol. wt. ranges. Further examination of the heat-shock response in H. volcanii revealed that heat-shock protein (hsp) synthesis was greatest at 60°C. Synthesis of hsps at this induction temperature was both rapid and transient. Cells recovered their normal protein synthesis patterns rapidly upon returning to their normal growth temperature following heat shock. H. volcanii cells also responded with a `heat shock-like' response to salt dilution, a natural environmental stress for these organisms. These results indicate that the heat shock or stress response which is charactertistic of eukaryotic and eubacterial cells is also present among members of the archaebacterial genus Halobacterium.
Keywords: heat shock, archaebacteria, stress response, evolution
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Gochnauer M. B., Kushner D. J. Growth and nutrition of extremely halophilic bacteria. Can J Microbiol. 1969 Oct;15(10):1157–1165. doi: 10.1139/m69-211. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanks K. W. Metabolite regulation of heat shock protein levels. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5325–5329. doi: 10.1073/pnas.80.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valera F., Ruiz-Berraquero F., Ramos-Cormenzana A. Behaviour of mixed populations of halophilic bacteria in continuous cultures. Can J Microbiol. 1980 Nov;26(11):1259–1263. doi: 10.1139/m80-210. [DOI] [PubMed] [Google Scholar]
- Tanguay R. M. Genetic regulation during heat shock and function of heat-shock proteins: a review. Can J Biochem Cell Biol. 1983 Jun;61(6):387–394. doi: 10.1139/o83-053. [DOI] [PubMed] [Google Scholar]
- Voellmy R., Bromley P., Kocher H. P. Structural similarities between corresponding heat-shock proteins from different eucaryotic cells. J Biol Chem. 1983 Mar 25;258(6):3516–3522. [PubMed] [Google Scholar]