Abstract
Background
Fractional exhaled nitric oxide (FENO) is used to assess of airway inflammation; diagnose asthma and monitor adherence to advised therapy. Reliable and accurate reference values for FENO are needed for both non-smoking and current smoking adults in the clinical setting. The present study was performed to establish reference adult FENO values among never-smokers, former smokers and current smokers.
Methods
FENO was measured in 5265 subjects aged 25–75 years in a general-population study, using a chemiluminescence (Niox ™) analyser according to the guidelines of the American Thoracic Society and the European Respiratory Society. Atopy was based on the presence of immunoglobulin E (IgE) antibodies to common inhalant allergens (measured using Phadiatop® test). Spirometry without bronchodilation was performed and forced vital capacity (FVC), forced expired volume in 1 s (FEV1) and the ratio of FEV1 to FVC values were obtained. After excluding subjects with asthma, chronic bronchitis, spirometric airway obstruction and current cold, 3378 subjects remained. Equations for predictions of FENO values were modelled using nonparametric regression models.
Results
FENO levels were similar in never-smokers and former smokers, and these two groups were therefore merged into a group termed “non-smokers”. Reference equations, including the 5th and 95th percentiles, were generated for female and male non-smokers, based on age, height and atopy. Regression models for current smokers were unstable. Hence, the proposed reference values for current smokers are based on the univariate distribution of FENO and fixed cut-off limits.
Conclusions
Reference values for FENO among respiratory healthy non-smokers should be outlined stratified for gender using individual reference values. For current smokers separate cut-off limits are proposed.
Electronic supplementary material
The online version of this article (doi:10.1186/s12890-017-0456-9) contains supplementary material, which is available to authorized users.
Keywords: Asthma, Atopy, FENO, General population, Nitric oxide, Normal values, Epidemiology
Background
In 2005, the European Respiratory Society (ERS) and the American Thoracic Society (ATS) jointly published recommendations for measuring the fractional excretion of exhaled nitric oxide (FENO). These recommendations were followed in 2011 by an updated ATS document on the clinical use of FENO [1, 2]. The 2011 guidelines suggested that an FENO level of >50 ppb indicated a high probability of eosinophilic airways inflammation, and that an FENO level 25–50 ppb should be evaluated further [2]. Subsequent publications have favoured the use of fixed cut-off limits to diagnose asthma, especially Th-2 driven airway inflammation, the most common motive for measuring FENO [3–5]. To date, however, no consensus has been reached regarding the reference values for FENO, although there is a tendency to support the use of fixed cut off limits [1, 6].
Moreover, there is a lack of knowledge regrading reference values in current smokers. A study published in 1995 reported that smokers exhaled lower concentrations of nitric oxide (NO) than non-smokers [7]. This finding has since been confirmed in other studies [8–10]. The usefulness of FENO values in assessing asthma and Th-2 driven airway inflammation in smokers is still unclear, although some studies have shown that smokers with asthma had higher FENO levels than healthy subjects who smoked [11]. Hence, there is a need to determine reference values for FENO among current smokers.
The primary aim of the present study was to establish reference values for FENO at an exhalation flow rate of 50 mL/s based on a large random population comprising never-smokers, former smokers and current smokers. The second aim of the study was to determine whether age, sex, height and atopy should to be considered when proposing reference values. The analysis was based on an expanded population sample of 5265 subjects; one segment of the cohort, 1803 never-smokers, was included in our previous paper [12].
Methods
The data for this study were extracted from the previously described ADONIX (Adult-Onset Asthma and Exhaled Nitric Oxide) random general-population study in Sweden, which included individuals aged 25–75 years [8, 12]. The clinical measurements were performed between 2002 and 2007. We included subjects with complete anthropometric data, smoking data, spirometry data and FENO levels measured at an exhalation flow rate of 50 mL/s (n = 5854). The FENO measurements were performed before spirometry. After excluding subjects whose FENO measurements did not meet the quality criteria (see below) 5265 subjects remained in the cohort.
