Abstract
Heart failure (HF) is a debilitating and life-threatening condition, with 5-year survival rate lower than breast or prostate cancer. It is the leading cause of hospital admission in over 65s, and these admissions are projected to rise by more than 50% over the next 25 years. Transthoracic echocardiography (TTE) is the first-line step in diagnosis in acute and chronic HF and provides immediate information on chamber volumes, ventricular systolic and diastolic function, wall thickness, valve function and the presence of pericardial effusion, while contributing to information on aetiology. Dilated cardiomyopathy (DCM) is the third most common cause of HF and is the most common cardiomyopathy. It is defined by the presence of left ventricular dilatation and left ventricular systolic dysfunction in the absence of abnormal loading conditions (hypertension and valve disease) or coronary artery disease sufficient to cause global systolic impairment. This document provides a practical approach to diagnosis and assessment of dilated cardiomyopathy that is aimed at the practising sonographer.
Keywords: dilated cardiomyopathy, echocardiography, left ventricular dysfunction
1. Introduction
1.1. The BSE Education Committee has previously published a minimum dataset for a standard adult transthoracic echocardiogram (TTE). These are published online in Echo Research and Practice (1). This document specifically states that the minimum dataset is usually only sufficient when the echocardiographic study is entirely normal. The aim of the Education Committee is to publish a series of appendices to cover specific pathologies to support this minimum dataset. This final document completes the cardiomyopathy series (hypertrophic (2), restrictive (3), ARVC (4) and dilated cardiomyopathies).
1.2. The intended benefits of such supplementary recommendations are to:
Support cardiologists and echocardiographers to develop local protocols and quality control programmes for adult transthoracic study.
Promote quality by defining a set of descriptive terms and measurements, in conjunction with a systematic approach to perform and report a study in specific disease states.
Facilitate the accurate comparison of serial echocardiograms performed in patients at the same or different sites.
1.3. The views and measurements are focussed upon dilated cardiomyopathy and are supplementary to those outlined in the TTE minimum dataset. This document gives recommendations for the image and analysis dataset required in patients either being assessed for or with a known diagnosis of dilated cardiomyopathy.
