Abstract
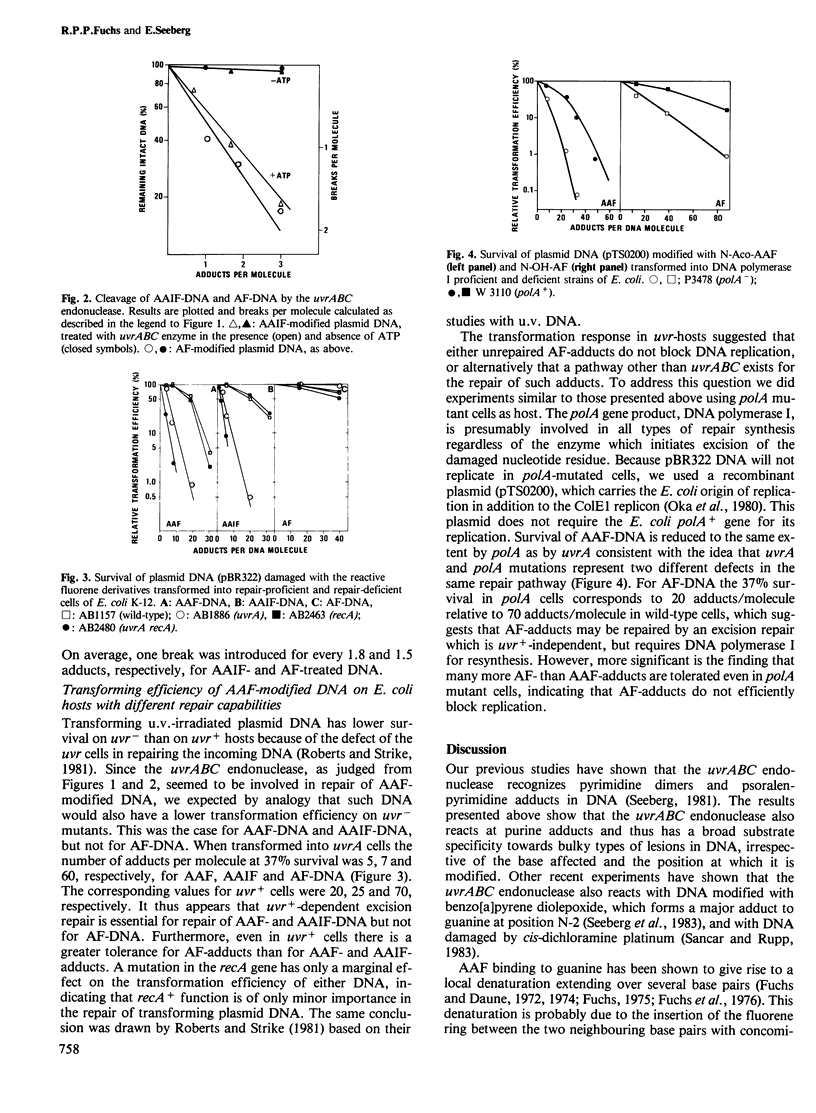
Covalently closed circular plasmid DNA was treated with three reactive derivatives of 2-acetylaminofluorene: N-acetoxy-N-2-acetylaminofluorene (N-Aco-AAF), its 7-iodo derivative (N-Aco- AAIF ) and N-hydroxy-N-2-aminofluorene (N-OH-AF), and tested as substrates for the Escherichia coli uvrABC endonuclease and for transformation frequencies on wild-type, uvrA, recA, uvrArecA and polA mutant strains. The uvrABC endonuclease reacted with all three substrates with high efficiency, implicating this enzyme in the repair of DNA containing all three types of adducts. However, only AAF- and AAIF -DNA showed greatly reduced survival on uvrA mutants (five adducts/lethal hit) relative to wild-type (20 adducts/lethal hit). AF-DNA survived equally well on uvrA mutant and wild-type cells, and at a much higher level of modification (60 adducts/lethal hit). A mutation in recA had only a minor effect on the survival of either DNA. The polA mutation reduced the survival of the AAF-treated DNA to the same extent as the uvrA mutation (five adducts/lethal hit). Also AF-DNA showed reduced survival on polA mutant cells versus wild-type. However, many more adducts (20/lethal hit) were tolerated than for AAF-DNA, indicating that AF lesions in the template do not efficiently block replication of DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Lee F. D., Durston W. E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Natl Acad Sci U S A. 1973 Mar;70(3):782–786. doi: 10.1073/pnas.70.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Broyde S., Hingerty B. Conformation of 2-aminofluorene-modified DNA. Biopolymers. 1983 Nov;22(11):2423–2441. doi: 10.1002/bip.360221109. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daune M. P., Fuchs R. P., Leng M. Structural modification and protein recognition of DNA modified by N-2-fluorenylacetamide, its 7-iodo derivative, and by N-2-fluorenamine. Natl Cancer Inst Monogr. 1981 Dec;(58):201–210. [PubMed] [Google Scholar]
- Evans F. E., Miller D. W., Beland F. A. Sensitivity of the conformation of deoxyguanosine to binding at the C-8 position by N-acetylated and unacetylated 2-aminofluorene. Carcinogenesis. 1980;1(11):955–959. doi: 10.1093/carcin/1.11.955. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Daune M. P. Dynamic structure of DNA modified with the carcinogen N-acetoxy-n-2-acetylaminofluorene. Biochemistry. 1974 Oct 8;13(21):4435–4440. doi: 10.1021/bi00718a028. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P. In vitro recognition of carcinogen-induced local denaturation sites native DNA by S1 endonuclease from Aspergillus oryzae. Nature. 1975 Sep 11;257(5522):151–152. doi: 10.1038/257151a0. [DOI] [PubMed] [Google Scholar]
- Fuchs R. P., Lefevre J. F., Pouyet J., Daune M. P. Comparative orientation of the fluorene residue in native DNA modified by N-acetoxy-N-2-acetylaminofluorene and two 7-halogeno derivatives. Biochemistry. 1976 Jul 27;15(15):3347–3351. doi: 10.1021/bi00660a027. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical basis of chemical carcinogenesis by N-2-fluorenylacetamide derivatives and analogs. FEBS Lett. 1973 Aug 15;34(2):295–298. doi: 10.1016/0014-5793(73)80815-4. [DOI] [PubMed] [Google Scholar]
- Fuchs R., Daune M. Physical studies on deoxyribonucleic acid after covalent binding of a carcinogen. Biochemistry. 1972 Jul 4;11(14):2659–2666. doi: 10.1021/bi00764a017. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Mount D. W. Mechanisms of DNA replication and mutagenesis in ultraviolet-irradiated bacteria and mammalian cells. Prog Nucleic Acid Res Mol Biol. 1981;25:53–126. doi: 10.1016/s0079-6603(08)60483-3. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Kriek E. On the interaction of N-2-fluorenylhydroxylamine with nucleic acids in vitro. Biochem Biophys Res Commun. 1965 Sep 22;20(6):793–799. doi: 10.1016/0006-291x(65)90088-4. [DOI] [PubMed] [Google Scholar]