FENO was measured with a chemiluminescence analyzer (NIOX, Aerocrine AB, Stockholm, Sweden) and the analyser was calibrated every other week with a certified calibration gas. All procedures were performed in accordance with the recent ATS recommendations and have been described previously in detail [13, 14]. Briefly, the subjects exhaled against a mouth pressure of 5 cm H2O at 50 mL/s for 10 s. NO was measured between the 6th and 10th second. Subjects were excluded if the exhaled flow was >55 mL/s or <45 mL/s. Blood samples were analysed for the presence of immunoglobulin E (IgE) antibodies to common inhalant allergens (Phadiatop, Pharmacia, Uppsala, Sweden) and the results were classified as negative (class 0) or positive (class 1) [15].
Height and weight were measured with light clothing and without shoes. Spirometry without bronchodilation was performed after the FENO measurements using a dry-wedge spirometer (Vitalograph, Maids Moreton, UK) and according to the ATS/ERS standards [16]. The forced vital capacity (FVC) and the forced expired volume in 1 s (FEV1) were obtained with the subjects in a sitting position, wearing a nose clip. The ratio of FEV1 to forced vital capacity (FVC) was calculated and expressed as FEV1/FVC%). Predicted normal values for the spirometric variables were obtained from the same population [17].
The subjects were classified as never-smokers, former smokers and current smokers, based on their responses to a questionnaire, as previously described [8]. A smoker who had refrained from smoking for >1 year was considered as a former smoker. Affirmative responses to the following questions or positive test results were used to define asthma, chronic bronchitis, atopy, and cold;
Asthma; Do you have or have you ever had asthma? or Have you ever had asthma diagnosed by a physician? [18].
Chronic bronchitis; Have you had persistent cough since the age of 15? and If yes, did any coughing period last at least 3 months? and If yes, have you experienced such coughing periods for at least two consecutive years? [19].
Atopy; Presence of IgE antibodies in blood sample tested using Phadiatop (class 1) [15].
Cold; Do you have a cold now? and/or Do you have a sore throat now?
Airway obstruction; FEV1/FVC ratio below the lower limit of normal [17].
We excluded subjects with asthma, chronic bronchitis, airway obstruction, and a cold, 3378 subjects remained in the cohort.
Statistical analyses were performed using SAS (Statistical Analysis System, version 9.3; SAS Institute Inc.; NC, USA). All comparisons between groups were analysed by the Mann-Whitney U test and two-sided p-values are presented. FENO levels were not normally distributed; hence all univariate analyses were performed using nonparametric tests. In preliminary multiple regression analyses on ln FENO the explanatory variables age, height, weight, body mass index (BMI) and atopy were included. Age, height and atopy were significant for both men and women and were therefore kept in the final multiple regression models (QUANTREG procedure) to obtain the estimated coefficients for median, 5th and 95th percentiles. To test whether the effect of age and height on FENO differed between never-smokers and former smokers we included smoking status, height, age, and the interactions between smoking status and both age and height. There was no significant effect of smoking status.
Results
Descriptive data, including FENO values for 3378 respiratory healthy subjects, are presented in Table 1. Median FENO values and the 5th and 95th percentiles according to smoking status and sex are presented in Table 2. In the whole population the median FENO value was 16.5 ppb with 7.2 ppb and 39.0 ppb as the 5th and 95th percentiles, respectively. FENO levels were significantly (p < 0.0001) higher in men than in women. Further, current smokers had significantly (p < 0.05) lower FENO levels (by around 6 ppb) than former smokers or never-smokers. FENO levels were similar among never-smokers and former smokers. In addition, linear regression did not find a significant effect of smoking status (never-smoker/former smoker) among women or men (data not shown). Hence, never-smokers and former smokers were merged into a group termed as “non-smokers” in subsequent analyses.
Table 1.