1.4. When the condition or acoustic windows of the patient prevent the acquisition of one or more components of the supplementary dataset or when measurements result in misleading information (e.g. off-axis measurements) this should be stated.
1.5. This document is a guideline for echocardiography in dilated cardiomyopathy and will be updated in accordance with changes directed by publications or changes in practice.
2. Dilated cardiomyopathy
2.1. Dilated cardiomyopathy (DCM) is a progressive disease of the heart muscle. It is characterised by chamber enlargement and contractile dysfunction of the left ventricle in the absence of chronic pressure and/or volume overload. Although not essential for diagnosis, the right ventricle may similarly be affected. Dilated cardiomyopathy is the third most common cause of heart failure and is the commonest type of cardiomyopathy (5). The phenotype of ventricular dilatation and abnormal contractile dysfunction can be the result of a range of pathological conditions such as infection, toxins or autoimmune disease. However, in 50% of cases, the aetiology remains unknown and is termed ‘idiopathic DCM’. It is recognised that between 20% and 35% of idiopathic cases may have a family history suggesting an inherited gene defect (6).
2.2. Echocardiography is the first-line imaging test in the assessment of patients with DCM. It provides pivotal information not only for diagnosis, risk stratification and guiding treatment, but also plays a key role in screening family members. This document provides recommendations for the acquisition of images and collection of a dataset to be analysed during echocardiography in the assessment of patients with suspected or known DCM.
2.3. In this document, the parameters used for diagnosis and risk stratification are set out systematically (Table 1). Appendix 1 provides additional information on some of these parameters, Appendix 2 describes the echocardiographic features that help to differentiate the commonly encountered conditions presenting as DCM and finally a list of useful references for further reading.
Table 1.
Parameters used for diagnosis and risk stratification.
| View | Measurements | Explanatory note | Image |
|---|---|---|---|
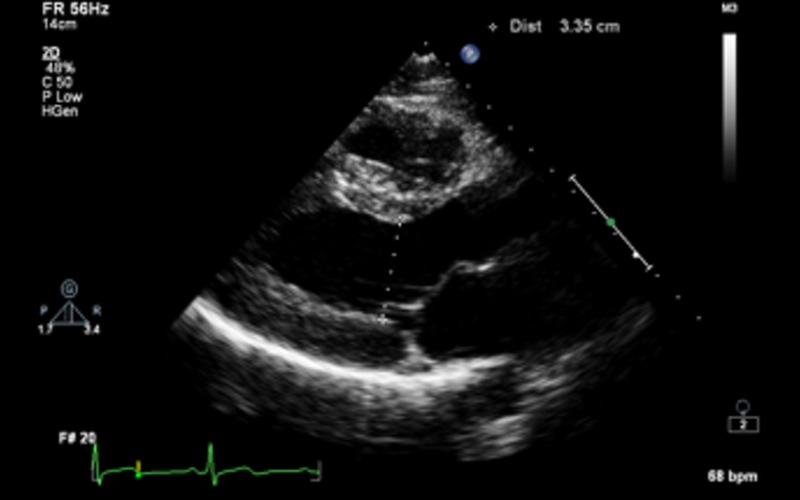
| PLAX 2D/M-mode | LVIDd/s, IVSd, LVPWd | LV cavity size, wall thickness and radial function. Measurements are taken just distal to the mitral valve tip If images are off axis, use 2D All measurements should be indexed to BSA (refer to chamber quantification guidelines for normal values) (7) |
