Abstract
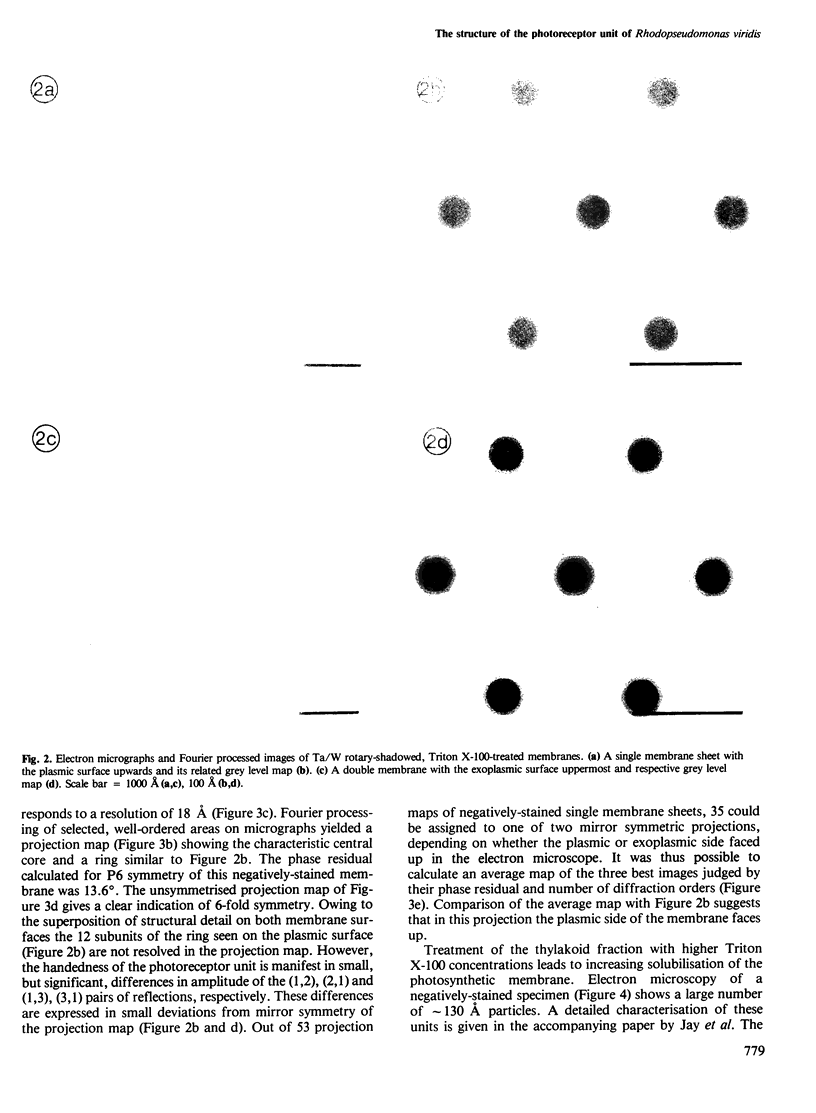
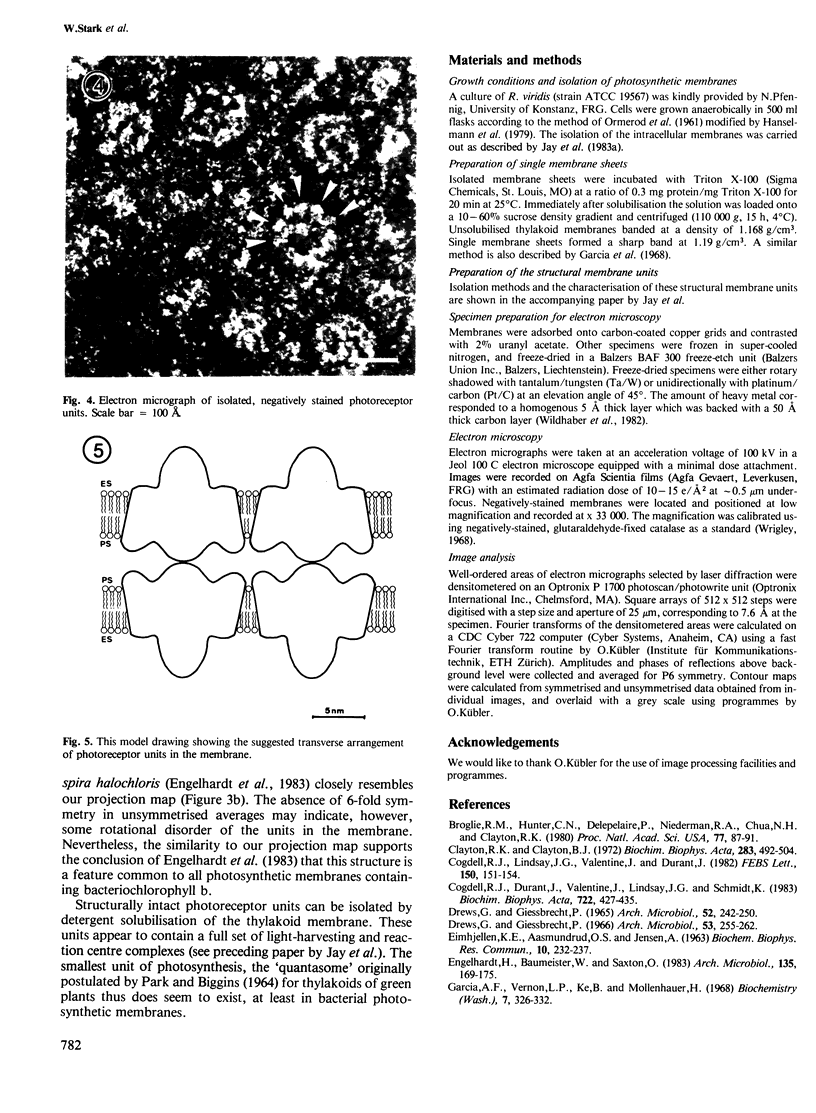
The thylakoid membrane of Rhodopseudomonas viridis contains extensive, regular arrays of photoreceptor complexes arranged on a hexagonal lattice with a repeat distance of ˜130 Å. Single membrane sheets were obtained by mild treatment of the thylakoid fraction with the detergent Triton X-100. Heavy metal shadowing and electron microscopy of isolated thylakoids indicated a strong asymmetry of the membrane, showing a smooth plasmic and a rough exoplasmic side. Fourier processing of rotary-shadowed specimens showed the different surface relief on both sides of the membrane. Structural units on both sides were roughly circular and showed 6-fold symmetry at a resolution close to 20 Å. The structural unit was characterised by a central core that seemed to extend through the membrane, protruding on the exoplasmic side. The core was surrounded by a ring showing 12 subunits on the plasmic side. Rotary-shadowed as well as negatively-stained membranes indicated a handedness of the structure. Treatment of thylakoid vesicles with higher detergent concentrations yielded a fraction of particles showing the same features as Fourier maps of the structural units. The isolated particles therefore appeared to represent structurally intact units of photosynthesis.
Keywords: electron microscopy, image analysis, photoreceptor unit, pigment-protein complex, Rhodopseudomonas viridis
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Clayton B. J. Relations between pigments and proteins in the photosynthetic membranes of Rhodopseudomonas spheroides. Biochim Biophys Acta. 1972 Dec 14;283(3):492–504. doi: 10.1016/0005-2728(72)90265-4. [DOI] [PubMed] [Google Scholar]
- Drews G., Giesbrecht P. Rhodopseudomonas viridis, nov. spec., ein neu isoliertes, obligat phototrophes Bakterium. Arch Mikrobiol. 1966 Mar 31;53(3):255–262. [PubMed] [Google Scholar]
- Garcia A., Vernon L. P., Ke B., Mollenhauer H. Some structural and photochemical properties of Rhodopseudomonas species NHTC 133 subchromatophore particles obtained by treatment with Triton X-100. Biochemistry. 1968 Jan;7(1):326–332. doi: 10.1021/bi00841a041. [DOI] [PubMed] [Google Scholar]