General characteristics and lung function of 3378 randomly selected healthy subjects
| All (n = 3378) | Women (n = 1741) | Men (n = 1637) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 51.4 | 11.2 | 50.9 | 11.3 | 52.0 | 11.1 |
| Height (cm) | 172.4 | 9.3 | 166.2 | 6.5 | 179.0 | 6.9 |
| Weight (kg) | 77.6 | 14.4 | 70.2 | 12.0 | 85.5 | 12.4 |
| BMI (kg/m2) | 26.0 | 3.9 | 25.4 | 4.2 | 26.7 | 3.5 |
| FVC (% pred) | 98.1 | 11.9 | 99.1 | 11.8 | 97.0 | 11.9 |
| FEV1 (% pred) | 98.6 | 12.1 | 99.7 | 12.0 | 97.6 | 12.1 |
| FEV1/FVC (%) | 79.5 | 4.7 | 79.7 | 4.7 | 79.3 | 4.7 |
| FEV1/FVC (% pred) | 100.5 | 5.6 | 100.4 | 5.4 | 100.6 | 5.7 |
Mean values and standard deviations (SD) are presented
Table 2.
FENO (ppb) of respiratory healthy subjects (n = 3378) according to smoking habits
| Smoking groups | Women (n = 1741) | Men (n = 1637) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | 5th perc. | 95th perc. | n | Median | 5th perc. | 95th perc. | p valuea | |
| Never smokers | 868 | 15.7 | 7.8 | 35.7 | 817 | 19.0 | 9.0 | 44.2 | <0.0001 |
| Former smokers | 581 | 16.3 | 7.6 | 35.6 | 615 | 18.9 | 9.2 | 39.9 | <0.0001 |
| Current smokers | 292 | 10.4b | 4.4 | 29.4 | 205 | 13.2 b | 6.2 | 34.3 | <0.0001 |
| All subjects | 1741 | 15.0 | 6.6 | 35.3 | 1637 | 18.2 | 8.2 | 41.3 | <0.001. |
a p values refer to differences between median values of FENO according to the Mann-Whitney
two sided test
bdenotes a significant difference from the other smoking categories ANOVA)
Number of subjects, median values and the 5th and 95th percentiles of FENO (ppb) are presented
Table 3 shows the FENO values for men and women stratified by age groups. Irrespective of age group, FENO levels were significantly (p < 0.05) higher among men than women. Furthermore, FENO levels increased with age among both men and women.
Table 3.
FENO results (ppb) of non-smokers, i e never-smokers and former smokers united (n = 2881), of the ADONIX database subdivided according to age class and sex
| Age class (years) | Women (n = 1449) | Men (n = 1432) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | 5th perc | 95th perc | n | Median | 5th perc | 95th perc | ||
| 25–34 | 103 | 12.9 | 5.9 | 30.3 | 88 | 16.8 | 9.4 | 39.5 | <.0001 |
| 35–44 | 320 | 14.3 | 6.8 | 31.3 | 296 | 17.5 | 7.9 | 38.9 | <.0001 |
| 45–54 | 406 | 15.4 | 7.7 | 33.6 | 417 | 19.1 | 8.8 | 41.7 | <.0001 |
| 55–64 | 411 | 18.1 | 8.3 | 37.9 | 418 | 20.0 | 9.3 | 43.2 | 0.010 |
| >64 | 209 | 19.5 | 8.3 | 42.2 | 213 | 21.1 | 9.2 | 50.6 | 0.028 |
p values refer to differences between median values according to the Mann-Whitney two- sided test
Number of subjects, median values and the 5th and 95th percentiles are presented
FENO values for non-smokers stratified by atopy and sex are presented in Table 4. FENO levels were significantly (p < 0.05) higher (by around 2 ppb) among subjects with atopy.
Table 4.