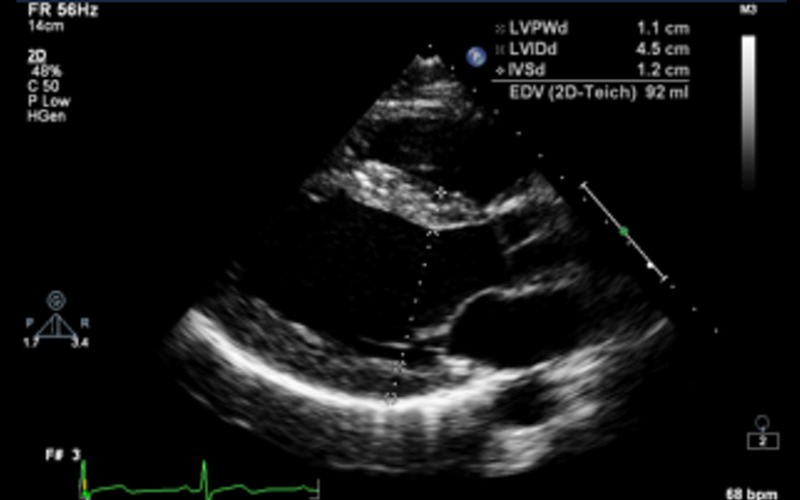 |
| Fractional shortening (FS) |
LVIDd >112% (2 s.d.) corrected for age and BSA is a diagnostic criterion for idiopathic DCM (refer to Appendix 1a) Value above 117% (2 s.d. plus 5%) increases the specificity and may be useful as a screening tool FS <25% is a criterion for the diagnosis of idiopathic DCM in the presence of a dilated ventricle |
 |
|
| PLAX M-mode | MV E-Septal separation (EPSS) | EPSS is defined as the minimal distance between E point (most anterior motion of the AML during diastole) and a line tangential to the most posterior excursion of the IVS within the same cardiac cycle Normal range 0–5.3 mm. Value above 7 mm is indicative of reduced systolic function but a normal value does not exclude LV dysfunction (8, 9) |
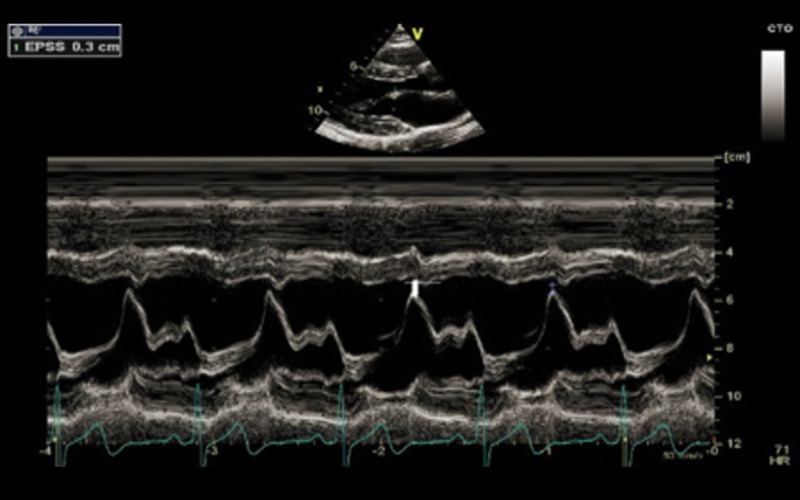 EPSS in a normal ventricle |
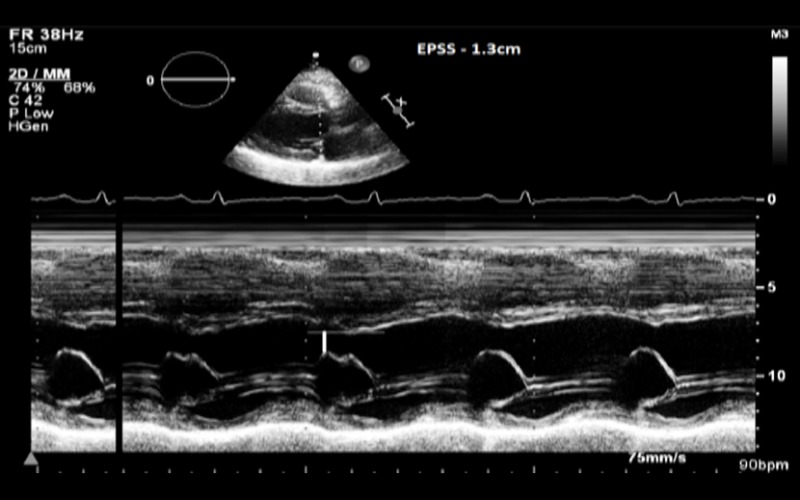 EPSS in a patient with DCM |
|||
| PLAX CFM | Mitral regurgitation | Assess mechanism and severity of mitral regurgitation | |
| See Appendix 1a and MR dataset (10) | |||
| PSAX base to apex 2D | Qualitative assessment of ventricular (LV and RV) structure and function with special reference to radial systolic function and to exclude regional wall motion abnormalities | Refer to Appendix 2 | 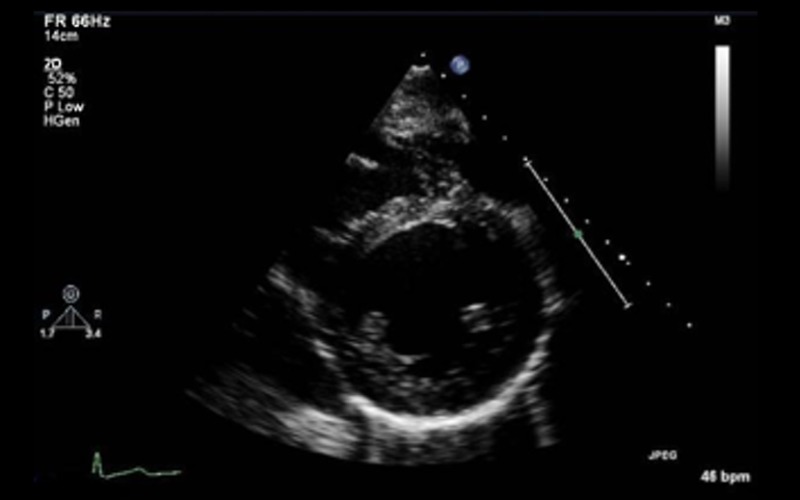 |
| A4C 2D | Qualitative assessment of ventricular (LV and RV) structure and function with special reference to radial and longitudinal function and regional wall motion abnormalities | Refer to Appendix 2 | |
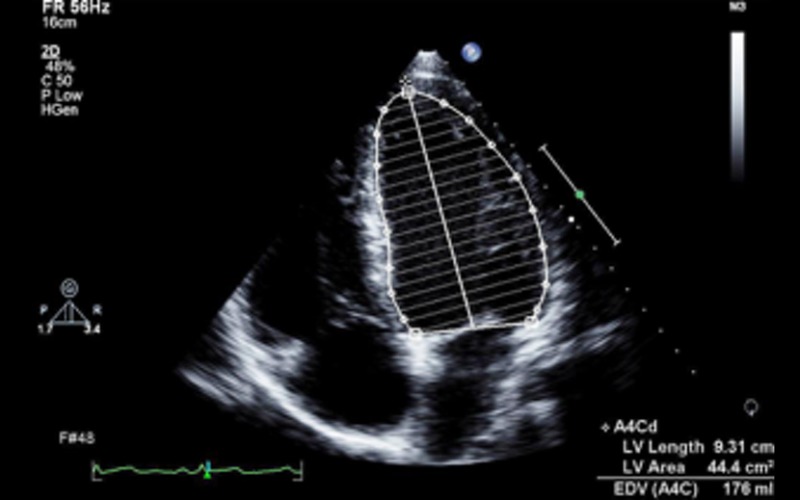
| LV volumes | Diastolic and systolic volumes indexed to BSA (see BSE chamber quantification guide (7)). 3DE is recommended as the optimal method if endocardial definition is adequate Consider use of echo contrast if poor endocardial definition |
 |
|
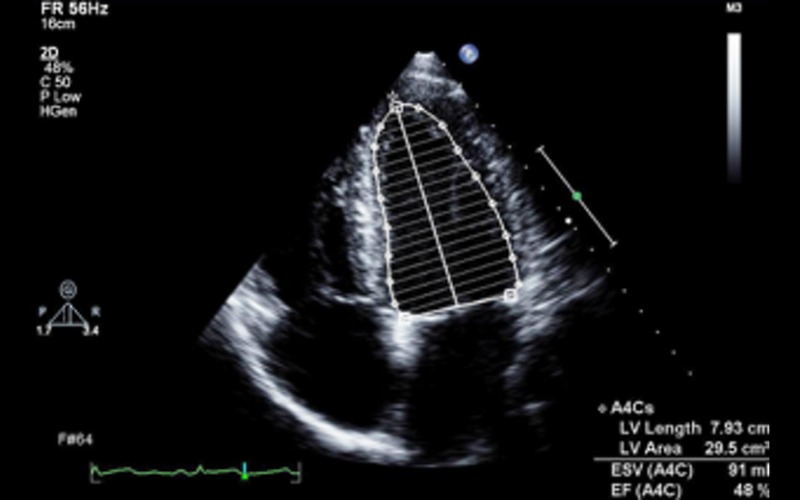
| Ejection fraction (EF) | Estimated using Bi-plane Simpsons. 3DE is the preferred modality for measurement of LVEF EF of less than 45% is a diagnostic criterion for idiopathic DCM in the presence of a dilated ventricle (see Appendix 1a) |
 |
|
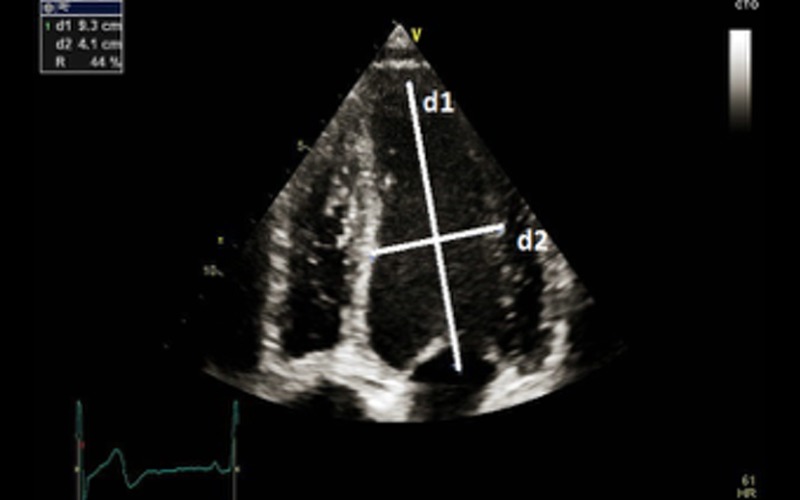
| Sphericity index (SI) | Ratio between the length (mitral annulus to apex in the apical view) and width (mid-cavity level in the A4C view |
 SI of 1.8 (d1/d2) in a non dilated LV with normal EF SI of 1.8 (d1/d2) in a non dilated LV with normal EF |
|