- Landolph J. R., Heidelberger C. Chemical carcinogens produce mutations to ouabain resistance in transformable C3H/10T1/2 Cl 8 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Feb;76(2):930–934. doi: 10.1073/pnas.76.2.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre J. F., Fuchs R. P., Daune M. P. Comparative studies on the 7-iodo and 7-fluoro derivatives of N-acetoxy-N-2-acetylaminofluorene: binding sites on DNA and conformational change of modified deoxytrinucleotides. Biochemistry. 1978 Jun 27;17(13):2561–2567. doi: 10.1021/bi00606a016. [DOI] [PubMed] [Google Scholar]
- Leng M., Ptak M., Rio P. Conformation of acetylaminofluorene and aminofluorene modified guanosine and guanosine derivatives. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1095–1102. doi: 10.1016/0006-291x(80)90064-9. [DOI] [PubMed] [Google Scholar]
- MILLER E. C., MILLER J. A., HARTMANN H. A. N-Hydroxy-2-acetylaminofluorene: a metabolite of 2-acetylaminofluorene with increased carcinogenic activity in the rat. Cancer Res. 1961 Jul;21:815–824. [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Strike P. Efficiency of Escherichia coli repair processes on uv-damaged transforming plasmid DNA. Plasmid. 1981 Mar;5(2):213–220. doi: 10.1016/0147-619x(81)90022-6. [DOI] [PubMed] [Google Scholar]
- Salles B., Lang M. C., Freund A. M., Paoletti C., Daune M., Fuchs R. P. Different levels of induction of RecA protein in E. coli (PQ 10) after treatment with two related carcinogens. Nucleic Acids Res. 1983 Aug 11;11(15):5235–5242. doi: 10.1093/nar/11.15.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Fuchs R. P., Grunberger D. Mutagenicity of 7-iodo and 7-fluoro derivatives of N-hydroxy-and N-acetoxy-N-2-acetylaminofluorene in the Salmonella typhimurium assay. Mutat Res. 1979 May;67(1):85–87. doi: 10.1016/0165-1218(79)90102-2. [DOI] [PubMed] [Google Scholar]
- Schmid S. E., Daune M. P., Fuchs R. P. Repair and mutagenesis of plasmid DNA modified by ultraviolet irradiation or N-acetoxy-N-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4133–4137. doi: 10.1073/pnas.79.13.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Nissen-Meyer J., Strike P. Incision of ultraviolet-irradiated DNA by extracts of E. coli requires three different gene products. Nature. 1976 Oct 7;263(5577):524–526. doi: 10.1038/263524a0. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Nordenskjöld M., Söderhäll S., Jernström B. Strand-break formation in DNA modified by benzo[alpha]pyrene diolepoxide. Quantitative cleavage by Escherichia coli uvrABC endonuclease. Mutat Res. 1983 Jun;112(3):139–145. doi: 10.1016/0167-8817(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Strand cleavage at psoralen adducts and pyrimidine dimers in DNA caused by interaction between semi-purified uvr+ gene products from Escherichia coli. Mutat Res. 1981 Jun;82(1):11–22. doi: 10.1016/0027-5107(81)90133-0. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Tang M., Lieberman M. W., King C. M. uvr Genes function differently in repair of acetylaminofluorene and aminofluorene DNA adducts. Nature. 1982 Oct 14;299(5884):646–648. doi: 10.1038/299646a0. [DOI] [PubMed] [Google Scholar]
- de Murcia G., Lang M. C., Freund A. M., Fuchs R. P., Duane M. P., Sage E., Leng M. Electron microscopic visualization of N-acetoxy-N-2-acetylaminofluorene binding sites in ColE1 DNA by means of specific antibodies. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6076–6080. doi: 10.1073/pnas.76.12.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]