FENO of healthy subjects of the ADONIX database (n = 3378) subdivided into subsets according to the outcome of the Phadiatop test
| Subsets | Phadiatop negative | Phadiatop positive | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Median | 5th perc | 95th perc | N | Median | 5th perc | 95th perc | ||
| All | 2210 | 16.9 | 7.9 | 36.7 | 671 | 19.1 | 8.8 | 49.7 | <.0001 |
| Women | 1133 | 15.7 | 7.7 | 33.6 | 316 | 17.1 | 7.5 | 46.1 | 0.0005 |
| Men | 1077 | 18.4 | 9.0 | 39.2 | 355 | 20.7 | 9.8 | 52.7 | <.0001 |
p values refer to differences between median values of FENO according to the Mann-Whitney two sided test
# denotes a significant difference from the other smoking categories (ANOVA)
Number of subjects, median values and the 5th and 95th percentiles of FENO (ppb) are presented
Table 5 presents FENO reference equations for men and women including median values and the 5th and 95th percentiles from quantile regression models for non-smokers (never-smokers and former smokers). Individual FENO values for men and women are plotted in Fig. 1a and b. The dashed lines represent the 95th percentile and the age dependence of the upper limit of normal, particularly among women. As an example, the predicted 95th percentile of FENO values for a 40 year-old non-atopic woman, height 170 cm, is 4.0 + 0.4081*40 + 0.1414*170–16.1097 = 28.2 ppb.
Table 5.
Coefficient estimates of reference equations for FENO. The 5th percentile, median value and the 95th percentiles are presented for females and males respectively
| Women (n = 1406) | Men (n = 1410) | |||||
|---|---|---|---|---|---|---|
| 5th perc. | Median | 95th perc. | 5th perc. | Median | 95th perc. | |
| Intercept | −6.9 | −12.7 | 4.0 | −5.1 | −14.1 | 84.7 |
| Age (years) | 0.0629 | 0.2205 | 0.4081 | 0.0508 | 0.1444 | 0.2682 |
| Height (cm) | 0.0736 | 0.1189 | 0.1414 | 0.0694 | 0.1559 | −0.2501 |
| Phadiatop positive | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Phadiatop negative | −0.9 | −2.7 | −16.1 | −1.1 | −2.8 | −15.0 |
Fig. 1.
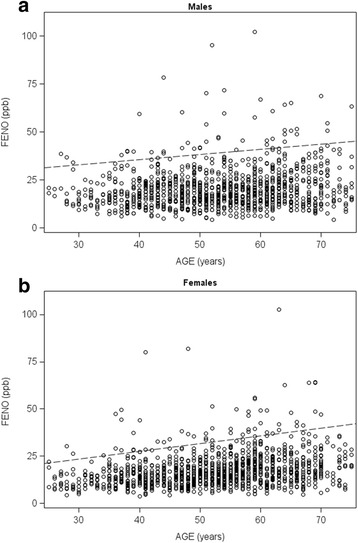
Plots of FENO against age for men (Fig. 1a) and women (Fig. 1b), respectively, which illustrate the age dependency of the upper limit normal. Only subjects with negative Phadiatop® results are presented (n = 2210). The y-axis range is restricted and excludes one female subject and three male subjects with FENO above 110 ppm. The dashed lines indicate the upper 95th percentiles assuming height of women and men to be 166 and 179 cm respectively
Regression modelling for the 420 current smokers in the cohort resulted in unstable models. Hence, the proposed reference values for current smokers are based on the univariate distribution of FENO values and 95th and 5th percentiles shown in Table 2. The proposed upper reference value for FENO is 29.4 ppb for female current smokers and 34.3 ppb for male current smokers.
The Additional file 1 includes an Excel-based FENO calculator for obtaining individual predicted reference values (median and 5th and 95th percentiles) after entering sex, age and height; Phadiatop blood test results (positive or negative) are an optional variable.