| With gradual dilatation, left ventricle becomes more spherical in DCM and the value approaches near 1 (normal >1.5) SI is a predictor of functional (exercise) capacity in patients with LV dysfunction and is an adverse prognostic marker (11) |
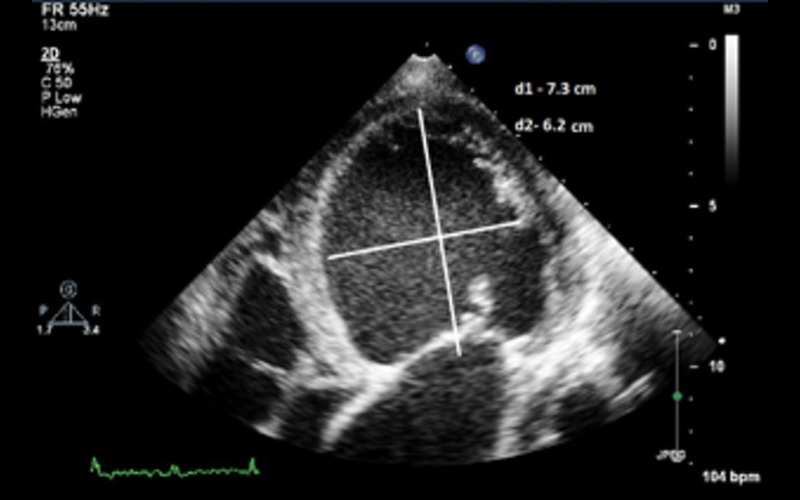 SI of 1.2 in a patient with DCM SI of 1.2 in a patient with DCM |
||
| A4C CFM | Mitral regurgitation | Assess mechanism and severity of MRRefer to Appendix 1a and MR dataset (10) | 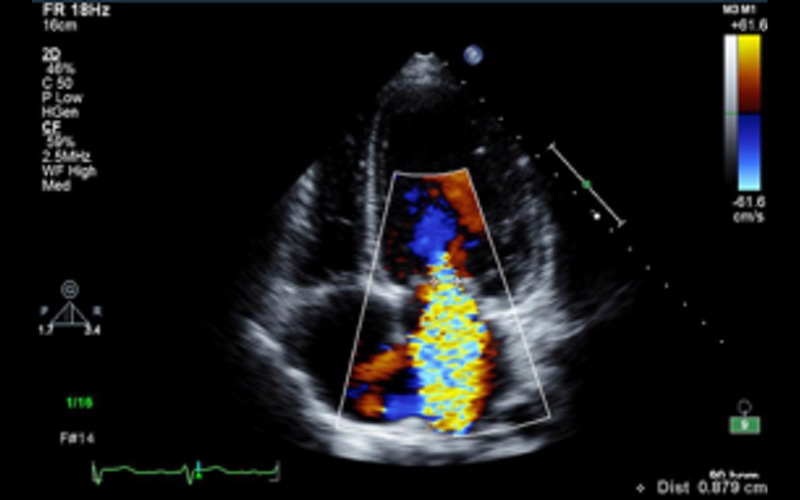 |
| A4C PW | LV inflow Doppler | E and A fusion is common in patients with DCM and indicates AV dysynchrony in the absence of sinus tachycardia | |
| Diastolic dysfunction | In patients with EF less than 45%, diastolic dysfunction coexists. In this group, grading of diastolic dysfunction can be performed using mitral inflow pattern alone and provides additional prognostic information See BSE diastolic function guidelines for further information (12) |
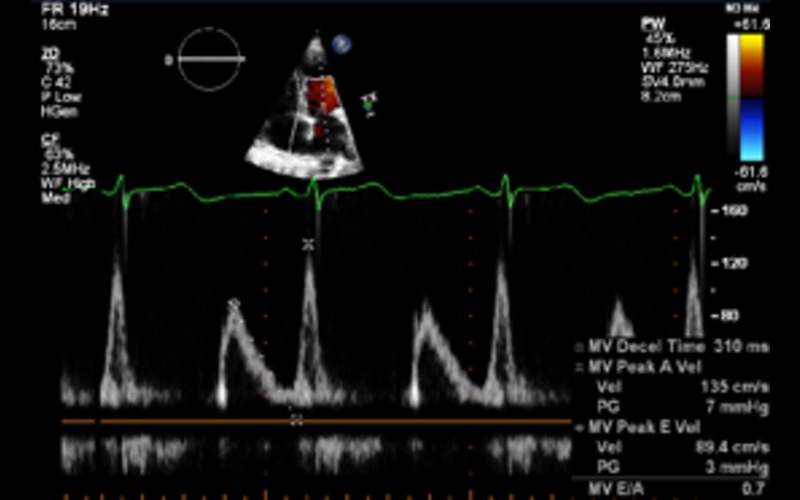 Grade I Grade I |
|
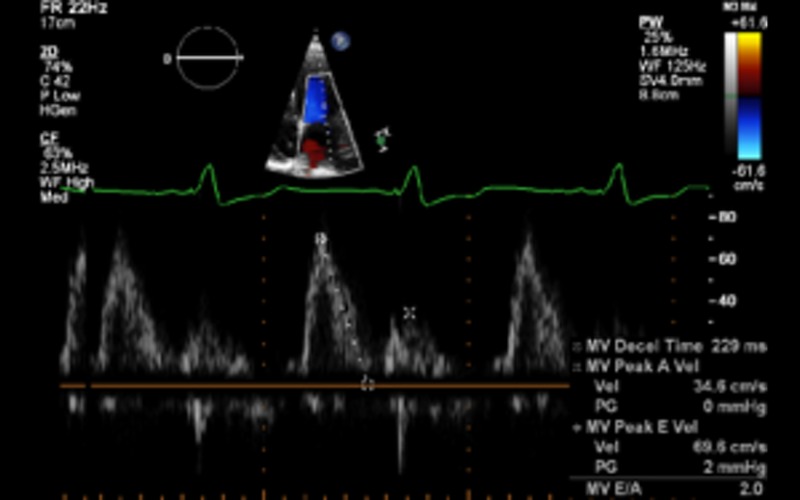 Grade II Grade II |
|||
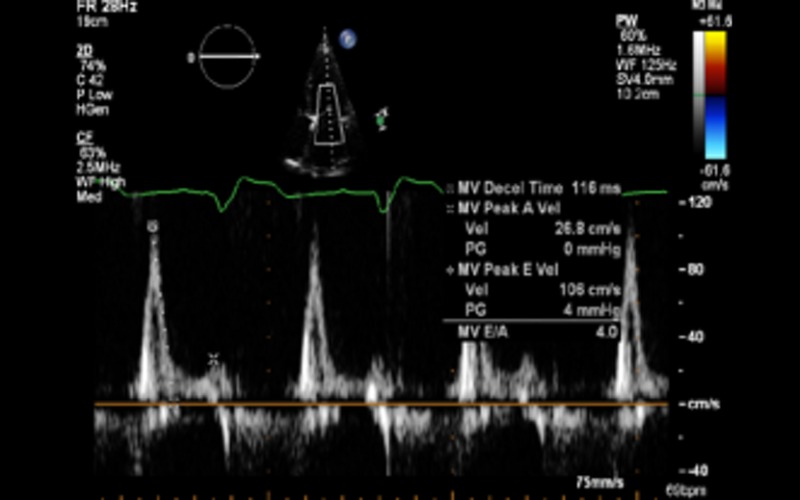 Grade III Grade III |
|||
| A4C tdi | S and e′ Calculate E/e′ ratio |
Both are decreased in DCM Refer to diastolic function guidelines for age adjusted values (9) e′: an average from 2 sites (septal and lateral) is used for calculation of this ratio Value above 13 suggests raised LA pressure (12) |
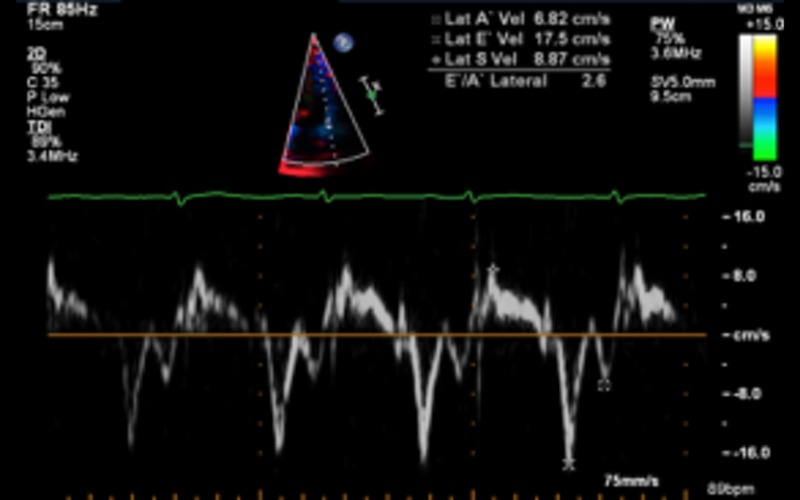 |
| A5C PW | Velocity time integral of LV outflow tract |
<18 cm is an adverse prognostic marker (13) Indicative of a low flow state |
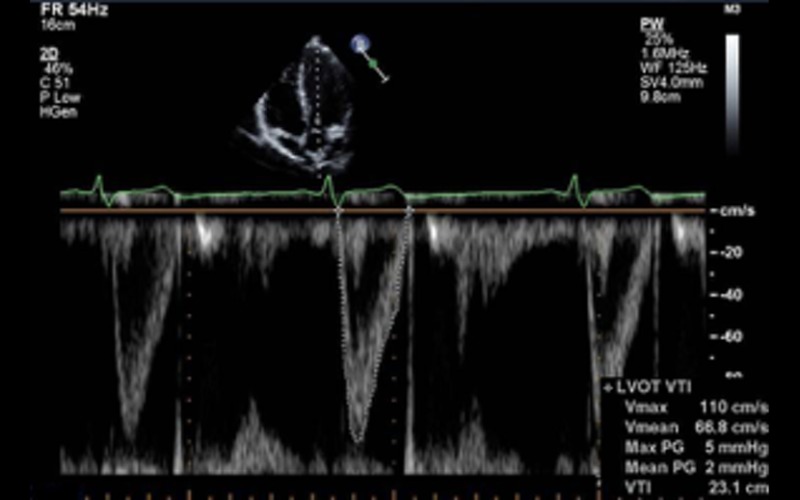 |
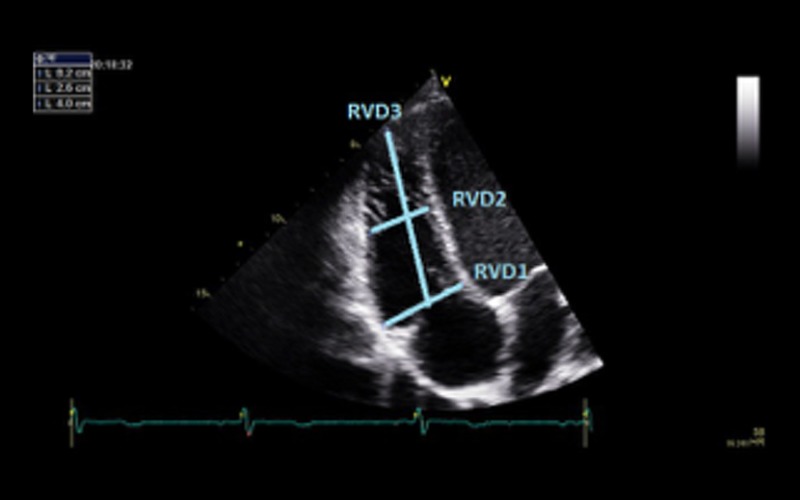
| Focussed RV view 2D | Basal LV:RV ratio | Qualitative assessment of RV sizeA ratio of >0.66 suggests RV dilatation | 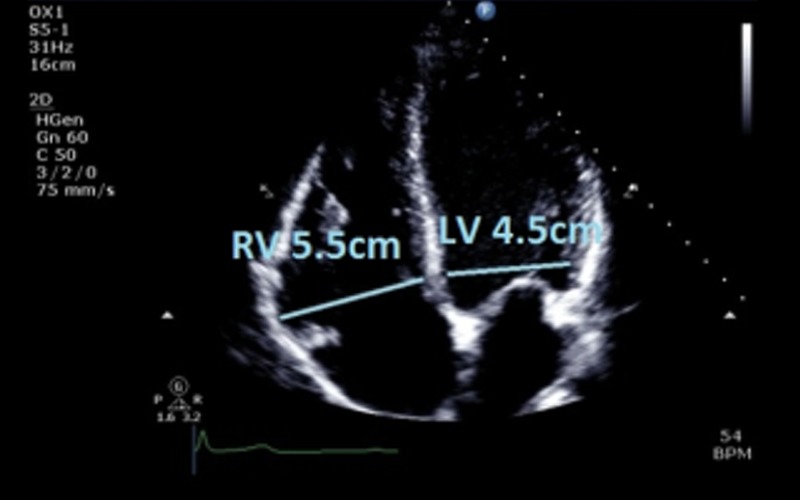 |
| RVD1 RVD2 RVD3 |
RVD1 >41 mm; RVD2 >35 mm; RVD3 >83 mm indicates RV dilatation Refer to BSE ARVC dataset for further information on RV measurements and assessment (4) |
 |
|
| Fractional area change (FAC) | FAC of <35% indicates RV dysfunction RV dysfunction is not essential for the diagnosis of DCM but when present is an adverse prognostic marker (14) |
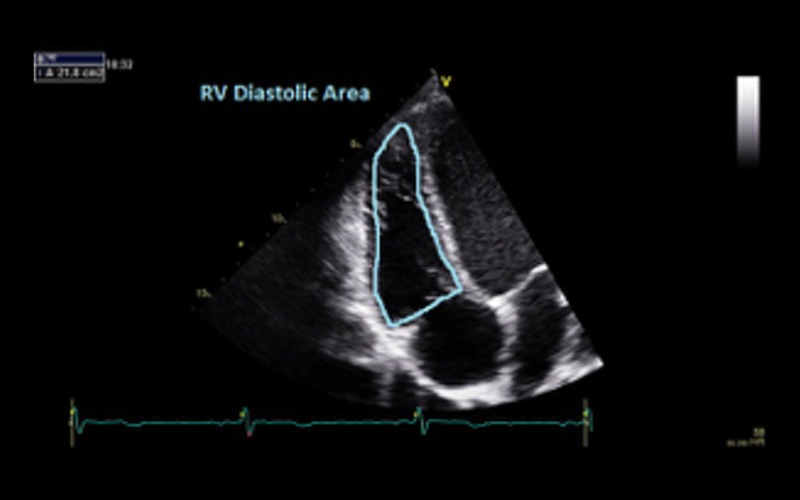 |
|
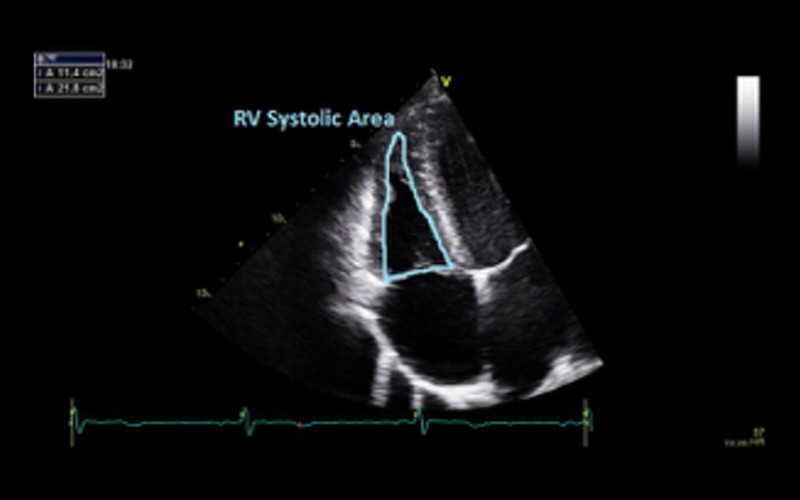 |
|||
| Focussed RV view M-mode | TAPSE | TAPSE of <17 mm indicates RV systolic dysfunction | 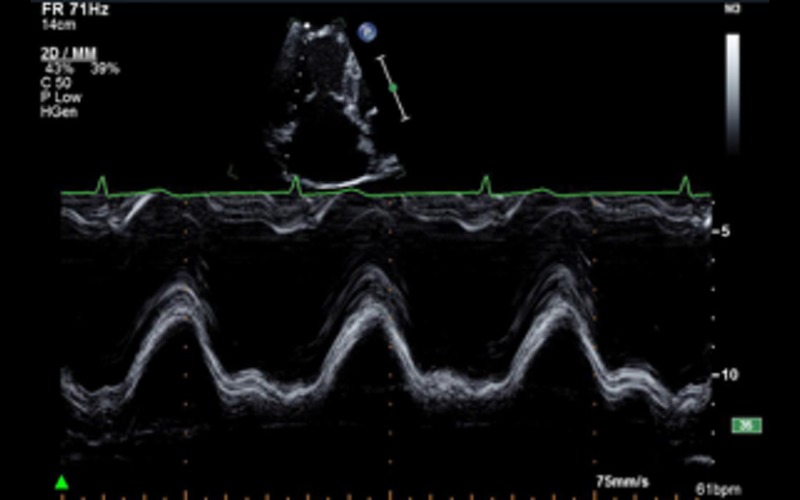 |
| Focussed RV view tdi | Tricuspid annulus S velocity | S velocity <9.5 cm/s indicates RV systolic dysfunction | 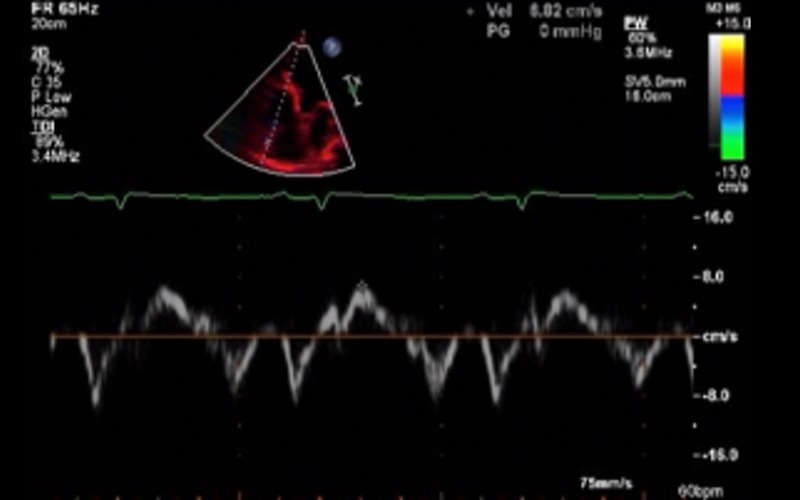 |