- Jacob J. S., Miller K. R. Structure of a bacterial photosynthetic membrane. Isolation, polypeptide composition, and selective proteolysis. Arch Biochem Biophys. 1983 May;223(1):282–290. doi: 10.1016/0003-9861(83)90593-3. [DOI] [PubMed] [Google Scholar]
- Jay F., Lambillotte M., Mühlethaler K. Localisation of Rhodopseudomonas viridis reaction centre and light harvesting proteins using ferritin-antibody labelling. Eur J Cell Biol. 1983 Mar;30(1):1–8. [PubMed] [Google Scholar]
- Michel H. Three-dimensional crystals of a membrane protein complex. The photosynthetic reaction centre from Rhodopseudomonas viridis. J Mol Biol. 1982 Jul 5;158(3):567–572. doi: 10.1016/0022-2836(82)90216-9. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Jacob J. S. Two-dimensional crystals formed from photosynthetic reaction centers. J Cell Biol. 1983 Oct;97(4):1266–1270. doi: 10.1083/jcb.97.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R. Structure of a bacterial photosynthetic membrane. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6415–6419. doi: 10.1073/pnas.76.12.6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Park R. B., Biggins J. Quantasome: Size and Composition. Science. 1964 May 22;144(3621):1009–1011. doi: 10.1126/science.144.3621.1009. [DOI] [PubMed] [Google Scholar]
- Pucheu N. L., Kerber N. L., García A. F. Isolation and purification of reaction center from Rhodopseudomonas viridis NHTC 133 by means of LDAO. Arch Microbiol. 1976 Sep 1;109(3):301–305. doi: 10.1007/BF00446642. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Cogdell R. J., Seftor R. E., Webster G. D. Further studies on the composition and spectral properties of the photochemical reaction centers of bacteriochlorophyll b-containing bacteria. Biochim Biophys Acta. 1980 Nov 5;593(1):60–75. doi: 10.1016/0005-2728(80)90008-0. [DOI] [PubMed] [Google Scholar]
- Wildhaber I., Gross H., Moor H. The control of freeze-drying with deuterium oxide (D2O). J Ultrastruct Res. 1982 Sep;80(3):367–373. doi: 10.1016/s0022-5320(82)80050-6. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]