Discussion
The present study found that FENO levels among non-smokers are significantly influenced by sex, height, age and atopy, and the reference equations imply that considerable differences in the reference values between a young woman and an older man would be considerable. The reference equations give the upper reference value for a non-atopic woman, 30 years old and 160 cm tall, as 23 ppb, and for a non-atopic man, 60 years old and 180 cm tall, as 41 ppb. Hence, we consider that the findings support the use of individual reference values among non-smokers rather than fixed cut-off limits. However, among current smokers fixed cut-off limits should still be used.
One approach to establishing criteria of normality has been the use of fixed cut-off values for abnormality based on the distribution among normal healthy individuals. Another approach is the production of reference values based on regression equations obtained from the distribution of factors of interest and important predictors, often anthropometric factors. However, the explanatory value of anthropometric factors for FENO is rather low, and that is one reason why cut-off values have often been recommended. The present results indicate, however, that there are considerable differences in the predicted reference values, which support the use of individual reference values based reference equations instead of a fixed cut-off value.
Whether subjects with atopy should be included in a healthy reference population is not clear. Most published studies have included subjects with atopy [2, 12, 20, 21]. At present, the clinical significance of increased production of specific IgE antibodies in non-asthmatic subjects is unclear, but certainly does not imply the presence of disease. Phadiatop test has been shown to exhibit higher sensitivity but lower specificity than the skin-prick test identifying subjects with allergic manifestations such as asthma, dermatitis or rhinitis [15]. However, the clinical significance is unclear. Subjects with atopy (positive Phadiatop test) had a 2 ppb higher median FENO, 13 ppb higher 95th percentile, Table 4. We therefore consider it important to present reference equations separately for atopics and non-atopics, which in the present study was based on Phadiatop testing. In a clinical situation with no information on the patient’s atopic status, we recommend to applying the reference equation for non-atopics (negative Phadiatop). We stress that a positive Phadiatop is not a determination of atopic disease. We should also add that our study population comprised subjects with atopic dermatitis, a condition that has been associated with increased FENO levels [22].
From the present results we can conclude that there are consistent differences between men and women, as women had lower FENO levels than men. The sex difference was not observed in the 1131 subjects included in our previous paper [12]. However, a sex difference was also numerically present in the previous study, but did not reach statistical significance because of the smaller sample-size. The lower FENO levels observed in women may not necessarily reflect lower NO production in women, as FENO is highly flow-dependent, and decreases substantially with increasing flow. For any given exhalation-flow from the mouth (we used 50 mL/s), the linear flow velocities within the airways will depend on lung size, and the smaller the lung the higher the linear flow velocities. As women have smaller lungs and consequently higher linear flow velocities in the airways, lower FENO levels may be expected. Furthermore, the airway surface area that produces NO is smaller among women. Accordingly, the sex-observed for FENO values in healthy subjects may to some extent be attributable to airway size difference rather than to differences in NO production.
The proposed reference values reflect the distribution of FENO levels, and may serve as a tool to enhance the identification of subjects with deviating, abnormal values, and possibly on-going Th-2 driven inflammation. The usefulness of reference values for FENO is indeed to determine what is abnormal. When using FENO to diagnose asthma, clinicians may also consider other factors. For a correct diagnosis, FENO values with the highest positive- and negative likelihood ratios must be identified, and those may be different for Th-2 and non-Th-2 driven asthma. However, the present study was not designed to define these diagnostic characteristics, and we lack other markers of T-helper-2 driven inflammation, for example in induced-sputum, for comparison. The accuracy of different fixed cut-off limits in relation to clinical asthma has recently been evaluated, showing that increasing cut-off limits increase the specificity [6]. These authors concluded that a reasonable balance between specificity and sensitivity would be a cut-off value >45 ppb. For non-smokers, we suggest the use of the individual upper 95th and lower 5th percentiles based on sex, age, atopy and height. The upper limits of normal calculated from the present findings are substantially lower than the upper limits suggested for diagnosing eosinophilic airways inflammation, suggested by the American Thoracic Society [2]. The ATS suggests that an FENO value exceeding 50 ppb indicates a high probability of eosinophilic airway-inflammation, and values of 25–50 ppb require further evaluation. However, our findings indicate that the upper limits of what is apparently normal can differ greatly between a young woman without atopy (around 22 ppb) and an elderly man (over 40 ppb). When evaluating FENO in clinical practice the presented upper limits of normal are important.