| Useful additional measurements | |||
| TR CW/CFM | Severity of TR and TR Vmax should be assessed in all views | See Appendix 1b Raised PA pressure is an adverse prognostic marker (15) |
|
| LA 2D/M-mode | LA dimension and volume | Measured at end systole and BSA indexed. LA dilatation suggests increased pressure and is an adverse prognostic marker (16) (please refer to BSE chamber quantification guidelines for reference values) (7) |
|
| dp/dt CW | Measured from MR jet indicates change in LV pressure over time during systole | Record MR spectral profile at a high sweep speed (typically 100 mm/s). The time interval between the points at which the velocity is 1 m/s and 3 m/s is measured. According to the Bernoulli equation, the pressure increase in the left ventricle between these points is 32 mmHg (ΔP = 4 × V22 − 4 × V12 = 4 × 32 − 4 × 12). Thus, dP/dt (in mmHg/s) is calculated from the following formula: LV dP/dt max = 32 mmHg/time (s) dp/dt is a measure of global LV contractility Normal value: 1000–1200 mmHg/s dp/dt <600 mmHg/s is an adverse prognostic marker (17) See Appendix 1d |
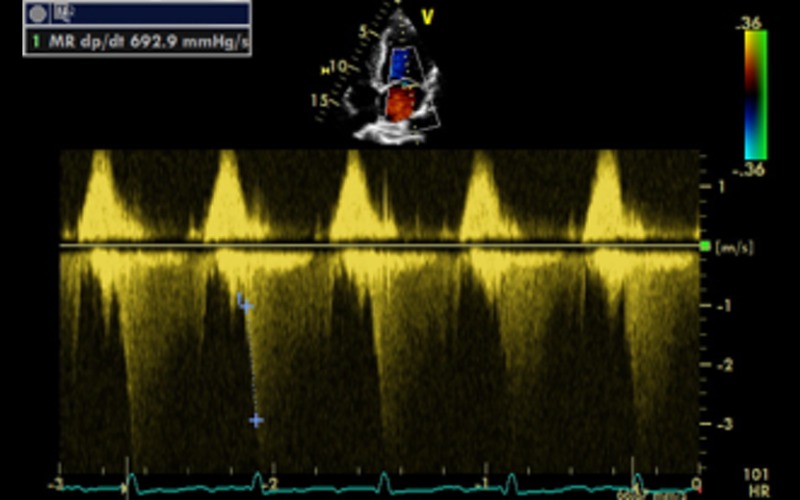 |
| Dysynchrony indices M-mode | Septal to posterior wall delay | Septal to posterior wall delay of more than 130 ms suggest intraventricular dysynchrony Value of more than 30 ms between right and left ventricular pre ejection time suggests inter ventricular dysynchrony |
|
| Dysynchrony indices PW | Difference between RV and LV ejection time | Average delay of more than 65 ms from onset of QRS to peak S wave from 4 basal segments suggests intra ventricular dysynchrony | |
| Dysynchrony indices TDI | Time from QRS onset to peak S wave from basal segments | Reduced aortic valve opening may be indicative of reduced stroke volume. However, if calcified may be due to primary AoV disease | |
| Aortic valve M-mode | Pattern of aortic valve opening | Spontaneous contrast is commonly seen in dilated, poorly functioning chambers and should prompt assessment for intra cardiac thrombus | |
| Thrombus 2D | |||
2.4. The diagnostic criteria for DCM require exclusion of chronic pressure overload (such as hypertension and left ventricular outflow obstruction), chronic volume overload states (intra-cardiac shunts and valvular regurgitation) and ischaemic heart disease.
Abbreviations
| Views | |
| A2C | Apical two chamber |
| A4C | Apical four chamber |
| A5C | Apical five chamber |
| A3C | Apical long axis or apical three chamber |
| PLAX | Parasternal long axis |
| PSAX | Parasternal short axis SC subcostal |