We consider the present proposed reference values for current smokers to be original. We confirmed results from previous studies, which reported that current smokers exhibited low FENO levels [23]. Several potential mechanisms underlying this finding have been proposed, such as the hypotheses that smoking may induce lower FENO metabolism or that smoking may induce a reduced active in nitric oxide synthases in the airways [23]. We modelled the data with various anthropometric variables, but because of the unstable models, we decided to propose sex-specific fixed cut-off limits based on a univariate analysis of our data. Further studies are needed to evaluate whether FENO levels in current smokers have clinical relevance [11].
Low FENO levels are of increasing interest. A study published in 1997 showed that 27 patients with cystic fibrosis had low FENO levels, with a mean level of 5.9 ppb [24]. FENO levels have also been studied in relation to BMI; low levels were observed in both obese and underweight individuals [25, 26]. Moreover, low FENO levels have been discussed in relation to certain asthma phenotypes [27]. These reports underscore the need to establish reference limits that define low levels of FENO. The present reference equations provide the lower limits of normal and ought to be of help in identifying subjects with abnormally low FENO.
Several small general-population studies from different countries have calculated reference values for FENO [4, 10, 12, 20, 21, 28–30]. There is, however, one large recently published general-population study from the US (the National Health and Nutrition Examination Survey 2007–2010) where the recommended cut-off values for diagnosing asthma in individuals aged 12–80 years was 39 ppb based on the 95th percentile, i.e. the upper and lower 2.5th percentiles [3]. The authors recommended the same value for men and women, and the statistical analyses were designed quite differently from the present study. The US study excluded only subjects with asthma and wheezing, but included smokers and subjects with atopy. The final regression model included age, height, sex, race, smoking status and passive smoking.
The present study was performed in a random adult population with a participation of approximately 40%; the highest non-participation was observed in young men and in smokers [31]. A possible selection bias may have been present and affected our analysis, as current smokers were overrepresented among non-participants. Another issue to note is that certain conditions which may affect the levels of FENO, such as cystic fibrosis and atopic dermatitis, were not excluded [22, 24]. Furthermore, we did not exclude subjects using inhaled or oral corticosteroids. However, this was a random population sample and subjects with asthma, airway obstruction and chronic bronchitis were excluded, reducing the bias in relation to inhaled or oral corticosteroids.
Conclusions
We conclude that the present reference values for female and male non-smokers (never-smokers and former smokers) that are based on age, height and atopy are more appropriate than fixed cut-off limits. However, for current smokers separate cut-off limits should still be used.
Acknowledgements
Not applicable
Funding
The study was supported by the Swedish Research Council for Health, Working Life and Welfare (Forte) and the Swedish Heart-Lung Foundation.
Availability of data and materials
The datasets used in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ADONIX
Adult-onset asthma and exhaled nitric oxide
- ATS
American thoracic society
- BMI
Body mass index
- ERS
European respiratory society
- FENO
Fractional excretion of exhaled nitric oxide
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- IgE
Immunoglobulin E
- NO
Nitric oxide
- ppb
Parts per billion
- SAS
Statistical analysis system
Additional file
Excel-based FENO calculator. (XLSX 19 kb)
Authors’ contributions
KT conceptualised, designed, and supervised the study. NM assisted with the study conception and drafted the manuscript. LS assisted with the manuscript conception and supervised the statistical analyses. BB assisted with the study conception, performed the statistical analyses and assisted with drafting the manuscript. ACO assisted with study conception and preparation of the manuscript and supervised the analyses and editing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethics committee of the University of Gothenburg approved the study and each subject provided written informed consent.
Consent for publication
Not applicable
Competing interests
All authors declared that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12890-017-0456-9) contains supplementary material, which is available to authorized users.