| SSN | Suprasternal |
| Modality | |
| CFM | Colour flow Doppler |
| CW | Continuous wave Doppler PW Pulse wave Doppler |
| TDI | Tissue Doppler imaging |
| Explanatory test | |
| 2D | 2-Dimensional echocardiography |
| 3DE | 3-Dimensional echocardiography |
| AML | Anterior mitral valve leaflet |
| ARVC | Arrhythmogenic right ventricular cardiomyopathy |
| AoV | Aortic valve |
| AV | Atrio-ventricular |
| BSA | Body surface area |
| BSE | British Society of Echocardiography |
| CRT | Cardiac resynchronisation therapy |
| DCM | dilated cardiomyopathy |
| dp/dt | LV pressure rise in systole divided by time (rate of pressure rise) |
| e′ | Early myocardial diastolic velocity on tissue Doppler imaging |
| E/e′ | Ratio of MV E Vmax/ tissue Doppler early myocardial relaxation velocity |
| EF | Ejection fraction |
| EPSS | E point septal separation |
| FAC | Fractional area change |
| FS | Fractional shortening |
| ICM | Ischaemic cardiomyopathy |
| IVS | Interventricular septum |
| IVSd | Interventricular septal thickness diastole |
| LA | Left atrium |
| LV | Left ventricle |
| LVIDd | Left ventricular internal dimension in diastole |
| LVIDs | Left ventricular internal dimension in systole |
| LVNC | Left ventricular non-compaction |
| LVPWd | Left ventricular posterior (inferolateral) wall thickness in diastole |
| MR | Mitral regurgitation |
| RV | Right ventricle |
| RVD1 | Right ventricular internal dimension |
| SI | Sphericity index |
| S′ | Peak myocardial systolic velocity on tissue Doppler imaging |
| TAPSE | Tricuspid annular plane systolic excursion |
| TR | Tricuspid regurgitation |
| PA | Pulmonary artery |
| RV | Right ventricle |
| RVD | Right ventricular dimension |
| RVSP | Right ventricular systolic pressure |
| TOE | Trans-oesophageal echocardiography |
Appendix 1: Important additional considerations
Diagnostic criteria: Diagnostic criteria of LV dilatation (>112% corrected to BSA and age) with reduced function (FS <25% and/or LVEF <45%) applies to ‘idiopathic’ DCM once secondary causes (hypertension, coronary artery disease, excess alcohol consumption, tachycardia-induced cardiomyopathy, systemic or pericardial disease, cor pulmonale and congenital heart disease) have been excluded (18, 19, 20). The value of 117% is equivalent to 2 s.d. from the upper normal limit corrected to age and BSA plus 5%. For the purposes of this guideline, it is recommended that a value above 2 s.d.s for age, sex and BSA should be used in diagnosis, using linear and volumetric methods, whether 2D or 3D.
-
Mitral regurgitation: MR is common in patients with DCM and is due to disease of the ventricle rather than a primary lesion of the mitral valve leaflets. Functional MR results from tethering of the leaflets secondary to a change in LV geometry leading to a tenting pattern. Identification of the mechanism of MR is important because chronic MR due to primary mitral valve disease can also present with findings similar to DCM. Echocardiographic features that suggest functional MR include the following (in the absence of any obvious morphological abnormality of the leaflets):
symmetric tenting of leaflets (asymmetric is more commonly seen in ischaemic CMP)
reduced leaflet coaptation zone*
central jet of MR
increased coaptation depth*
increased tenting area*
-
dilated mitral valve annulus
*see European Association of Cardiovascular Imaging Valve Regurgitation Guidelines (21).
Transthoracic echocardiography usually differentiates primary from functional MR, but in a small proportion of patients, trans-oesophageal echocardiography may be required for further clarification.
Tricuspid regurgitation (TR): Functional TR in DCM is a marker of RV dilatation, RV dysfunction or pulmonary hypertension. Pulmonary artery pressure measured from TR velocity provides additional prognostic information in patients with DCM and should be assessed in all cases. Peak TR velocity of more than 2.5 m/s is associated with increased mortality, increased hospitalisation and higher incidence of heart failure (15).
Role of dysynchrony indices: Mechanical dysynchrony is common in patients with DCM and detection of dysynchrony is important to identify patients who may benefit from cardiac resynchronisation therapy (CRT). Several echocardiographic parameters have been used to assess mechanical dysynchrony in small subsets of patients; however, the wider role of echocardiography in selecting patients for CRT is still debated. Currently, the role of echo is limited to patients with borderline QRS duration (120–149 ms) in which the presence of inter or intraventricular dysynchrony may provide additional information (22). Assessment of dysynchrony is therefore not part of the routine dataset for patients with DCM and should be used as an option in selected cases only.
Stress echo and contractile reserve: Assessment of contractile reserve by low-dose dobutamine stress echocardiography may refine prognosis in patients with DCM and may be used when cardiopulmonary exercise stress testing is not available or in patients who are unable to exercise. An increase in LV EF from rest to peak stress by ≥5% or a percentage change from baseline of ≥20% indicates the presence of contractile reserve. Its absence is an adverse prognostic marker (23).
Role of echo in family screening: Asymptomatic first-degree relatives of patients with idiopathic DCM should have an echocardiogram every 3–5 years or anytime signs or symptoms occur (24).
Appendix 2: Other related cardiomyopathic conditions
Ischaemic cardiomyopathy (ICM): Ischaemic cardiomyopathy can be differentiated from dilated cardiomyopathy by echocardiography in the vast majority of cases. LV dilatation in ICM usually occurs due to adverse remodelling after transmural myocardial infarction. During echocardiography, this is manifested by thinned, akinetic myocardial segments in the infarcted territory with cavity dilatation and mild compensatory hypertrophy of the non-infarcted segments. Infrequently, ICM can present as global hypokinesis mimicking DCM. When this occurs, it is usually in the presence of severe three-vessel coronary artery disease. In these situations, further assessment with stress echocardiography, stress cardiac MRI, coronary angiography or CT coronary angiography can be considered to exclude an ischaemic cause.
-
Left ventricular non-compaction cardiomyopathy (LVNC): LVNC is an unclassified cardiomyopathy and advanced cases can present with findings similar to DCM (i.e., a dilated and impaired left ventricle). Similarly, patients with DCM may have some echocardiographic features of LVNC which, if not recognised, can lead to over diagnosis. Echocardiographic criteria for the diagnosis of non-compaction include (25):
A two-layer structure with a compacted epicardial and non-compacted endocardial layer with maximal end systolic ratio of non-compacted to compacted layers of >2.
Predominant localisation of pathology to the apex, mid-lateral and mid-inferior segments.
Colour Doppler evidence of blood flow amid the myocardial wall deep recesses and LV cavity.
Absence of any other coexisting cardiac abnormalities (e.g. primary valve disease).
Peri-partum cardiomyopathy (PPCM): PPCM is an idiopathic cardiomyopathy that presents with heart failure secondary to left ventricular systolic dysfunction; usually seen towards the end of pregnancy or in the first 5 months after delivery; in the absence of any other cause of heart failure. A reduction in LV EF (usually <45%) is required to establish the diagnosis but the left ventricle may or may not be dilated. Other features are similar to DCM.
Toxic causes: A number of substances can be directly toxic to cardiac myocytes and result in depressed cardiac function and a phenotype of DCM. Most common agents include alcohol and chemotherapeutic drugs used for the treatment of cancers. Alcoholic cardiomyopathy most typically affects men between the ages of 35 and 50 years although women can also be affected. There is usually a long and significant history of heavy alcohol use. In addition to standard heart failure treatment, it is essential that affected patients abstain completely from alcohol. If identified at an early stage, cardiac function can improve with abstinence from alcohol but if severe LV dysfunction has developed, the damage may be irreversible. Chemotherapeutic drugs such as trastuzumab (Herceptin), anthracyclines and cyclophosphamide are most commonly implicated in causing impaired LV function. Regular monitoring for cardiotoxicity with serial echocardiography is usually recommended, and the chemotherapeutic agent may need to be stopped or changed if signs of cardiotoxicity develop. In addition conditions that result in excess iron deposition within the heart can lead to a phenotype of DCM. These include conditions such as hemochromatosis (genetic condition that results in excessive iron absorption) or conditions in which repeated blood transfusions are required (secondary iron overload). Treatment of this includes iron chelation agents, and in some cases, repeated venesection. Recreational drug use, particularly with cocaine can also result in DCM.
Viral/infective causes: Viral infection can result in acute and chronic myocarditis with myocyte death. In the acute phase, this can be occasionally very severe with the development of severe LV dysfunction, occasionally necessitating mechanical circulatory support or cardiac transplantation. It has also been suggested that viral persistence is implicated in the development of idiopathic DCM (26). Infection with human immunodeficiency virus (HIV) can result in a number of cardiac manifestations including the development of DCM. If DCM develops in the context of HIV, it carries a particularly unfavourable prognosis.