Contributor Information
Kjell Torén, Email: kjell.toren@amm.gu.se.
Nicola Murgia, Email: Nicola.Murgia@unipg.it.
Linus Schiöler, Email: linus.schioler@amm.gu.se.
Björn Bake, Email: bjorn.bake@vgregion.se.
Anna-Carin Olin, Email: anna-carin.olin@amm.gu.se.
References
- 1.ATS/ERS recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–930. [DOI] [PubMed]
- 2.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See KC, Christiani DC. Normal values and thresholds for the clinical interpretation of exhaled nitric oxide levels in the US general population: Results from the National Health and Nutrition Examination Survey 2007-2010. Chest. 2013;143:107–116. doi: 10.1378/chest.12-0416. [DOI] [PubMed] [Google Scholar]
- 4.Jo E-J, Song W-J, Kim T-W, Park H-W, Chang Y-S, Kim T-B, et al. Reference ranges and determinant factors for exhaled nitric oxide in a healthy Korean elderly population. Allergy Asthma Immunol Res. 2014;6:504–510. doi: 10.4168/aair.2014.6.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacenti G, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014;108:830–841. doi: 10.1016/j.rmed.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Karrasch S, Linde K, Rücker G, Sommer H, Karsch-Völk M, Kleijnen J, et al. Accuracy of FENO for diagnosing asthma: A systematic review. Thorax. 2016; [E-pub Jul 7] [DOI] [PubMed]
- 7.Kharitonov SA, Robbins RA, Yates D, Keatings V, Barnes PJ. Acute and chronic effects of cigarette smoking on exhaled nitric oxide. Am J Respir Crit Care Med. 1995;152:609–612. doi: 10.1164/ajrccm.152.2.7543345. [DOI] [PubMed] [Google Scholar]
- 8.Olin AC, Rosengren A, Thelle DS, Lissner L, Bake B, Torén K. Height, age, and atopy are associated with the fraction of exhaled nitric oxide in a large adult general population sample. Chest. 2006;130:1319–1325. doi: 10.1378/chest.130.5.1319. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DR, Mandhane P, Greene JM, Hancox RJ, Filsell S, McLachlan CR, et al. Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res. 2007;8:82. doi: 10.1186/1465-9921-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karrasch S, Ernst K, Behr J, Heinrich J, Huber RM, Nowak D, et al. Exhaled nitric oxide and influencing factors in a random population sample. Respir Med. 2011;105:713–718. doi: 10.1016/j.rmed.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Malinovschi A, Backer V, Harving H, Porsbjerg C. The value of exhaled nitric oxide to identify asthma in smoking patients with asthma-like symptoms. Respir Med. 2012;106:794–801. doi: 10.1016/j.rmed.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Olin AC, Bake B, Torén K. Normal equations for fraction of exhaled nitric oxide. Chest. 2007;131:1852–1856. doi: 10.1378/chest.06-2928. [DOI] [PubMed] [Google Scholar]
- 13.Olin A-C, Aldenbratt A, Ekman A, Ljungkvist G, Alving K, Torén K. Increased nitric oxide in exhaled air after intake of a nitrate rich meal. Respir Med. 2001;95:153–158. doi: 10.1053/rmed.2000.1010. [DOI] [PubMed] [Google Scholar]
- 14.Olin A-C, Alving K, Torén K. Exhaled nitric oxide: Relation to sensitization and respiratory symptoms. Clin Exp Allergy. 2004;34:221–236. doi: 10.1111/j.1365-2222.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 15.Tschopp JM, Sistek D, Schindler C, Leuenberger P, Perruchoud AP, Wütrich B, et al. Current allergic asthma and rhinitis: Diagnostic efficiency of three commonly used atopic markers (IgE, skin prick tests and Phadiatop®) Allergy. 1998;53:608–613. doi: 10.1111/j.1398-9995.1998.tb03937.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Brisman J, Kim J-L, Olin A-C, Torén K, Bake B. Spirometric reference equations for Swedish adults. Clin Physiol Funct Imag. 2016 [Feb 10] [E-pub ahead of print]. [DOI] [PubMed]
- 18.Torén K, Palmqvist M, Löwhagen O, Balder B, Tunsäter A. Accuracy of self-reported year of asthma-onset. J Clin Epidemiol. 2006;59:90–93. doi: 10.1016/j.jclinepi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Holm M, Torén K, Andersson E. Incidence of chronic bronchitis: A prospective study in a large general population. Int J Tuberc Lung Dis. 2014;18:870–875. doi: 10.5588/ijtld.13.0652. [DOI] [PubMed] [Google Scholar]
- 20.Travers J, Marsh S, Aldington S, Williams M, Shirtcliffe P, Pritchard A, et al. Reference ranges for exhaled nitric oxide derived from a random community survey of adults. Am J Respir Crit Care Med. 2007;176:238–242. doi: 10.1164/rccm.200609-1346OC. [DOI] [PubMed] [Google Scholar]
- 21.Dressel H, de la Motte D, Reichert J, Ochman U, Petru R, Angerer P, Holz O, Nowak D, Jörres RA. Exhaled nitric oxide: Independent effects of atopy, smoking, respiratory tract infections, gender and height. Respir Med. 2008;102:962–969. doi: 10.1016/j.rmed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Zinelli C, Caffarelli C, Strid J, Jaffe A, Atherton DJ. Measurement of nitric oxide and 8-isoprostane in exhaled breath of children with atopic eczema. Clin Exp Dermatol. 2009;34:607–612. doi: 10.1111/j.1365-2230.2008.03142.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Hu H, Kearney GD, Kan H, Carrillo G, Chen X. A population-based study of smoking, serum cotinine and exhaled nitric oxide among asthmatics and a healthy population in the USA. Inhal Toxicol. 2016;28:724–730. doi: 10.1080/08958378.2016.1264502. [DOI] [PubMed] [Google Scholar]
- 24.Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol. 1997;24:173–177. doi: 10.1002/(SICI)1099-0496(199709)24:3<173::AID-PPUL2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 25.Maniscalo M, Zedda A, Faraone S, Cristiano S, Sofia M, Motta A. Low alveolar and bronchial nitric oxide in severe uncomplicated obesity. Obes Res Clin Pract. 2015;9:603–608. doi: 10.1016/j.orcp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Uppalapati A, Gogineni S, Espiritu R. Association between body mass index (BMI) and fraction of exhaled nitric oxide (FeNO) levels in the National Health and Nutrition Survey (NHANES) 2007–2010. Obes Res Clin Pract. 2016;10:652–58. [DOI] [PubMed]
- 27.Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brody DJ, Zhang X, Kit BK, Dillon CF. Reference values and factors associated with exhaled nitric oxide: U.S. youth and adults. Respir Med. 2013;107:1682–1691. doi: 10.1016/j.rmed.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Kim TH, Sohn JW, Yoon HJ, Shin DH, Park SS. Reference values and determinants of exhaled nitric oxide in healthy Korean adults. J Asthma. 2010;47:563–567. doi: 10.3109/02770901003702840. [DOI] [PubMed] [Google Scholar]
- 30.Ko FW, Leung TF, Wong GW, Chu JH, Sy HY, Hui DS. Determinants of, and reference equation for, exhaled nitric oxide in the Chinese population. Eur Respir J. 2013;42:767–775. doi: 10.1183/09031936.00130112. [DOI] [PubMed] [Google Scholar]
- 31.Strandhagen E, Berg C, Lissner L, Nunez L, Torén K, et al. Selection bias in a population survey with registry linkage: Potential effect on socioeconomic gradient in cardiovascular risk. Eur J Epidemiol. 2010;25:163–172. doi: 10.1007/s10654-010-9427-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in the current study are available from the corresponding author upon reasonable request.