Systemic disease: A number of other systemic diseases can be associated with DCM. These include generalised muscles disease such as muscular dystrophy, connective tissue diseases and vasculitis. Again, the presence of cardiac involvement in these conditions is an adverse prognostic marker.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this guideline.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, Lloyd G, Masani N, Mathew T, Oxborough D, et al. 2015. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Research and Practice 2 G9–G24. ( 10.1530/ERP-14-0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith N, Steeds RP, Masani N, Sandoval J, Sharton G, Allen J, Chambers R, Jones G, Lloyd B, Rana K, et al. 2015. A systematic approach to echocardiography in hypertrophic cardiomyopathy: a guideline protocol from the British Society of Echocardiography. Echo Research and Practice 2 G1–G7. ( 10.1530/ERP-14-0115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight D, Patel K, Whelan C, Steeds RP, Harkness A, Jones R, Kanagala P, Lloyd G, Mathew T, O’Gallagher K, et al. 2013. A guideline protocol for the assessment of restrictive cardiomyopathy. London, UK: British Society of Echocardiography; (available at: http://www.bsecho.org/assessment-of-restrictive-cardiomyopathy/) [Google Scholar]
- 4.Oxborough D, Zaidi A, Sharma S, Somauroo J, Steeds RP, Bradlow W, Carr A, Jones R, Kanagala P, Knight D, et al. 2013. The Echocardiographic Assessment of the Right Ventricle with particular reference to Arrhythmogenic Right Ventricular Cardiomyopathy – A Protocol of the British Society of Echocardiography. London, UK: British Society of Echocardiography. (available at: http://www.bsecho.org/arrhythmogenic-right-ventricular-cardiomyopathy/) [Google Scholar]
- 5.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav J, Clunie S, Messere J, Cox GF, Paul R, Lurie PR, et al. 2006. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296 1867–1876. ( 10.1001/jama.296.15.1867) [DOI] [PubMed] [Google Scholar]
- 6.Hershberger RE, Morales A, Siegfried JD. 2010. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genetics in Medicine 12 655–667. ( 10.1097/GIM.0b013e3181f2481f) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masani N, Wharton G, Allen J, Chambers J, Graham J, Jones R, Rana B, Steeds R, the British Society of Echocardiography Education Committee 2011. Echocardiography: Guidelines for Chamber Quantification. London, UK: British Society of Echocardiography (available at: https://www.bsecho.org/media/40506/chamber-final-2011_2_.pdf) [Google Scholar]
- 8.Lew W, Henning H, Schelbert H, Karliner JS. 1978. Assessment of mitral valve E point-septal separation as an index of left ventricular performance in patients with acute and previous myocardial infarction. American Journal of Cardiology 41 836–845. ( 10.1016/0002-9149(78)90722-1) [DOI] [PubMed] [Google Scholar]
- 9.McKaigney CJ, Krantz MJ, La Rocque CL, Hurst ND, Buchanan MS, Kendall JL. 2014. E-point septal separation: a bedside tool for emergency physician assessment of left ventricular ejection fraction. American Journal of Emergency Medicine 32 493–497. ( 10.1016/j.ajem.2014.01.045) [DOI] [PubMed] [Google Scholar]
- 10.Steeds RP, Rana B, Allen J, Chambers J, Jones R, Lloyd G, Smith N, Sandoval J, Wharton G. 2011. A Guideline Protocol for the Assessment of the Mitral Valve With a View to Repair. London, UK: British Society of Echocardiography; (available at: http://www.bsecho.org/mitral-valve-repair) [Google Scholar]
- 11.Tischler MD, Niggel J, Borowski DT, LeWinter MM. 1993. Relation between left ventricular shape and exercise capacity in patients with left ventricular dysfunction. Journal of the American College of Cardiology 22 751–757. ( 10.1016/0735-1097(93)90187-6) [DOI] [PubMed] [Google Scholar]
- 12.Mathew T, Steeds RP, Jones R, Kanagala P, Lloyd G, Knight D, O’Gallagher K, Oxborough D, Rana B, Ring L, et al. 2013. A Guideline Protocol for the Echocardiographic assessment of Diastolic Dysfunction. London, UK: British Society of Echocardiography; (available at: http://www.bsecho.org/diastolic-dysfunction/) [Google Scholar]
- 13.Ristow B, Na B, Ali S, Whooley MA, Schiller NB. 2011. Left ventricular outflow tract and pulmonary artery stroke distances independently predict heart failure hospitalization and mortality: the Heart and Soul Study. Journal of American Society of Echocardiography 24 565–572. ( 10.1016/j.echo.2010.12.024) [DOI] [PubMed] [Google Scholar]
- 14.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. 1998. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. Journal of American College of Cardiology 32 948 ( 10.1016/S0735-1097(98)00337-4) [DOI] [PubMed] [Google Scholar]
- 15.Hung J, Koelling T, Semigran MJ, Dec GW, Levine RA, Di Salvo TG. 1998. Usefulness of echocardiographic determined tricuspid regurgitation in predicting event-free survival in severe heart failure secondary to idiopathic-dilated cardiomyopathy or to ischemic cardiomyopathy. American Journal of Cardiology 82 1301–1303, A10. ( 10.1016/S0002-9149(98)00624-9) [DOI] [PubMed] [Google Scholar]
- 16.Ferreira F, Galrinho A, Soares R, Branco L, Abreu J, Feliciano J, Papoila AL, Virella D, Leal A, Cruz Ferreira R. 2013. Prognostic value of left atrial volume in patients with dilated cardiomyopathy. Revista Portuguesa de Cardiologia 32 865–872. ( 10.1016/j.repce.2013.10.028) [DOI] [PubMed] [Google Scholar]
- 17.Kolias TJ, Aaronson KD, Armstrong WF. 2000. Doppler-derived dP/dt and −dP/dt predict survival in congestive heart failure. Journal of the American College of Cardiology 36 1594 ( 10.1016/S0735-1097(00)00908-6) [DOI] [PubMed] [Google Scholar]
- 18.Henry WL, Gardin JM, Ware JH. 1980. Echocardiographic measurements in normal subjects from infancy to old age. Circulation 62 1054–1061. ( 10.1161/01.CIR.62.5.1054) [DOI] [PubMed] [Google Scholar]
- 19.Manolio TA, Baughman KL, Rodeheffer R, Pearson TA, Bristow JD, Michels VV, Abelmann WH, Harlan WR. 1992. Prevalence and etiology of idiopathic dilated cardiomyopathy (summary of a National Heart, Lung and Blood Institute Workshop). American Journal of Cardiology 69 1459–1466. ( 10.1016/0002-9149(92)90901-a) [DOI] [PubMed] [Google Scholar]
- 20.Mestroni L, Maisch B, McKenna WJ, Schwartz K, Charron P, Rocco C, Tesson F, Richter A, Wilke A, Komajda M. 1999. Collaborative research group of the European human and capital mobility project on familial dilated cardiomyopathy. Guidelines for the study of familial dilated cardiomyopathies. European Heart Journal 20 93–102. ( 10.1053/euhj.1998.1145) [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. 2013. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging 14 611–644. ( 10.1093/ehjci/jet105) [DOI] [PubMed] [Google Scholar]
- 22.Gorcsan J, III, Abraham T, Agler DA, Bax JJ, Dedrumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM. 2008. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting – a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. Journal of the American Society of Echocardiography 2 192–211. [DOI] [PubMed] [Google Scholar]
- 23.Neskovic AN, Otasevic P. 2005. Stress-echocardiography in idiopathic dilated cardiomyopathy: instructions for use. Cardiovascular Ultrasound 3 1476–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA. 2009. Genetic evaluation of cardiomyopathy – a Heart Failure Society of America practice guideline. Heart Failure Society of America. Journal of Cardiac Failure 15 83 ( 10.1016/j.cardfail.2009.01.006) [DOI] [PubMed] [Google Scholar]
- 25.Jenni R, Oeschlin EN, van der Loo B. 2007. Isolated ventricular non-compaction of the myocardium in adults. Heart 93 11–15. ( 10.1136/hrt.2005.082271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazebroek M, Dennert R, Heymans S. 2012. Idiopathic dilated cardiomyopathy: possible triggers and treatment strategies. Netherlands Heart Journal 20 332–335. ( 10.1007/s12471-012-0285-7) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a